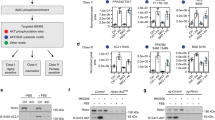
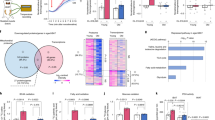
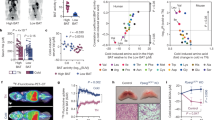
Abstract
Fatty acid synthase (FASN) predominantly generates straight-chain fatty acids using acetyl-CoA as the initiating substrate. However, monomethyl branched-chain fatty acids (mmBCFAs) are also present in mammals but are thought to be primarily diet derived. Here we demonstrate that mmBCFAs are de novo synthesized via mitochondrial BCAA catabolism, exported to the cytosol by adipose-specific expression of carnitine acetyltransferase (CrAT), and elongated by FASN. Brown fat exhibits the highest BCAA catabolic and mmBCFA synthesis fluxes, whereas these lipids are largely absent from liver and brain. mmBCFA synthesis is also sustained in the absence of microbiota. We identify hypoxia as a potent suppressor of BCAA catabolism that decreases mmBCFA synthesis in obese adipose tissue, such that mmBCFAs are significantly decreased in obese animals. These results identify adipose tissue mmBCFA synthesis as a novel link between BCAA metabolism and lipogenesis, highlighting roles for CrAT and FASN promiscuity influencing acyl-chain diversity in the lipidome.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Newgard, C. B. et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 9, 311–326 (2009).
Wang, T. J. et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 17, 448–453 (2011).
Tönjes, M. et al. BCAT1 promotes cell proliferation through amino acid catabolism in gliomas carrying wild-type IDH1. Nat. Med. 19, 901–908 (2013).
Overmyer, K. A. et al. Maximal oxidative capacity during exercise is associated with skeletal muscle fuel selection and dynamic changes in mitochondrial protein acetylation. Cell Metab. 21, 468–478 (2015).
Herman, M. A., She, P., Peroni, O. D., Lynch, C. J. & Kahn, B. B. Adipose tissue branched chain amino acid (BCAA) metabolism modulates circulating BCAA levels. J. Biol. Chem. 285, 11348–11356 (2010).
Crown, S. B., Marze, N. & Antoniewicz, M. R. Catabolism of branched chain amino acids contributes significantly to synthesis of odd-chain and even-chain fatty acids in 3T3-L1 adipocytes. PLoS ONE. 10, e0145850 (2015).
Green, C. R. et al. Branched-chain amino acid catabolism fuels adipocyte differentiation and lipogenesis. Nat. Chem. Biol. 12, 15–21 (2016).
Ran-Ressler, R. R., Bae, S., Lawrence, P., Wang, D. H. & Brenna, J. T. Branched-chain fatty acid content of foods and estimated intake in the USA. Br. J. Nutr. 112, 565–572 (2014).
Jenkins, B., West, J. A. & Koulman, A. A review of odd-chain fatty acid metabolism and the role of pentadecanoic Acid (c15: 0) and heptadecanoic Acid (c17:0) in health and disease. Molecules 20, 2425–2444 (2015).
Mercier, R., Domínguez-Cuevas, P. & Errington, J. Crucial role for membrane fluidity in proliferation of primitive cells. Cell Rep. 1, 417–423 (2012).
Kniazeva, M., Zhu, H., Sewell, A. K. & Han, M. A lipid-TORC1 pathway promotes neuronal development and foraging behavior under both fed and fasted conditions in C. elegans. Dev. Cell 33, 260–271 (2015).
Gibson, R. A. & Kneebone, G. M. Fatty acid composition of human colostrum and mature breast milk. Am. J. Clin. Nutr. 34, 252–257 (1981).
Grigor, M. R., Dunckley, G. G. & Purves, H. D. The synthesis of the branched-chain fatty acids of rat skin surface lipid. Biochim. Biophys. Acta 218, 389–399 (1970).
Ran-Ressler, R. R., Devapatla, S., Lawrence, P. & Brenna, J. T. Branched chain fatty acids are constituents of the normal healthy newborn gastrointestinal tract. Pediatr. Res. 64, 605–609 (2008).
Su, X. et al. Adipose tissue monomethyl branched-chain fatty acids and insulin sensitivity: effects of obesity and weight loss. Obesity (Silver Spring) 23, 329–334 (2015).
Mika, A. et al. A comprehensive study of serum odd- and branched-chain fatty acids in patients with excess weight. Obesity (Silver Spring) 24, 1669–1676 (2016).
Lackey, D. E. et al. Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity. Am. J. Physiol. Endocrinol. Metab. 304, E1175–E1187 (2013).
Kishimoto, Y., Williams, M., Moser, H. W., Hignite, C. & Biermann, K. Branched-chain and odd-numbered fatty acids and aldehydes in the nervous system of a patient with deranged vitamin B 12 metabolism. J. Lipid Res. 14, 69–77 (1973).
Svensson, R. U. et al. Inhibition of acetyl-CoA carboxylase suppresses fatty acid synthesis and tumor growth of non-small-cell lung cancer in preclinical models. Nat. Med. 22, 1108–1119 (2016).
Hiltunen, J. K. et al. Mitochondrial fatty acid synthesis type II: more than just fatty acids. J. Biol. Chem. 284, 9011–9015 (2009).
Lewis, C. A. et al. Tracing compartmentalized NADPH metabolism in the cytosol and mitochondria of mammalian cells. Mol. Cell 55, 253–263 (2014).
Horning, M. G., Martin, D. B., Karmen, A. & Vagelos, P. R. Fatty acid synthesis in adipose tissue. II. Enzymatic synthesis of branched chain and odd-numbered fatty acids. J. Biol. Chem. 236, 669–672 (1961).
Oku, H., Yagi, N., Nagata, J. & Chinen, I. Precursor role of branched-chain amino acids in the biosynthesis of iso and anteiso fatty acids in rat skin. Biochim. Biophys. Acta 1214, 279–287 (1994).
Liu, L. et al. Human fetal intestinal epithelial cells metabolize and incorporate branched chain fatty acids in a structure specific manner. Prostaglandins Leukot. Essent. Fatty Acids 116, 32–39 (2017).
Obayashi, M. et al. Estrogen controls branched-chain amino acid catabolism in female rats. J. Nutr. 134, 2628–2633 (2004).
Kaneda, T. Iso- and anteiso-fatty acids in bacteria: biosynthesis, function, and taxonomic significance. Microbiol. Rev. 55, 288–302 (1991).
Macfarlane, G. T., Gibson, G. R., Beatty, E. & Cummings, J. H. Estimation of short-chain fatty acid production from protein by human intestinal bacteria based on branched-chain fatty acid measurements. FEMS Microbiol. Lett. 101, 81–88 (1992).
Previs, S. F. et al. New methodologies for studying lipid synthesis and turnover: looking backwards to enable moving forwards. Biochim. Biophys. Acta 1842, 402–413 (2014).
Abdelmagid, S. A. et al. Comprehensive profiling of plasma fatty acid concentrations in young healthy Canadian adults. PLoS ONE. 10, e0116195 (2015).
Sailer, M. et al. Increased plasma citrulline in mice marks diet-induced obesity and may predict the development of the metabolic syndrome. PLoS ONE. 8, e63950 (2013).
Jenkins, B. J. et al. Odd chain fatty acids; new insights of the relationship between the gut microbiota, dietary intake, biosynthesis and glucose Intolerance. Sci. Rep. 7, 44845 (2017).
Eissing, L. et al. De novo lipogenesis in human fat and liver is linked to ChREBP-β and metabolic health. Nat. Commun. 4, 1528 (2013).
Duarte, J. A. et al. A high-fat diet suppresses de novo lipogenesis and desaturation but not elongation and triglyceride synthesis in mice. J. Lipid Res. 55, 2541–2553 (2014).
Sanchez-Gurmaches, J. et al. Brown fat AKT2 is a cold-induced kinase that stimulates ChREBP-mediated de novo lipogenesis to optimize fuel storage and thermogenesis. Cell Metab. 27, 195–209.e6 (2018).
Violante, S. et al. Substrate specificity of human carnitine acetyltransferase: implications for fatty acid and branched-chain amino acid metabolism. Biochim. Biophys. Acta 1832, 773–779 (2013).
Geiger, T. et al. Initial quantitative proteomic map of 28 mouse tissues using the SILAC mouse. Mol. Cell. Proteomics. 12, 1709–1722 (2013).
Trayhurn, P., Wang, B. & Wood, I. S. Hypoxia in adipose tissue: a basis for the dysregulation of tissue function in obesity? Br. J. Nutr. 100, 227–235 (2008).
Robciuc, M. R. et al. VEGFB/VEGFR1-induced expansion of adipose vasculature counteracts obesity and related metabolic complications. Cell Metab. 23, 712–724 (2016).
Ye, J., Gao, Z., Yin, J. & He, Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am. J. Physiol. Endocrinol. Metab. 293, E1118–E1128 (2007).
Mayers, J. R. et al. Tissue of origin dictates branched-chain amino acid metabolism in mutant Kras-driven cancers. Science 353, 1161–1165 (2016).
Jang, C. et al. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat. Med. 22, 421–426 (2016).
Anderson, K. A. et al. SIRT4 Is a lysine deacylase that controls leucine metabolism and insulin secretion. Cell Metab. 25, 838–855.e15 (2017).
Hellerstein, M. K., Neese, R. A. & Schwarz, J. M. Model for measuring absolute rates of hepatic de novo lipogenesis and reesterification of free fatty acids. Am. J. Physiol. 265, E814–E820 (1993).
Shin, A. C. et al. Brain insulin lowers circulating BCAA levels by inducing hepatic BCAA catabolism. Cell Metab. 20, 898–909 (2014).
Seiler, S. E. et al. Carnitine acetyltransferase mitigates metabolic inertia and muscle fatigue during exercise. Cell Metab. 22, 65–76 (2015).
Davies, M. N. et al. The acetyl group buffering action of carnitine acetyltransferase offsets macronutrient-induced lysine acetylation of mitochondrial proteins. Cell Rep. 14, 243–254 (2016).
Mardinoglu, A. et al. Integration of clinical data with a genome-scale metabolic model of the human adipocyte. Mol. Syst. Biol. 9, 649 (2013).
Kniazeva, M., Euler, T. & Han, M. A branched-chain fatty acid is involved in post-embryonic growth control in parallel to the insulin receptor pathway and its biosynthesis is feedback-regulated in C. elegans. Genes Dev. 22, 2102–2110 (2008).
Yan, Y. et al. BCFA suppresses LPS induced IL-8 mRNA expression in human intestinal epithelial cells. Prostaglandins Leukot. Essent. Fatty Acids 116, 27–31 (2017).
Ran-Ressler, R. R. et al. Branched chain fatty acids reduce the incidence of necrotizing enterocolitis and alter gastrointestinal microbial ecology in a neonatal rat model. PLoS ONE. 6, e29032 (2011).
Gantner, M. L., Hazen, B. C., Eury, E., Brown, E. L. & Kralli, A. Complementary roles of estrogen-related receptors in brown adipocyte thermogenic function. Endocrinology 157, 4770–4781 (2016).
Vacanti, N. M. et al. Regulation of substrate utilization by the mitochondrial pyruvate carrier. Mol. Cell 56, 425–435 (2014).
Cabrales, P. & Tsai, A. G. Plasma viscosity regulates systemic and microvascular perfusion during acute extreme anemic conditions. Am. J. Physiol. Heart Circ. Physiol. 291, H2445–H2452 (2006).
Decaris, M. L. et al. Identifying nonalcoholic fatty liver disease patients with active fibrosis by measuring extracellular matrix remodeling rates in tissue and blood. Hepatology 65, 78–88 (2017).
Fernandez, C. A., Des Rosiers, C., Previs, S. F., David, F. & Brunengraber, H. Correction of 13C mass isotopomer distributions for natural stable isotope abundance. J. Mass. Spectrom. 31, 255–262 (1996).
Young, J. D. INCA: a computational platform for isotopically non-stationary metabolic flux analysis. Bioinformatics 30, 1333–1335 (2014).
Lee, W. N. et al. In vivo measurement of fatty acids and cholesterol synthesis using D2O and mass isotopomer analysis. Am. J. Physiol. 266, E699–E708 (1994).
Louie, S. M. et al. GSTP1 Is a driver of triple-negative breast cancer cell metabolism and pathogenicity. Cell Chem. Biol. 23, 567–578 (2016).
Acknowledgements
This work was supported, in part, by US National Institutes of Health (NIH) grants R01CA188652 (C.M.M.), NIH R01CA172667 (D.K.N.), P01-HL110900 (P.C.) and R01-HL126945 (P.C.), a Searle Scholar Award (C.M.M.), a Camille and Henry Dreyfus Teacher-Scholar Award (C.M.M.), an NSF CAREER Award (#1454425 to C.M.M.), and an Ajinomoto Innovation Alliance Program Grant (C.M.M). The project was funded (in part) by a seed grant made available through the UC San Diego Larsson-Rosenquist Foundation Mother-Milk-Infant Center of Research Excellence (M.W). J.S.G. is supported by AHA award 18CDA34080527. This material is the result of work supported with resources and the use of facilities at the VA San Diego Medical Center. The contents do not represent the views of the US Department of Veterans Affairs or the United States Government. We would like to thank M. Gantner for helping with primary brown adipose tissue isolation and culture.
Author information
Authors and Affiliations
Contributions
M.W. and C.M.M. conceived and designed the study. C.R.G. carried out knock down and analysis of ACAD enzymes and assisted with design and execution of all 3T3L1 experiments; L.S.R. and D.K.N. carried out lipidomic analysis of 13C-traced adipocytes; and J.L.M., Y.M.L. and J.S.A. provided germ-free and SPF samples. T.P.C. and S.A.P. isolated and cultured human primary cells. N.M. and J.M.G. assisted with GC–MS analysis of in vivo samples; J.D.H. carried out gene expression analysis of tissue samples, J.S.G. and D.A.G. carried out D2O thermovariation experiments in mice. P.C. carried out in vivo oxygen tension and arterial blood flow studies in mice. M.W. performed all other experiments. R.L. designed and executed the clinical study of NAFL and NASH patients. M.W. and C.M.M. wrote the paper with help from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Tables and Figures
Supplementary Tables 1–7, Supplementary Figures 1–12
Rights and permissions
About this article
Cite this article
Wallace, M., Green, C.R., Roberts, L.S. et al. Enzyme promiscuity drives branched-chain fatty acid synthesis in adipose tissues. Nat Chem Biol 14, 1021–1031 (2018). https://doi.org/10.1038/s41589-018-0132-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-018-0132-2
This article is cited by
-
BCAA metabolism in pancreatic cancer affects lipid balance by regulating fatty acid import into mitochondria
Cancer & Metabolism (2024)
-
Branched-chain amino acid catabolism in muscle affects systemic BCAA levels but not insulin resistance
Nature Metabolism (2023)
-
NME4 mediates metabolic reprogramming and promotes nonalcoholic fatty liver disease progression
EMBO Reports (2023)
-
Lipid remodeling of adipose tissue in metabolic health and disease
Experimental & Molecular Medicine (2023)
-
The metabolomic profiling of total fat and fat distribution in a multi-cohort study of women and men
Scientific Reports (2023)