Abstract
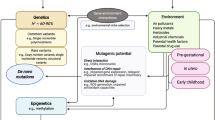
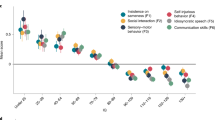
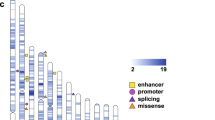
The genetic etiology of autism spectrum disorder (ASD) is multifactorial, but how combinations of genetic factors determine risk is unclear. In a large family sample, we show that genetic loads of rare and polygenic risk are inversely correlated in cases and greater in females than in males, consistent with a liability threshold that differs by sex. De novo mutations (DNMs), rare inherited variants and polygenic scores were associated with various dimensions of symptom severity in children and parents. Parental age effects on risk for ASD in offspring were attributable to a combination of genetic mechanisms, including DNMs that accumulate in the paternal germline and inherited risk that influences behavior in parents. Genes implicated by rare variants were enriched in excitatory and inhibitory neurons compared with genes implicated by common variants. Our results suggest that a phenotypic spectrum of ASD is attributable to a spectrum of genetic factors that impact different neurodevelopmental processes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
WGS data from the SSC and exome and SNP genotyping data from SPARK are available from SFARI (https://www.sfari.org/resource/autism-cohorts). Summary genetic data from WGS and exomes, including individual counts for dnLoF, dnMIS, inhLoF and PSs for all subjects in the present study and input data files for all analysis code, are also available from SFARI. WGS data from the REACH project are available from the National Institute of Mental Health Data Archive (NDA), including the structural variant callset and raw sequence (FASTQ), alignment (BAM) and VCF files from the REACH cohort (https://nda.nih.gov/edit_collection.html?id=2019). GWAS summary statistics are available from the Psychiatric Genomics Consortium (ASD and SZ) (https://www.med.unc.edu/pgc/download-results) and the Social Science Genetic Association Consortium (EA) (http://www.thessgac.org/data). Bulk tissue expression data on ASD susceptibility genes was obtained from the BrainSpan developmental transcriptome dataset (v.0; https://www.brainspan.org/api/v2/well_known_file_download/267666525). Cell-type expression levels of ASD susceptibility genes in fetal cortex were obtained through the web interface of the CoDEx viewer (http://solo.bmap.ucla.edu/shiny/webapp).
Code availability
Analysis code for all major statistical genetic analyses in the paper and for generating Figs. 1–7 is available as a Google Colab notebook on Github (https://github.com/sebatlab/Antaki2021).
Change history
29 June 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41588-022-01145-5
References
Sebat, J. et al. Strong association of de novo copy number mutations with autism. Science 316, 445–449 (2007).
Iossifov, I. et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature 515, 216–221 (2014).
Grove, J. et al. Identification of common genetic risk variants for autism spectrum disorder. Nat. Genet. 51, 431–444 (2019).
Sebat, J., Levy, D. L. & McCarthy, S. E. Rare structural variants in schizophrenia: one disorder, multiple mutations; one mutation, multiple disorders. Trends Genet. 25, 528–535 (2009).
Bergen, S. E. et al. Joint contributions of rare copy number variants and common SNPs to risk for schizophrenia. Am. J. Psychiatry 176, 29–35 (2019).
Davies, R. W. et al. Using common genetic variation to examine phenotypic expression and risk prediction in 22q11.2 deletion syndrome. Nat. Med. 26, 1912–1918 (2020).
Lim, E. T. et al. Rare complete knockouts in humans: population distribution and significant role in autism spectrum disorders. Neuron 77, 235–242 (2013).
Zhao, X. et al. A unified genetic theory for sporadic and inherited autism. Proc. Natl Acad. Sci. USA 104, 12831–12836 (2007).
Werling, D. M. & Geschwind, D. H. Recurrence rates provide evidence for sex-differential, familial genetic liability for autism spectrum disorders in multiplex families and twins. Mol. Autism 6, 27 (2015).
Robinson, E. B., Lichtenstein, P., Anckarsater, H., Happe, F. & Ronald, A. Examining and interpreting the female protective effect against autistic behavior. Proc. Natl Acad. Sci. USA 110, 5258–5262 (2013).
Sanders, S. J. et al. Insights into autism spectrum disorder genomic architecture and biology from 71 risk loci. Neuron 87, 1215–1233 (2015).
Desachy, G. et al. Increased female autosomal burden of rare copy number variants in human populations and in autism families. Mol. Psychiatry 20, 170–175 (2015).
Jacquemont, S. et al. A higher mutational burden in females supports a ‘female protective model’ in neurodevelopmental disorders. Am. J. Hum. Genet. 94, 415–425 (2014).
De Rubeis, S. et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 515, 209–215 (2014).
Brandler, W. M. et al. Paternally inherited cis-regulatory structural variants are associated with autism. Science 360, 327–331 (2018).
Krumm, N. et al. Excess of rare, inherited truncating mutations in autism. Nat. Genet. 47, 582–588 (2015).
Weiner, D. J. et al. Polygenic transmission disequilibrium confirms that common and rare variation act additively to create risk for autism spectrum disorders. Nat. Genet. 49, 978–985 (2017).
Grove, J. et al. Identification of common genetic risk variants for autism spectrum disorder. Nat. Genet. 51, 431–444 (2019).
Spark Consortium. SPARK: a US cohort of 50,000 families to accelerate autism research. Neuron 97, 488–493 (2018).
Lloyd-Jones, L. R. et al. Improved polygenic prediction by Bayesian multiple regression on summary statistics. Nat. Commun. 10, 5086 (2019).
Werling, D. M. The role of sex-differential biology in risk for autism spectrum disorder. Biol. Sex. Differ. 7, 58 (2016).
Falconer, D. S. Inheritance of liability to certain diseases estimated from incidence among relatives. Ann. Hum. Genet. 29, 51–76 (1965).
Reich, T., Morris, C. A. & James, J. W. Use of multiple thresholds in determining mode of transmission of semi-continuous traits. Ann. Hum. Genet. 36, 163 (1972).
Antaki, D. et al. A phenotypic spectrum of autism is attributable to the combined effects of rare variants, polygenic risk and sex. Preprint at medRxiv https://doi.org/2021.03.30.21254657 (2021).
Robinson, E. B. et al. Genetic risk for autism spectrum disorders and neuropsychiatric variation in the general population. Nat. Genet. 48, 552–555 (2016).
Buja, A. et al. Damaging de novo mutations diminish motor skills in children on the autism spectrum. Proc. Natl Acad. Sci. USA 115, E1859–E1866 (2018).
Satterstrom, F. K. et al. Large-scale exome sequencing study implicates both developmental and functional changes in the neurobiology of autism. Cell 180, 568–584.e23 (2020).
Michaelson, J. J. et al. Whole-genome sequencing in autism identifies hot spots for de novo germline mutation. Cell 151, 1431–1442 (2012).
Kong, A. et al. Rate of de novo mutations and the importance of father’s age to disease risk. Nature 488, 471–475 (2012).
Goriely, A. & Wilkie, A. O. Missing heritability: paternal age effect mutations and selfish spermatogonia. Nat. Rev. Genet. 11, 589 (2010).
Gratten, J. et al. Risk of psychiatric illness from advanced paternal age is not predominantly from de novo mutations. Nat. Genet. 48, 718–724 (2016).
Mullins, N. et al. Reproductive fitness and genetic risk of psychiatric disorders in the general population. Nat. Commun. 8, 15833 (2017).
He, X. et al. Integrated model of de novo and inherited genetic variants yields greater power to identify risk genes. PLoS Genet. 9, e1003671 (2013).
Li, M. et al. Integrative functional genomic analysis of human brain development and neuropsychiatric risks. Science 362, eaat7615 (2018).
Polioudakis, D. et al. A single-cell transcriptomic atlas of human neocortical development during mid-gestation. Neuron 103, 785–801.e8 (2019).
Wainschtein, P. et al. Assessing the contribution of rare variants to complex trait heritability from whole-genome sequence data. Nat. Genet. 54, 263–273 (2022).
Turner, T. N. et al. Sex-based analysis of de novo variants in neurodevelopmental disorders. Am. J. Hum. Genet. 105, 1274–1285 (2019).
Russell, G., Steer, C. & Golding, J. Social and demographic factors that influence the diagnosis of autistic spectrum disorders. Soc. Psychiatry Psychiatr. Epidemiol. 46, 1283–1293 (2011).
Werling, D. M. & Geschwind, D. H. Sex differences in autism spectrum disorders. Curr. Opin. Neurol. 26, 146–153 (2013).
D’Angelo, D. et al. Defining the effect of the 16p11.2 duplication on cognition, behavior, and medical comorbidities. JAMA Psychiatry 73, 20–30 (2016).
Malaspina, D. et al. Paternal age and intelligence: implications for age-related genomic changes in male germ cells. Psychiatr. Genet. 15, 117–125 (2005).
Lyall, K. et al. The association between parental age and autism-related outcomes in children at high familial risk for autism. Autism Res. 13, 998–1010 (2020).
Frans, E. M. et al. Autism risk across generations: a population-based study of advancing grandpaternal and paternal age. JAMA Psychiatry 70, 516–521 (2013).
Lampi, K. M. et al. Parental age and risk of autism spectrum disorders in a Finnish national birth cohort. J. Autism Dev. Disord. 43, 2526–2535 (2013).
Lundstrom, S. et al. Trajectories leading to autism spectrum disorders are affected by paternal age: findings from two nationally representative twin studies. J. Child Psychol. Psychiatry 51, 850–856 (2010).
Fulco, C. J., Henry, K. L., Rickard, K. M. & Yuma, P. J. Time-varying outcomes associated with maternal age at first birth. J. Child Fam. Stud. 29, 1537–1547 (2020).
Boyle, E. A., Li, Y. I. & Pritchard, J. K. An expanded view of complex traits: from polygenic to omnigenic. Cell 169, 1177–1186 (2017).
Liu, X., Li, Y. I. & Pritchard, J. K. Trans effects on gene expression can drive omnigenic inheritance. Cell 177, 1022–1034.e6 (2019).
Maurano, M. T. et al. Systematic localization of common disease-associated variation in regulatory DNA. Science 337, 1190–1195 (2012).
Jacquemont, S. et al. Genes to mental health (G2MH): a framework to map the mombined effects of rare and common variants on dimensions of cognition and psychopathology. Am. J. Psychiatry 179, 189–203 (2022).
Brandler, W. M. et al. Paternally inherited cis-regulatory structural variants are associated with autism. Science 360, 327–331 (2018).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
Lam, M. et al. RICOPILI: Rapid Imputation for COnsortias PIpeLIne. Bioinformatics 36, 930–933 (2020).
Lee, J. J. et al. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat. Genet. 50, 1112–1121 (2018).
Choi, S. W. & O’Reilly, P. F. PRSice-2: polygenic risk score software for biobank-scale data. Gigascience 8, giz082 (2019).
Lian, A., Guevara, J., Xia, K. & Sebat, J. Customized de novo mutation detection for any variant calling pipeline: SynthDNM. Bioinformatics 37, 3640–3641 (2021).
Feliciano, P. et al. Exome sequencing of 457 autism families recruited online provides evidence for autism risk genes. npj Genom. Med. 4, 19 (2019).
Antaki, D., Brandler, W. M. & Sebat, J. SV2: accurate structural variation genotyping and de novo mutation detection from whole genomes. Bioinformatics 34, 1774–1777 (2018).
Samocha, K. E. et al. Regional missense constraint improves variant deleteriousness prediction. Preprint at bioRxiv (2017).
Kosmicki, J. A. et al. Refining the role of de novo protein-truncating variants in neurodevelopmental disorders by using population reference samples. Nat. Genet. 49, 504–510 (2017).
Brainstorm Consortium et al. Analysis of shared heritability in common disorders of the brain. Science 360, eaap8757 (2018).
Hagenaars, S. P. et al. Shared genetic aetiology between cognitive functions and physical and mental health in UK Biobank (N = 112 151) and 24 GWAS consortia. Mol. Psychiatry 21, 1624–1632 (2016).
Clarke, T. K. et al. Common polygenic risk for autism spectrum disorder (ASD) is associated with cognitive ability in the general population. Mol. Psychiatry 21, 419–425 (2016).
Samocha, K. E. et al. A framework for the interpretation of de novo mutation in human disease. Nat. Genet. 46, 944–950 (2014).
Sey, N. Y. A. et al. A computational tool (H-MAGMA) for improved prediction of brain-disorder risk genes by incorporating brain chromatin interaction profiles. Nat. Neurosci. 23, 583–593 (2020).
Macosko, E. Z. et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161, 1202–1214 (2015).
Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018).
Acknowledgements
We thank the families who participated in the genetic studies of the REACH, SSC and SPARK cohorts. The large samples used in the present study were made possible through the strong commitment to sharing of individual-level data by SFARI, the National Institutes of Health (NIH) and UCSD, and the sharing of summary-level data by the Psychiatric Genomics Consortium. We thank W. Pfeiffer, the San Diego Supercomputer Center and Amazon Web Services for hosting the computing infrastructure necessary for completing this project. We thank W. Chung and SFARI for providing data and materials for validation of DNM calls. We thank N. Baya for assistance with data processing. We thank H. Wong for providing the list of high-confidence GWAS genes. This work was supported by grants to J.S. from the SFARI (grant no. SFARI 606768), NIH (grant nos. MH113715, MH119746, 1MH109501) and the Escher fund for Autism (grant no. 20171603), a grant to C.M.N. from the NIH (grant no. MH106595), and a grant to A.R.M. from the NIH (MH127077). L.M.I. was supported by grants from the NIH (MH109885, MH108528, MH105524, MH104766) and SFARI (#345469). D.A. was supported by a T32 training grant from the NIH (grant no. GM008666). M.K. was supported by grants from the Dutch Research Council (grant nos. 09150162010073 and 45219212).
Author information
Authors and Affiliations
Contributions
J.S. conceived and coordinated the study. D.A., M.G. and J. Guevara performed data processing, variant calling and annotation of WGS and exome datasets. J.S., C.M.N. and A.X.M. supervised statistical genetic analyses. D.A., J. Guevara, A.X.M., M.K. and J.S. performed statistical genetic analyses. J. Guevara, D.A., L.M.I. and J.S. performed analysis of RNA-sequencing datasets. E.R., C.E.C. and J. Grove performed analysis of SNP genotypes and meta-analysis of summary statistics. J.S., C.C., K.K.V., A.H., M.J.A., K.P., E.C., J.G.G., A.R.M. and O.H. coordinated recruitment and DNA sample processing for the UCSD dataset.
Corresponding author
Ethics declarations
Competing interests
A.R.M. is a co-founder and has an equity interest in TISMOO, a company focusing on applications of genetics and human brain organoids to personalized medicine. The terms of this arrangement have been reviewed and approved by the UCSD, in accordance with its conflict-of-interest policies. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Genetics thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Rates of de novo mutations stratified by cohort and evaluation of potential confounders.
a, Rates of de novo synonymous (dnSyn) variants were not associated with ASD in the combined sample, but were enriched 1.1-fold in the SPARK cohort (P = 0.021). b, We evaluated whether quality metrics or other confounders could explain the slight excess of dnSyn variants in SPARK cases. Quality metrics did not differ in cases and controls including coverage, transition:transversion ratio (Ti/Tv) or ratio of heterozygous calls (Het/Hom). c, Paternal age did not differ significantly between cases and controls.
Extended Data Fig. 2 The combined effects of dnLoF, inhLoF and sex on the transmission of rare variants in families.
a, A significant liability threshold for rare variants was evident based on a negative correlation of dnLoF and inhLoF (linear regression P = 0.03), and this effect did not differ significantly by sex. b, Case-control odds ratios were compared for the transmission rates in families by sex (father-daughter, mother-daughter, father-son, mother-son). Both maternal and paternal rare variants contribute to ASD with a significant over-transmission from mother to daughter and from father to son. We did not observe a significant sex bias in the transmission of rare variants in families. In particular, we did not observe an enriched transmission from mother to male cases as we have previously hypothesized8.
Extended Data Fig. 3 Sex differences in the correlation of rare variant and common variant risk was not robust across multiple polygenic scoring methods.
a, An early analysis of this dataset using polygenic score estimates from PRSice observed that the negative correlation of RVRS and CVRS was stronger in males than in females, consistent with males having less tolerance of genetic risk. The heatmap displays the correlations between polygenic scores and rare variants in males and females separately. Correlations were tested by linear regression controlling for cohort, case status and ancestry PCs, and a gene-by-sex interaction was tested in the combined sample (ǂgene-by-sex P < 0.05). b, With polygenic scores calculated using SBayesR, there was a similar trend with the correlation of CVRS and RVRS being stronger in males; however, the gene-by-sex interaction was not statistically significant.
Extended Data Fig. 4 Correlation of de novo mutation rate with parental age.
a,b, Correlation of total autosomal de novo SNVs with age of fathers (a) and mothers (b). See also Fig. 6a. n = 4,518 trios for which age-at-birth was available for the mother and father.
Extended Data Fig. 5 Comparison of the predictive values of polygenic scoring methods PRSice and SBayesR.
Polygenic scores calculated using SBayesR had greater predictive value for polygenic scores for ASD (PSASD), schizophrenia (PSSZ) and educational attainment (PSEA).
Supplementary information
Supplementary Information
Supplementary Note.
Supplementary Tables
Supplementary Tables 1–21.
Rights and permissions
About this article
Cite this article
Antaki, D., Guevara, J., Maihofer, A.X. et al. A phenotypic spectrum of autism is attributable to the combined effects of rare variants, polygenic risk and sex. Nat Genet 54, 1284–1292 (2022). https://doi.org/10.1038/s41588-022-01064-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-022-01064-5
This article is cited by
-
Statistical methods for assessing the effects of de novo variants on birth defects
Human Genomics (2024)
-
Structural models of genome-wide covariance identify multiple common dimensions in autism
Nature Communications (2024)
-
Pathogenic/likely pathogenic mutations identified in Vietnamese children diagnosed with autism spectrum disorder using high-resolution SNP genotyping platform
Scientific Reports (2024)
-
Genetic modifiers of rare variants in monogenic developmental disorder loci
Nature Genetics (2024)
-
Gene–brain–behavior mechanisms underlying autism spectrum disorder: implications for precision psychiatry
Neuropsychopharmacology (2024)