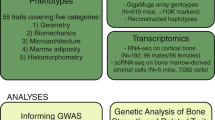
Abstract
Osteoporosis is a common aging-related disease diagnosed primarily using bone mineral density (BMD). We assessed genetic determinants of BMD as estimated by heel quantitative ultrasound in 426,824 individuals, identifying 518 genome-wide significant loci (301 novel), explaining 20% of its variance. We identified 13 bone fracture loci, all associated with estimated BMD (eBMD), in ~1.2 million individuals. We then identified target genes enriched for genes known to influence bone density and strength (maximum odds ratio (OR) = 58, P = 1 × 10−75) from cell-specific features, including chromatin conformation and accessible chromatin sites. We next performed rapid-throughput skeletal phenotyping of 126 knockout mice with disruptions in predicted target genes and found an increased abnormal skeletal phenotype frequency compared to 526 unselected lines (P < 0.0001). In-depth analysis of one gene, DAAM2, showed a disproportionate decrease in bone strength relative to mineralization. This genetic atlas provides evidence linking associated SNPs to causal genes, offers new insight into osteoporosis pathophysiology, and highlights opportunities for drug development.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Human genotype and phenotype data on which the results of this study were based are available upon application from the UK Biobank (http://www.ukbiobank.ac.uk). GWAS summary statistics for eBMD and fracture can be downloaded from the GEFOS website (http://www.gefos.org/). RNA-seq and ATAC-seq data generated for human osteoblast cell lines, including re-called DHS peaks from human primary osteoblasts, can be downloaded from the Gene Expression Omnibus (accession number GSE120755). Mouse phenotype data are available online from the IMPC (http://www.mousephenotype.org) and OBCD (http://www.boneandcartilage.com).
Change history
15 April 2019
In the version of this article initially published, in Fig. 5a, the data in the right column of ‘DAAM2 gRNA1’ were incorrectly plotted as circles indicating ‘untreated’ rather than as squares indicating ‘treated’. The error has been corrected in the HTML and PDF versions of the article.
References
World Health Organization. Consensus development conference: Prophylaxis and treatment of osteoporosis. Osteoporos. Int. 1, 114–117 (1991).
Richards, J. B., Zheng, H.-F. & Spector, T. D. Genetics of osteoporosis from genome-wide association studies: advances and challenges. Nat. Rev. Genet. 13, 576–588 (2012).
Johnell, O. et al. Predictive value of BMD for hip and other fractures. J. Bone Miner. Res. 20, 1185–1194 (2005).
Kemp, J. P. et al. Identification of 153 new loci associated with heel bone mineral density and functional involvement of GPC6 in osteoporosis. Nat. Genet. 49, 1468–1475 (2017).
Arden, N. K., Baker, J., Hogg, C., Baan, K. & Spector, T. D. The heritability of bone mineral density, ultrasound of the calcaneus and hip axis length: a study of postmenopausal twins. J. Bone Miner. Res. 11, 530–534 (1996).
Hunter, D. J. et al. Genetic variation in bone mineral density and calcaneal ultrasound: A study of the influence of menopause using female twins. Osteoporos. Int. 12, 406–411 (2001).
Bauer, D. C. Broadband ultrasound attenuation predicts fractures strongly and independently of densitometry in older women. Arch. Intern. Med. 157, 629 (1997).
Bauer, D. C. et al. Quantitative ultrasound predicts hip and non-spine fracture in men: The MrOS study. Osteoporos. Int. 18, 771–777 (2007).
Karasik, D. et al. Mapping of quantitative ultrasound of the calcaneus bone to chromosome 1 by genome-wide linkage analysis. Osteoporos. Int. 13, 796–802 (2002).
Medina-Gomez, C. et al. Life-course genome-wide association study meta-analysis of total body BMD and assessment of age-specific effects. Am. J. Hum. Genet. 102, 88–102 (2018).
McCloskey, E. V. et al. Predictive ability of heel quantitative ultrasound for incident fractures: an individual-level meta-analysis. Osteoporos. Int. 26, 1979–1987 (2015).
Timpson, N. J., Greenwood, C. M. T., Soranzo, N., Lawson, D. J. & Richards, J. B. Genetic architecture: the shape of the genetic contribution to human traits and disease. Nat. Rev. Genet. 19, 110–124 (2018).
Yang, J., Lee, S. H., Goddard, M. E. & Visscher, P. M. GCTA: a tool for genome-wide complex trait analysis. Am. J. Hum. Genet. 88, 76–82 (2011).
Yang, J. et al. Conditional and joint multiple-SNP analysis of GWAS summary statistics identifies additional variants influencing complex traits. Nat. Genet. 44, 369–375 (2012).
Benner, C. et al. FINEMAP: Efficient variable selection using summary data from genome-wide association studies. Bioinformatics 32, 1493–1501 (2016).
Thurman, R. E. et al. The accessible chromatin landscape of the human genome. Nature 489, 75–82 (2012).
Rivadeneira, F. & Mäkitie, O. Osteoporosis and bone mass disorders: from gene pathways to treatments. Trends. Endocrinol. Metab. 27, 262–281 (2016).
Watanabe, K., Taskesen, E., Van Bochoven, A. & Posthuma, D. Functional mapping and annotation of genetic associations with FUMA. Nat. Commun. 8, 1826 (2017).
Kutmon, M. et al. WikiPathways: Capturing the full diversity of pathway knowledge. Nucleic Acids Res. 44, D488–D494 (2016).
Dallas, S. L. & Bonewald, L. F. Dynamics of the transition from osteoblast to osteocyte. Ann. NY Acad. Sci. 1192, 437–443 (2010).
Youlten, S. et al. Osteocytes express a unique transcriptome that underpins skeletal homeostasis.J. Bone Min. Res. 32 (Suppl 1), S55–S56 (2017).
Lee, H. K. & Deneen, B. Daam2 Is required for dorsal patterning via modulation of canonical Wnt signaling in the developing spinal cord. Dev. Cell. 22, 183–196 (2012).
Lee, H. K. et al. Daam2-PIP5K is a regulatory pathway for Wnt signaling and therapeutic target for remyelination in the CNS. Neuron 85, 1227–1243 (2015).
Visscher, P. M. et al. 10 years of GWAS discovery: biology, function, and translation. Am. J. Hum. Genet. 101, 5–22 (2017).
Nelson, M. R. et al. The support of human genetic evidence for approved drug indications. Nat. Genet. 47, 856–860 (2015).
Bone, H. G. et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 5, 513–523 (2017).
Lawlor, D. A., Tilling, K. & Smith, G. D. Triangulation in aetiological epidemiology. Int. J. Epidemiol. 45, 1866–1886 (2016).
Moayyeri, A. et al. Quantitative ultrasound of the heel and fracture risk assessment: an updated meta-analysis. Osteoporos. Int. 23, 143–153 (2012).
Grundberg, E. et al. Population genomics in a disease targeted primary cell model. Genome Res. 19, 1942–1952 (2009).
Sudlow, C. et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 12, e1001779 (2015).
Loh, P. R. et al. Efficient Bayesian mixed-model analysis increases association power in large cohorts. Nat. Genet. 47, 284–290 (2015).
Chang, C. C. et al. Second-generation PLINK: Rising to the challenge of larger and richer datasets. Gigascience 4, 7 (2015).
Winkler, T. W. et al. EasyStrata: Evaluation and visualization of stratified genome-wide association meta-Analysis data. Bioinformatics 31, 259–261 (2015).
Cochran, W. G. The combination of estimates from different experiments. Biometrics 10, 101 (1954).
Willer, C. J., Li, Y. & Abecasis, G. R. METAL: Fast and efficient meta-analysis of genomewide association scans. Bioinformatics 26, 2190–2191 (2010).
Quinlan, A. R. & Hall, I. M. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842 (2010).
Bulik-Sullivan, B. et al. LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 47, 291–295 (2015).
Witte, J. S., Visscher, P. M. & Wray, N. R. The contribution of genetic variants to disease depends on the ruler. Nat. Rev. Genet. 15, 765–776 (2014).
Chapman, J. M., Cooper, J. D., Todd, J. A. & Clayton, D. G. Detecting disease associations due to linkage disequilibrium using haplotype tags: a class of tests and the determinants of statistical power. Hum. Hered. 56, 18–31 (2003).
Spencer, C. C. A., Su, Z., Donnelly, P. & Marchini, J. Designing genome-wide association studies: Sample size, power, imputation, and the choice of genotyping chip. PLoS Genet. 5, e1000477 (2009).
Finucane, H. K. et al. Partitioning heritability by functional annotation using genome-wide association summary statistics. Nat. Genet. 47, 1228–1235 (2015).
Hart, T., Komori, H. K., LaMere, S., Podshivalova, K. & Salomon, D. R. Finding the active genes in deep RNA-seq gene expression studies. BMC Genomics 14, 778 (2013).
Buenrostro, J. D., Giresi, P. G., Zaba, L. C., Chang, H. Y. & Greenleaf, W. J. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat. Methods 10, 1213–1218 (2013).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Anders, S., Pyl, P. T. & Huber, W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Schmitt, A. D. et al. A compendium of chromatin contact maps reveals spatially active regions in the human genome. Cell Reports 17, 2042–2059 (2016).
Rao, S. S. P. et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell 159, 1665–1680 (2014).
Belaghzal, H., Dekker, J. & Gibcus, J. H. Hi-C 2.0: An optimized Hi-C procedure for high-resolution genome-wide mapping of chromosome conformation. Methods 123, 56–65 (2017).
Servant, N. et al. HiC-Pro: An optimized and flexible pipeline for Hi-C data processing. Genome. Biol. 16, 259 (2015).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010).
Mifsud, B. et al. GOTHiC, a probabilistic model to resolve complex biases and to identify real interactions in Hi-C data. PLoS One 12, e0174744 (2017).
Durand, N. C. et al. Juicer provides a one-click system for analyzing loop-resolution Hi-C experiments. Cell Syst. 3, 95–98 (2016).
O’Leary, N. A. et al. Reference sequence (RefSeq) database at NCBI: Current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 44, D733–D745 (2016).
Ran, F. A. et al. Genome engineering using the CRISPR–Cas9 system. Nat. Protoc. 8, 2281–2308 (2013).
Doench, J. G. et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat. Biotechnol. 34, 184–191 (2016).
Haeussler, M. et al. Evaluation of off-target and on-target scoring algorithms and integration into the guide RNA selection tool CRISPOR. Genome. Biol. 17, 148 (2016).
Acknowledgements
This research has been conducted using the UK Biobank Resource (accession IDs: 24268, 12703 and 4580). J.B.R. was supported by the Canadian Institutes of Health Research, the Canadian Foundation for Innovation and the Fonds de Recherche Santé Québec (FRSQ), and a FRQS Clinical Research Scholarship. TwinsUK is funded by the Wellcome Trust, Medical Research Council, European Union, the National Institute for Health Research (NIHR)-funded BioResource, Clinical Research Facility, and Biomedical Research Centre based at Guy’s and St Thomas’s NHS Foundation Trust in partnership with King’s College London. J.A.M. was funded by the Canadian Institutes of Health Research. D.M.E. was funded by a National Health and Medical Research Council Senior Research Fellowship (APP1137714) and funded by a Medical Research Council Programme Grant (MC_UU_12013/4). J.P.K. was funded by a University of Queensland Development Fellowship (UQFEL1718945). C.L.G. was funded by Arthritis Research UK (ref; 20000). G.R.W., J.H.D.B., and P.I.C. were funded by the Wellcome Trust (Strategic Award grant number 101123; project grant 094134), and P.I.C. was also funded by the Mrs. Janice Gibson and the Ernest Heine Family Foundation. D.K. was supported by Israel Science Foundation grant #1283/14. Y-H.H. was funded by US NIH NIAMS 1R01AR072199. F.R., C.M-G., and K.T. were funded by the Netherlands Organization for Health Research and Development (ZonMw VIDI 016.136.361 grant). C.L.A-B. was funded by NIH/NIAMS AR063702 AR060981. D.P.K. was funded by grants from the National Institute of Arthritis Musculoskeletal and Skin Diseases R01 AR041398, R01 AR072199. S.Y. was funded by the Australian Government Research Training Program Scholarship. J.R. and S.K. were funded by the Genetic Factors of Osteoporosis-GEFOS EU FP7 Integrated Project Grant Reference: 201865 2008-12 and 2007-12 UK NIHR Biomedical Research Centre Grant (Musculoskeletal theme) to Cambridge Clinical School. C.O. was supported by the Swedish Research Council, Swedish Foundation for Strategic Research, ALF/LUA research grant from the Sahlgrenska University Hospital, Lundberg Foundation, European Calcified Tissue Society, Torsten and Ragnar Söderberg’s Foundation, Novo Nordisk Foundation, and Knut and Alice Wallenberg Foundation. M.T.M. was supported by NIH grant R35 GM119703. We thank M. Schull for assistance with high-performance computing at the University of Queensland Diamantina Institute and T. Winkler for invaluable technical support for the EasyStrata Software used in this study. We thank the Sanger Institute’s Research Support Facility, Mouse Pipelines and Mouse Informatics Group who generated the mice and collected materials for this manuscript. We would like to thank the research participants and employees of 23andMe, Inc. for making this work possible.
Author information
Authors and Affiliations
Consortia
Contributions
J.A.M., J.P.K., A.P., C.L.A-B., C.L.G., C.O., D.K., D.P.K., E.E., E.G., F.R., G.R.W., J.H.D.B., J.H.T., M.T.M., N.C.H., P.I.C., V.F., Y-H.H., D.M.E. and J.B.R. conceived of and designed experiments. J.A.M., J.P.K., A.K., A.S.P., A.-T.A., C.C., D.A.H., D.G., D.S.K.K.-E., E.E.N., E.J.G., H.F.D., J.G.L., J.R., K.F.C., K.T., M.-J.G.B., N.A.V., N.C.B., N.S.M., P.C.S., R.C.C., S.E.Y., S.M.V., S.K., T.A.D.H., V.D.L., A.P., C.L.A.-B., C.L.G., D.M.E., E.G., G.R.W., J.H.D.B., M.T.M., N.C.H., V.F., Y.-H.H. and J.B.R. performed data analysis. J.A.M., J.P.K, A-L.L., A-T.A., C.J.L., C.M-G., C.M.S., D.G., David J. Adams, Douglas J. Adams, E.J.G., H.F.D., J.G.L., J.Q., J.V., K.F.C., L.L., L.N-Y., M.-J.G.B., M-M.S., N.S.M., P.B., P.C.S., R.C.C., S.E.Y., S.T.M., A.P., C.L.A.-B., and Y.-H.H. conducted experiments. J.A.M., J.P.K., G.R.W., J.H.D.B., D.M.E. and J.B.R. wrote the manuscript. J.A.M. and J.P.K. were the lead analysts. All authors revised and reviewed the paper.
Corresponding authors
Ethics declarations
Competing interests
A.K. and D.A.H. are employees of 23andMe, Inc.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–23 and Supplementary Note
Supplementary Tables
Supplementary Tables 1–22
Rights and permissions
About this article
Cite this article
Morris, J.A., Kemp, J.P., Youlten, S.E. et al. An atlas of genetic influences on osteoporosis in humans and mice. Nat Genet 51, 258–266 (2019). https://doi.org/10.1038/s41588-018-0302-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-018-0302-x
This article is cited by
-
Diabetes and osteoporosis: a two-sample mendelian randomization study
BMC Musculoskeletal Disorders (2024)
-
Genetic evidence of the causal relationship between chronic liver diseases and musculoskeletal disorders
Journal of Translational Medicine (2024)
-
Transcriptional reprogramming during human osteoclast differentiation identifies regulators of osteoclast activity
Bone Research (2024)
-
Long noncoding RNA Malat1 protects against osteoporosis and bone metastasis
Nature Communications (2024)
-
Leveraging electronic health records and knowledge networks for Alzheimer’s disease prediction and sex-specific biological insights
Nature Aging (2024)