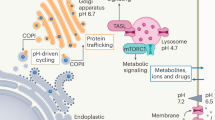
Abstract
α-Ketoglutarate (αKG) is a key node in many important metabolic pathways. The αKG analog N-oxalylglycine (NOG) and its cell-permeable prodrug dimethyloxalylglycine (DMOG) are extensively used to inhibit αKG-dependent dioxygenases. However, whether NOG interference with other αKG-dependent processes contributes to its mode of action remains poorly understood. Here we show that, in aqueous solutions, DMOG is rapidly hydrolyzed, yielding methyloxalylglycine (MOG). MOG elicits cytotoxicity in a manner that depends on its transport by monocarboxylate transporter 2 (MCT2) and is associated with decreased glutamine-derived tricarboxylic acid–cycle flux, suppressed mitochondrial respiration and decreased ATP production. MCT2-facilitated entry of MOG into cells leads to sufficiently high concentrations of NOG to inhibit multiple enzymes in glutamine metabolism, including glutamate dehydrogenase. These findings reveal that MCT2 dictates the mode of action of NOG by determining its intracellular concentration and have important implications for the use of (D)MOG in studying αKG-dependent signaling and metabolism.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Accession codes and relevant web links can be found in the respective legends and methods sections.
Change history
23 October 2019
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Zhang, J., Pavlova, N. N. & Thompson, C. B. Cancer cell metabolism: the essential role of the nonessential amino acid, glutamine. EMBO J. 36, 1302–1315 (2017).
Altman, B. J., Stine, Z. E. & Dang, C. V. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat. Rev. Cancer 16, 619–634 (2016).
Still, E. R. & Yuneva, M. O. Hopefully devoted to Q: targeting glutamine addiction in cancer. Br. J. Cancer 116, 1375–1381 (2017).
Zdzisińska, B., Żurek, A. & Kandefer-Szerszeń, M. Alpha-ketoglutarate as a molecule with pleiotropic activity: well-known and novel possibilities of therapeutic use. Arch. Immunol. Ther. Exp. (Warsz.) 65, 21–36 (2017).
Anastasiou, D. & Cantley, L. C. Breathless cancer cells get fat on glutamine. Cell Res. 22, 443–446 (2012).
Jiang, L. et al. Reductive carboxylation supports redox homeostasis during anchorage-independent growth. Nature 532, 255–258 (2016).
Loenarz, C. & Schofield, C. J. Physiological and biochemical aspects of hydroxylations and demethylations catalyzed by human 2-oxoglutarate oxygenases. Trends Biochem. Sci. 36, 7–18 (2011).
Ivan, M. & Kaelin, W. G. Jr. The EGLN-HIF O2-sensing system: multiple inputs and feedbacks. Mol. Cell 66, 772–779 (2017).
Chan, M. C., Holt-Martyn, J. P., Schofield, C. J. & Ratcliffe, P. J. Pharmacological targeting of the HIF hydroxylases: a new field in medicine development. Mol. Aspects Med. 47-48, 54–75 (2016).
Son, J. et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 496, 101–105 (2013).
Jin, L. et al. Glutamate dehydrogenase 1 signals through antioxidant glutathione peroxidase 1 to regulate redox homeostasis and tumor growth. Cancer Cell 27, 257–270 (2015).
Mariño, G. et al. Regulation of autophagy by cytosolic acetyl-coenzyme A. Mol. Cell 53, 710–725 (2014).
Taniguchi, C. M. et al. Cross-talk between hypoxia and insulin signaling through Phd3 regulates hepatic glucose and lipid metabolism and ameliorates diabetes. Nat. Med. 19, 1325–1330 (2013).
Aragonés, J. et al. Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat. Genet. 40, 170–180 (2008).
Olenchock, B. A. et al. EGLN1 inhibition and rerouting of α-ketoglutarate suffice for remote ischemic protection. Cell 164, 884–895 (2016).
Eltzschig, H. K., Bratton, D. L. & Colgan, S. P. Targeting hypoxia signalling for the treatment of ischaemic and inflammatory diseases. Nat. Rev. Drug Discov. 13, 852–869 (2014).
Rose, N. R., McDonough, M. A., King, O. N., Kawamura, A. & Schofield, C. J. Inhibition of 2-oxoglutarate dependent oxygenases. Chem. Soc. Rev. 40, 4364–4397 (2011).
Cunliffe, C. J., Franklin, T. J., Hales, N. J. & Hill, G. B. Novel inhibitors of prolyl 4-hydroxylase. 3. Inhibition by the substrate analogue N-oxaloglycine and its derivatives. J. Med. Chem. 35, 2652–2658 (1992).
Jaakkola, P. et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292, 468–472 (2001).
Ivan, M. et al. HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292, 464–468 (2001).
Hamada, S. et al. Synthesis and activity of N-oxalylglycine and its derivatives as Jumonji C-domain-containing histone lysine demethylase inhibitors. Bioorg. Med. Chem. Lett. 19, 2852–2855 (2009).
Baader, E., Tschank, G., Baringhaus, K. H., Burghard, H. & Günzler, V. Inhibition of prolyl 4-hydroxylase by oxalyl amino acid derivatives in vitro, in isolated microsomes and in embryonic chicken tissues. Biochem. J. 300, 525–530 (1994).
Fraisl, P., Aragonés, J. & Carmeliet, P. Inhibition of oxygen sensors as a therapeutic strategy for ischaemic and inflammatory disease. Nat. Rev. Drug Discov. 8, 139–152 (2009).
Leite de Oliveira, R. et al. Gene-targeting of Phd2 improves tumor response to chemotherapy and prevents side-toxicity. Cancer Cell 22, 263–277 (2012).
Fukuda, R. et al. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell 129, 111–122 (2007).
Kim, J. W., Tchernyshyov, I., Semenza, G. L. & Dang, C. V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 3, 177–185 (2006).
Sun, R. C. & Denko, N. C. Hypoxic regulation of glutamine metabolism through HIF1 and SIAH2 supports lipid synthesis that is necessary for tumor growth. Cell Metab. 19, 285–292 (2014).
Garnett, M. J. et al. Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature 483, 570–575 (2012).
Halestrap, A. P. The SLC16 gene family: structure, role and regulation in health and disease. Mol. Aspects Med. 34, 337–349 (2013).
Pérez-Escuredo, J. et al. Monocarboxylate transporters in the brain and in cancer. Biochim. Biophys. Acta 1863, 2481–2497 (2016).
Hudson, R. F. The perturbation treatment of chemical reactivity. Angew. Chem. Int. Edn Engl. 12, 36–56 (1973).
Sekine, N. et al. Low lactate dehydrogenase and high mitochondrial glycerol phosphate dehydrogenase in pancreatic beta-cells: potential role in nutrient sensing. J. Biol. Chem. 269, 4895–4902 (1994).
Bröer, S. et al. Characterization of the high-affinity monocarboxylate transporter MCT2 in Xenopus laevis oocytes. Biochem. J. 341, 529–535 (1999).
Fan, J. et al. Glutamine-driven oxidative phosphorylation is a major ATP source in transformed mammalian cells in both normoxia and hypoxia. Mol. Syst. Biol. 9, 712 (2013).
Zhdanov, A. V., Okkelman, I. A., Collins, F. W., Melgar, S. & Papkovsky, D. B. A novel effect of DMOG on cell metabolism: direct inhibition of mitochondrial function precedes HIF target gene expression. Biochim. Biophys. Acta 1847, 1254–1266 (2015).
Rendina, A. R. et al. Mutant IDH1 enhances the production of 2-hydroxyglutarate due to its kinetic mechanism. Biochemistry 52, 4563–4577 (2013).
Kell, D. B. Finding novel pharmaceuticals in the systems biology era using multiple effective drug targets, phenotypic screening and knowledge of transporters: where drug discovery went wrong and how to fix it. FEBS J. 280, 5957–5980 (2013).
Gong, L., Goswami, S., Giacomini, K. M., Altman, R. B. & Klein, T. E. Metformin pathways: pharmacokinetics and pharmacodynamics. Pharmacogenet. Genomics 22, 820–827 (2012).
Gan, L. et al. Metabolic targeting of oncogene MYC by selective activation of the proton-coupled monocarboxylate family of transporters. Oncogene 35, 3037–3048 (2016).
Pértega-Gomes, N. et al. Monocarboxylate transporter 2 (MCT2) as putative biomarker in prostate cancer. Prostate 73, 763–769 (2013).
Christen, S. et al. Breast cancer-derived lung metastases show increased pyruvate carboxylase-dependent anaplerosis. Cell Rep. 17, 837–848 (2016).
Avril, N. GLUT1 expression in tissue and (18)F-FDG uptake. J. Nucl. Med. 45, 930–932 (2004).
Kulkarni, A. et al. Glucose metabolism and oxygen availability govern reactivation of the latent human retrovirus HTLV-1. Cell Chem. Biol. 24, 1377–1387.e1373 (2017).
Daberkow, R. L., White, B. R., Cederberg, R. A., Griffin, J. B. & Zempleni, J. Monocarboxylate transporter 1 mediates biotin uptake in human peripheral blood mononuclear cells. J. Nutr. 133, 2703–2706 (2003).
Overington, J. P., Al-Lazikani, B. & Hopkins, A. L. How many drug targets are there? Nat. Rev. Drug Discov. 5, 993–996 (2006).
Hopkins, A. L., Mason, J. S. & Overington, J. P. Can we rationally design promiscuous drugs? Curr. Opin. Struct. Biol. 16, 127–136 (2006).
Knight, Z. A., Lin, H. & Shokat, K. M. Targeting the cancer kinome through polypharmacology. Nat. Rev. Cancer 10, 130–137 (2010).
Zhang, J. et al. EglN2 associates with the NRF1-PGC1α complex and controls mitochondrial function in breast cancer. EMBO J. 34, 2953–2970 (2015).
Papandreou, I., Cairns, R. A., Fontana, L., Lim, A. L. & Denko, N. C. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 3, 187–197 (2006).
Ran, F. A. et al. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281–2308 (2013).
Starck, S. R., Green, H. M., Alberola-Ila, J. & Roberts, R. W. A general approach to detect protein expression in vivo using fluorescent puromycin conjugates. Chem. Biol. 11, 999–1008 (2004).
Allen, A., Kwagh, J., Fang, J., Stanley, C. A. & Smith, T. J. Evolution of glutamate dehydrogenase regulation of insulin homeostasis is an example of molecular exaptation. Biochemistry 43, 14431–14443 (2004).
Frezza, C., Cipolat, S. & Scorrano, L. Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat. Protoc. 2, 287–295 (2007).
Zhang, T., Creek, D. J., Barrett, M. P., Blackburn, G. & Watson, D. G. Evaluation of coupling reversed phase, aqueous normal phase, and hydrophilic interaction liquid chromatography with Orbitrap mass spectrometry for metabolomic studies of human urine. Anal. Chem. 84, 1994–2001 (2012).
Schleucher, J. et al. A general enhancement scheme in heteronuclear multidimensional NMR employing pulsed field gradients. J. Biomol. NMR 4, 301–306 (1994).
Bax, A. & Summers, M. F. H-1 and C-13 assignments from sensitivity-enhanced detection of heteronuclear multiple-bond connectivity by 2D multiple quantum NMR. J. Am. Chem. Soc. 108, 2093–2094 (1986).
Hwang, T. L. & Shaka, A. J. Water suppression that works: excitation sculpting using arbitrary wave-forms and pulsed-field gradients. J. Magn. Reson. 112, 275–279 (1995).
Dalvit, C., Fogliatto, G., Stewart, A., Veronesi, M. & Stockman, B. WaterLOGSY as a method for primary NMR screening: practical aspects and range of applicability. J. Biomol. NMR 21, 349–359 (2001).
London, R. E. & Gabel, S. A. Determination of membrane potential and cell volume by 19F NMR using trifluoroacetate and trifluoroacetamide probes. Biochemistry 28, 2378–2382 (1989).
Acknowledgements
We thank all members of the laboratory of D.A. for valuable discussions and input throughout this work, particularly J. Macpherson and N. Bevan for technical help. We are grateful to L. Cantley for advice during early stages of this work. We acknowledge S. O’Callaghan (Bio21 Institute, University of Melbourne) for the algorithm to correct for natural isotope abundance in metabolomics data. We thank J. Kleinjung for advice with statistical methods, M. Howell for advice and help with cell proliferation and viability measurements, J. Cerveira for help with flow cytometry measurements and A. Gould for comments on the manuscript. We are grateful to the staff at the Medical Research Council National Biomedical NMR Centre at the Francis Crick Institute, where NMR data were obtained. This work was funded by the MRC (MC_UP_1202/1) and by the Francis Crick Institute, which receives its core funding from Cancer Research UK (FC001033), the UK Medical Research Council (FC001033) and the Wellcome Trust (FC001033) to D.A.
Author information
Authors and Affiliations
Contributions
P.C.D. and T.J.R. performed NMR experiments; F.G. generated HIF1α-mutant cell lines and wrote scripts for metabolomics data analysis and visualization; A.J. and P.M.N. performed respiration experiments; M.G. assisted with the development of LC–MS analytical methods; G.D. generated and characterized cell lines; M.S.d.S. and J.I.M. assisted with and advised on metabolomics experiments; A.J.W. did flux modeling; G.P. and N.O’R. synthesized DM-[13C5]αKG; S.C. and D.H. synthesized MOG and advised on chemistry; C.H.B. contributed to the large-scale DMOG sensitivity screen; K.D.C. performed experiments and analyzed data; and L.F. and D.A. designed and performed experiments, analyzed data and wrote the manuscript. All authors reviewed and commented on the manuscript. Work by A.J. was supported by the MetaRNA Marie Skłodowska-Curie Innovative Training Nerwork (642738).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1 and 2, and Supplementary Figures 1–13
Supplementary Note
Synthetic Procedures
Supplementary Dataset 1
Spearman’s rank correlation coefficients of gene transcripts with IC50DMOG, determined as described in Methods. Genes identified as positively or negatively correlating with sensitivity (as determined by a 5% FDR) are highlighted.
Supplementary Dataset 2
Spearman’s rank correlation coefficients of gene transcripts with IC50DMOG, using only the top quartile of SLC16A7-expressing cell lines (213 lines) used in Fig. 6e, f. Genes identified as positively or negatively correlating with sensitivity (as determined by a 5% FDR) are highlighted.
Rights and permissions
About this article
Cite this article
Fets, L., Driscoll, P.C., Grimm, F. et al. MCT2 mediates concentration-dependent inhibition of glutamine metabolism by MOG. Nat Chem Biol 14, 1032–1042 (2018). https://doi.org/10.1038/s41589-018-0136-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-018-0136-y
This article is cited by
-
Mechanism of substrate hydrolysis by the human nucleotide pool sanitiser DNPH1
Nature Communications (2023)
-
Vitamin B5 supports MYC oncogenic metabolism and tumor progression in breast cancer
Nature Metabolism (2023)
-
MOG analogues to explore the MCT2 pharmacophore, α-ketoglutarate biology and cellular effects of N-oxalylglycine
Communications Biology (2022)
-
Spontaneous hydrolysis and spurious metabolic properties of α-ketoglutarate esters
Nature Communications (2021)
-
New tricks for an old drug
Nature Chemical Biology (2018)