Abstract
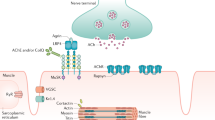
Myasthenia gravis (MG) is an autoimmune disorder that affects the neuromuscular junction, leading to muscle weakness and fatigue. MG is caused by antibodies against the acetylcholine receptor (AChR), the muscle-specific kinase (MuSK) or other AChR-related proteins that are expressed in the postsynaptic muscle membrane. The standard therapeutic approach for MG has relied on acetylcholinesterase inhibitors, corticosteroids and immunosuppressants, which have shown good efficacy in improving MG-related symptoms in most people with the disease; however, these therapies can carry a considerable burden of long-term adverse effects. Moreover, up to 15% of individuals with MG exhibit limited or no response to these standard therapies. The emergence of molecular therapies, including monoclonal antibodies, B cell-depleting agents and chimeric antigen receptor T cell-based therapies, has the potential to revolutionize the MG treatment landscape. This Review provides a comprehensive overview of the progress achieved in molecular therapies for MG associated with AChR antibodies and MuSK antibodies, elucidating both the challenges and the opportunities these therapies present to the field. The latest developments in MG treatment are described, exploring the potential for personalized medicine approaches.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Claytor, B., Cho, S. M. & Li, Y. Myasthenic crisis. Muscle Nerve 68, 8–19 (2023).
Gilhus, N. E. et al. Myasthenia gravis. Nat. Rev. Dis. Prim. 5, 30 (2019).
Higuchi, O., Hamuro, J., Motomura, M. & Yamanashi, Y. Autoantibodies to low-density lipoprotein receptor-related protein 4 in myasthenia gravis. Ann. Neurol. 69, 418–422 (2011).
Li, Y. et al. Anti-LRP4 autoantibodies in Chinese patients with myasthenia gravis. Muscle Nerve 56, 938–942 (2017).
Zisimopoulou, P. et al. A comprehensive analysis of the epidemiology and clinical characteristics of anti-LRP4 in myasthenia gravis. J. Autoimmun. 52, 139–145 (2014).
Klein, C. J. et al. LRP4-IgG service line testing in seronegative myasthenia gravis and controls. J. Neuroimmunol. 368, 577895 (2022).
Narayanaswami, P. et al. International consensus guidance for management of myasthenia gravis: 2020 update. Neurology 96, 114–122 (2021).
Huscher, D. et al. Dose-related patterns of glucocorticoid-induced side effects. Ann. Rheum. Dis. 68, 1119–1124 (2009).
Lascano, A. M. & Lalive, P. H. Update in immunosuppressive therapy of myasthenia gravis. Autoimmun. Rev. 20, 102712 (2021).
Machkhas, H & Harati, Y. Side effects of immunosuppressant therapies used in neurology. Neurol. Clin. 16, 171–188 (1998).
Mantegazza, R. & Antozzi, C. When myasthenia gravis is deemed refractory: clinical signposts and treatment strategies. Ther. Adv. Neurol. Disord. 11, 1756285617749134 (2018).
Chia, R. et al. Identification of genetic risk loci and prioritization of genes and pathways for myasthenia gravis: a genome-wide association study. Proc. Natl Acad. Sci. USA 119, e2108672119 (2022).
Yasumizu, Y. et al. Myasthenia gravis-specific aberrant neuromuscular gene expression by medullary thymic epithelial cells in thymoma. Nat. Commun. 13, 4230 (2022).
Ingelfinger, F. et al. Single-cell profiling of myasthenia gravis identifies a pathogenic T cell signature. Acta Neuropathol. 141, 901–915 (2021).
Fichtner, M. L., Jiang, R., Bourke, A., Nowak, R. J. & O’Connor, K. C. Autoimmune pathology in myasthenia gravis disease subtypes is governed by divergent mechanisms of immunopathology. Front. Immunol. 11, 776 (2020).
Masi, G. & O’Connor, K. C. Novel pathophysiological insights in autoimmune myasthenia gravis. Curr. Opin. Neurol. 35, 586–596 (2022).
Elsner, R. A. & Shlomchik, M. J. Germinal center and extrafollicular B cell responses in vaccination, immunity, and autoimmunity. Immunity 53, 1136–1150 (2020).
Zografou, C., Vakrakou, A. G. & Stathopoulos, P. Short- and long-lived autoantibody-secreting cells in autoimmune neurological disorders. Front. Immunol. 12, 686466 (2021).
Stathopoulos, P., Kumar, A., Nowak, R. J. & O’Connor, K. C. Autoantibody-producing plasmablasts after B cell depletion identified in muscle-specific kinase myasthenia gravis. JCI Insight 2, e94263 (2017).
Marino, M. et al. Long-lasting rituximab-induced reduction of specific–but not total–IgG4 in MuSK-positive myasthenia gravis. Front. Immunol. 11, 613 (2020).
Evoli, A. & Iorio, R. Controversies in ocular myasthenia gravis. Front. Neurol. 11, 605902 (2020).
Kaminski, H. J., Li, Z., Richmonds, C., Lin, F. & Medof, M. E. Complement regulators in extraocular muscle and experimental autoimmune myasthenia gravis. Exp. Neurol. 189, 333–342 (2004).
Vilquin, J. T., Bayer, A. C., Le Panse, R. & Berrih-Aknin, S. The muscle is not a passive target in myasthenia gravis. Front. Neurol. 10, 1343 (2019).
Punga, A. R., Maddison, P., Heckmann, J. M., Guptill, J. T. & Evoli, A. Epidemiology, diagnostics, and biomarkers of autoimmune neuromuscular junction disorders. Lancet Neurol. 21, 176–188 (2022).
Marx, A. et al. Thymus and autoimmunity. Semin. Immunopathol. 43, 45–64 (2021).
Gregersen, P. K. et al. Risk for myasthenia gravis maps to a 151Pro->Ala change in TNIP1 and to human leukocyte antigen-B*08. Ann. Neurol. 72, 927–935 (2012).
Maniaol, A. H. et al. Late onset myasthenia gravis is associated with HLA DRB1*15:01 in the Norwegian population. PLoS ONE 7, e36603 (2012).
Iorio, R., Spagni, G. & Evoli, A. Paraneoplastic neurological syndromes associated with mediastinal tumors. Mediastinum https://doi.org/10.21037/med.2018.01.03 (2018).
Iorio, R., Evoli, A., Lauriola, L. & Batocchi, A. P. A B3 type-thymoma in a 7-year-old child with myasthenia gravis. J. Thorac. Oncol. 7, 937–938 (2012).
Cetin, H., Beeson, D., Vincent, A. & Webster, R. The structure, function, and physiology of the fetal and adult acetylcholine receptor in muscle. Front. Mol. Neurosci. 13, 581097 (2020).
Luo, J. et al. Main immunogenic region structure promotes binding of conformation-dependent myasthenia gravis autoantibodies, nicotinic acetylcholine receptor conformation maturation, and agonist sensitivity. J. Neurosci. 29, 13898–13908 (2009).
Lennon, V. A., Lambert, E. H., Leiby, K. R., Okarma, T. B. & Talib, S. Recombinant human acetylcholine receptor ɑ-subunit induces chronic experimental autoimmune myasthenia gravis. J. Immunol. 146, 2245–2248 (1991).
Pham, M. C. et al. Individual myasthenia gravis autoantibody clones can efficiently mediate multiple mechanisms of pathology. Acta Neuropathol. 146, 319–336 (2023).
Vincent, A. Unravelling the pathogenesis of myasthenia gravis. Nat. Rev. Immunol. 2, 797–804 (2002).
Sahashi, K., Engel, A. G., Lambert, E. H. & Howard, F. M. Jr. Ultrastructural localization of the terminal and lytic ninth complement component (C9) at the motor end-plate in myasthenia gravis. J. Neuropathol. Exp. Neurol. 39, 160–172 (1980).
Sahashi, K., Engel, A. G., Linstrom, J. M., Lambert, E. H. & Lennon, V. A. Ultrastructural localization of immune complexes (IgG and C3) at the end-plate in experimental autoimmune myasthenia gravis. J. Neuropathol. Exp. Neurol. 37, 212–223 (1978).
Reis, E. S., Mastellos, D. C., Hajishengallis, G. & Lambris, J. D. New insights into the immune functions of complement. Nat. Rev. Immunol. 19, 503–516 (2019).
Mastellos, D. C., Hajishengallis, G. & Lambris, J. D. A guide to complement biology, pathology and therapeutic opportunity. Nat. Rev. Immunol. https://doi.org/10.1038/s41577-023-00926-1 (2023).
Engel, A. G., Lambert, E. H. & Howard, F. M. Immune complexes (IgG and C3) at the motor end-plate in myasthenia gravis: ultrastructural and light microscopic localization and electrophysiologic correlations. Mayo Clin. Proc. 52, 267–280 (1977).
Engel, A. G. et al. Passively transferred experimental autoimmune myasthenia gravis. Sequential and quantitative study of the motor end-plate fine structure and ultrastructural localization of immune complexes (IgG and C3), and of the acetylcholine receptor. Neurology 29, 179–188 (1979).
Chamberlain-Banoub, J., Neal, J. W., Mizuno, M., Harris, C. L. & Morgan, B. P. Complement membrane attack is required for endplate damage and clinical disease in passive experimental myasthenia gravis in Lewis rats. Clin. Exp. Immunol. 146, 278–286 (2006).
Tuzun, E., Scott, B. G., Goluszko, E., Higgs, S. & Christadoss, P. Genetic evidence for involvement of classical complement pathway in induction of experimental autoimmune myasthenia gravis. J. Immunol. 171, 3847–3854 (2003).
Christadoss, P. C5 gene influences the development of murine myasthenia gravis. J. Immunol. 140, 2589–2592 (1988).
Tuzun, E., Li, J., Saini, S. S., Yang, H. & Christadoss, P. Pros and cons of treating murine myasthenia gravis with anti-C1q antibody. J. Neuroimmunol. 182, 167–176 (2007).
Zhou, Y. et al. Anti-C5 antibody treatment ameliorates weakness in experimentally acquired myasthenia gravis. J. Immunol. 179, 8562–8567 (2007).
Biesecker, G. & Gomez, C. M. Inhibition of acute passive transfer experimental autoimmune myasthenia gravis with Fab antibody to complement C6. J. Immunol. 142, 2654–2659 (1989).
Kusner, L. L. et al. Investigational RNAi therapeutic targeting C5 is efficacious in pre-clinical models of myasthenia gravis. Mol. Ther. Methods Clin. Dev. 13, 484–492 (2019).
Huda, R., Tuzun, E. & Christadoss, P. Complement C2 siRNA mediated therapy of myasthenia gravis in mice. J. Autoimmun. 42, 94–104 (2013).
Qi, Y., Zhang, R., Lu, Y., Zou, X. & Yang, W. Aire and Fezf2, two regulators in medullary thymic epithelial cells, control autoimmune diseases by regulating TSAs: partner or complementer? Front. Immunol. 13, 948259 (2022).
Weigert, C. Pathologisch-anatomischer Beitrag zur Erb’schen Krankheit (myasthenia gravis). Neurologisches Centralblatt. Jg 20, 597–601 (1901).
Wolfe, G. I. et al. Randomized trial of thymectomy in myasthenia gravis. N. Engl. J. Med. 375, 511–522 (2016).
Sims, G. P., Shiono, H., Willcox, N. & Stott, D. I. Somatic hypermutation and selection of B cells in thymic germinal centers responding to acetylcholine receptor in myasthenia gravis. J. Immunol. 167, 1935–1944 (2001).
Bernasconi, P. et al. Increased toll-like receptor 4 expression in thymus of myasthenic patients with thymitis and thymic involution. Am. J. Pathol. 167, 129–139 (2005).
Cavalcante, P. et al. Increased expression of Toll-like receptors 7 and 9 in myasthenia gravis thymus characterized by active Epstein-Barr virus infection. Immunobiology 221, 516–527 (2016).
Rose, N. et al. Receptor clustering and pathogenic complement activation in myasthenia gravis depend on synergy between antibodies with multiple subunit specificities. Acta Neuropathol. 144, 1005–1025 (2022).
Fujii, Y., Monden, Y., Hashimoto, J., Nakahara, K. & Kawashima, Y. Acetylcholine receptor antibody-producing cells in thymus and lymph nodes in myasthenia gravis. Clin. Immunol. Immunopathol. 34, 141–146 (1985).
Fujii, Y., Monden, Y., Hashimoto, J., Nakahara, K. & Kawashima, Y. Acetylcholine receptor antibody production by bone marrow cells in a patient with myasthenia gravis. Neurology 35, 577–579 (1985).
Lisak, R. P., Laramore, C., Zweiman, B. & Moskovitz, A. In vitro synthesis of antibodies to acetylcholine receptor by peripheral blood mononuclear cells of patients with myasthenia gravis. Neurology 33, 604–608 (1983).
DeChiara, T. M. et al. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell 85, 501–512 (1996).
Lacazette, E., Le Calvez, S., Gajendran, N. & Brenner, H. R. A novel pathway for MuSK to induce key genes in neuromuscular synapse formation. J. Cell Biol. 161, 727–736 (2003).
Kim, N. et al. Lrp4 is a receptor for Agrin and forms a complex with MuSK. Cell 135, 334–342 (2008).
Burden, S. J., Yumoto, N. & Zhang, W. The role of MuSK in synapse formation and neuromuscular disease. Cold Spring Harb. Perspect. Biol. 5, a009167 (2013).
Zong, Y. & Jin, R. Structural mechanisms of the agrin-LRP4-MuSK signaling pathway in neuromuscular junction differentiation. Cell Mol. Life Sci. 70, 3077–3088 (2013).
Evoli, A. et al. Clinical correlates with anti-MuSK antibodies in generalized seronegative myasthenia gravis. Brain 126, 2304–2311 (2003).
Farrugia, M. E. et al. MRI and clinical studies of facial and bulbar muscle involvement in MuSK antibody-associated myasthenia gravis. Brain 129, 1481–1492 (2006).
Evoli, A. & Iorio, R. Characteristics of myasthenia gravis with antibodies to muscle‐specific kinase and low‐density lipoprotein‐related receptor protein 4. Clin. Exp. Neuroimmunol. 6, 40–48 (2015).
Evoli, A. et al. Myasthenia gravis with antibodies to MuSK: an update. Ann. N. Y. Acad. Sci. 1412, 82–89 (2018).
Koneczny, I., Cossins, J., Waters, P., Beeson, D. & Vincent, A. MuSK myasthenia gravis IgG4 disrupts the interaction of LRP4 with MuSK but both IgG4 and IgG1-3 can disperse preformed agrin-independent AChR clusters. PLoS ONE 8, e80695 (2013).
van der Neut Kolfschoten, M. et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science 317, 1554–1557 (2007).
Huijbers, M. G. et al. MuSK myasthenia gravis monoclonal antibodies: valency dictates pathogenicity. Neurol. Neuroimmunol. Neuroinflamm 6, e547 (2019).
Damato, V. et al. Clinical value of cell-based assays in the characterisation of seronegative myasthenia gravis. J. Neurol. Neurosurg. Psychiatry 93, 995–1000 (2022).
Hoffmann, S. et al. Complement deposition at the neuromuscular junction in seronegative myasthenia gravis. Acta Neuropathol. 139, 1119–1122 (2020).
Evoli, A. et al. Italian recommendations for the diagnosis and treatment of myasthenia gravis. Neurol. Sci. 40, 1111–1124 (2019).
Murai, H. et al. The Japanese clinical guidelines 2022 for myasthenia gravis and Lambert–Eaton myasthenic syndrome. Clin. Exp. Neuroimmunol. 14, 19–27 (2023).
Melzer, N. et al. Clinical features, pathogenesis, and treatment of myasthenia gravis: a supplement to the Guidelines of the German Neurological Society. J. Neurol. 263, 1473–1494 (2016).
Modoni, A., Mastrorosa, A., Spagni, G. & Evoli, A. Cholinergic hyperactivity in patients with myasthenia gravis with MuSK antibodies: a neurophysiological study. Clin. Neurophysiol. 132, 1845–1849 (2021).
Evoli, A., Alboini, P. E., Damato, V. & Iorio, R. 3,4-Diaminopyridine may improve myasthenia gravis with MuSK antibodies. Neurology 86, 1070–1071 (2016).
Ipe, T. S., Davis, A. R. & Raval, J. S. Therapeutic plasma exchange in myasthenia gravis: a systematic literature review and meta-analysis of comparative evidence. Front. Neurol. 12, 662856 (2021).
Rozsa, C., Lovas, G., Fornadi, L., Szabo, G. & Komoly, S. Safety of long-term combined immunosuppressive treatment in myasthenia gravis – analysis of adverse effects of 163 patients. Eur. J. Neurol. 13, 947–952 (2006).
Verwijst, J., Westerberg, E. & Punga, A. R. Cancer in myasthenia gravis subtypes in relation to immunosuppressive treatment and acetylcholine receptor antibodies: a Swedish nationwide register study. Eur. J. Neurol. 28, 1706–1715 (2021).
Kosmas, C., Stamatopoulos, K., Stavroyianni, N., Tsavaris, N. & Papadaki, T. Anti-CD20-based therapy of B cell lymphoma: state of the art. Leukemia 16, 2004–2015 (2002).
Lee, D. S. W., Rojas, O. L. & Gommerman, J. L. B cell depletion therapies in autoimmune disease: advances and mechanistic insights. Nat. Rev. Drug. Discov. 20, 179–199 (2021).
Schioppo, T. & Ingegnoli, F. Current perspective on rituximab in rheumatic diseases. Drug. Des. Devel Ther. 11, 2891–2904 (2017).
Whittam, D. H. et al. Rituximab in neurological disease: principles, evidence and practice. Pract. Neurol. 19, 5–20 (2019).
Pavlasova, G. & Mraz, M. The regulation and function of CD20: an “enigma” of B-cell biology and targeted therapy. Haematologica 105, 1494–1506 (2020).
Klasener, K. et al. CD20 as a gatekeeper of the resting state of human B cells. Proc. Natl Acad. Sci. USA 118, e2021342118 (2021).
Leandro, M. J., Cooper, N., Cambridge, G., Ehrenstein, M. R. & Edwards, J. C. Bone marrow B-lineage cells in patients with rheumatoid arthritis following rituximab therapy. Rheumatology 46, 29–36 (2007).
Kamburova, E. G. et al. A single dose of rituximab does not deplete B cells in secondary lymphoid organs but alters phenotype and function. Am. J. Transpl. 13, 1503–1511 (2013).
Iorio, R., Damato, V., Alboini, P. E. & Evoli, A. Efficacy and safety of rituximab for myasthenia gravis: a systematic review and meta-analysis. J. Neurol. 262, 1115–1119 (2015).
Dos Santos, A. et al. Efficacy and safety of rituximab in myasthenia gravis: a French multicentre real-life study. Eur. J. Neurol. 27, 2277–2285 (2020).
Hehir, M. K. et al. Rituximab as treatment for anti-MuSK myasthenia gravis: multicenter blinded prospective review. Neurology 89, 1069–1077 (2017).
Topakian, R. et al. High efficacy of rituximab for myasthenia gravis: a comprehensive nationwide study in Austria. J. Neurol. 266, 699–706 (2019).
Brauner, S. et al. Comparison between rituximab treatment for new-onset generalized myasthenia gravis and refractory generalized myasthenia gravis. JAMA Neurol. 77, 974–981 (2020).
Nowak, R. J. et al. Phase 2 trial of rituximab in acetylcholine receptor antibody-positive generalized myasthenia gravis: the BeatMG study. Neurology 98, e376–e389 (2021).
Piehl, F. et al. Efficacy and safety of rituximab for new-onset generalized myasthenia gravis: the RINOMAX randomized clinical trial. JAMA Neurol. 79, 1105–1112 (2022).
Jiang, R. et al. Single-cell repertoire tracing identifies rituximab-resistant B cells during myasthenia gravis relapses. JCI Insight 5, e136471 (2020).
Hauser, S. L. et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N. Engl. J. Med. 376, 221–234 (2017).
Hauser, S. L. et al. Ofatumumab versus teriflunomide in multiple sclerosis. N. Engl. J. Med. 383, 546–557 (2020).
Zhang, B. Ofatumumab. MAbs 1, 326–331 (2009).
Steinman, L. et al. Ublituximab versus teriflunomide in relapsing multiple sclerosis. N. Engl. J. Med. 387, 704–714 (2022).
Le Garff-Tavernier, M. et al. Antibody-dependent cellular cytotoxicity of the optimized anti-CD20 monoclonal antibody ublituximab on chronic lymphocytic leukemia cells with the 17p deletion. Leukemia 28, 230–233 (2014).
Tedder, T. F. CD19: a promising B cell target for rheumatoid arthritis. Nat. Rev. Rheumatol. 5, 572–577 (2009).
Cree, B. A. C. et al. Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): a double-blind, randomised placebo-controlled phase 2/3 trial. Lancet 394, 1352–1363 (2019).
Khaled, A., Kaminski, H. J. & Howard, J. F. Complement inhibitor therapy for myasthenia gravis. Front. Immunol. https://doi.org/10.3389/fimmu.2020.00917 (2020).
Holers, V. M. Complement and its receptors: new insights into human disease. Annu. Rev. Immunol. 32, 433–459 (2014).
Killick, J., Morisse, G., Sieger, D. & Astier, A. L. Complement as a regulator of adaptive immunity. Semin. Immunopathol. 40, 37–48 (2018).
Li, Y. et al. Cellular changes in eculizumab early responders with generalized myasthenia gravis. Clin. Immunol. 231, 108830 (2021).
Dubois, E. A. & Cohen, A. F. Eculizumab. Br. J. Clin. Pharmacol. 68, 318–319 (2009).
Hillmen, P. et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N. Engl. J. Med. 355, 1233–1243 (2006).
Legendre, C. M. et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N. Engl. J. Med. 368, 2169–2181 (2013).
Howard, J. F. Jr. et al. Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol. 16, 976–986 (2017).
Pittock, S. J. et al. Eculizumab in aquaporin-4-positive neuromyelitis optica spectrum disorder. N. Engl. J. Med. 381, 614–625 (2019).
Mantegazza, R. et al. Post-intervention status in patients with refractory myasthenia gravis treated with eculizumab during REGAIN and its open-label extension. Neurology 96, e610–e618 (2021).
Andersen, H. et al. Eculizumab improves fatigue in refractory generalized myasthenia gravis. Qual. Life Res. 28, 2247–2254 (2019).
McNamara, L. A. et al. High risk for invasive meningococcal disease among patients receiving eculizumab (Soliris) despite receipt of meningococcal vaccine. MMWR Morb. Mortal. Wkly. Rep. 66, 734–737 (2017).
Bozio, C. H., Isenhour, C. & McNamara, L. A. Characteristics of and meningococcal disease prevention strategies for commercially insured persons receiving eculizumab in the United States. PLoS ONE 15, e0241989 (2020).
Gäckler, A. et al. Failure of first meningococcal vaccination in patients with atypical haemolytic uraemic syndrome treated with eculizumab. Nephrol. Dial. Transpl. 35, 298–303 (2020).
Nishimura, J. et al. Genetic variants in C5 and poor response to eculizumab. N. Engl. J. Med. 370, 632–639 (2014).
Vu, T. et al. Ravulizumab pharmacokinetics and pharmacodynamics in patients with generalized myasthenia gravis. J. Neurol. 270, 3129–3137 (2023).
Lee, J. W. et al. Ravulizumab (ALXN1210) vs eculizumab in adult patients with PNH naive to complement inhibitors: the 301 study. Blood 133, 530–539 (2019).
Tuan, V. et al. Terminal complement inhibitor ravulizumab in generalized myasthenia gravis. NEJM Evid. 1, EVIDoa2100066 (2022).
Navarro-Carrera, P., Garcia-Rodriguez, J. & Cendejas-Bueno, E. Detection of Neisseria meningitidis in a patient receiving ravulizumab by the FilmArray(R) Meningitis/Encephalitis Panel – a case report. J. Infect. 82, e22–e23 (2021).
Tang, G. Q. et al. Zilucoplan, a macrocyclic peptide inhibitor of human complement component 5, uses a dual mode of action to prevent terminal complement pathway activation. Front. Immunol. 14, 1213920 (2023).
Mastellos, D. C., Ricklin, D. & Lambris, J. D. Clinical promise of next-generation complement therapeutics. Nat. Rev. Drug. Discov. 18, 707–729 (2019).
Howard, J. F. Jr et al. Safety and efficacy of zilucoplan in patients with generalised myasthenia gravis (RAISE): a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Neurol. 22, 395–406 (2023).
Pyzik, M., Kozicky, L. K., Gandhi, A. K. & Blumberg, R. S. The therapeutic age of the neonatal Fc receptor. Nat. Rev. Immunol. 23, 415–432 (2023).
Morell, A., Terry, W. D. & Waldmann, T. A. Metabolic properties of IgG subclasses in man. J. Clin. Invest. 49, 673–680 (1970).
Blumberg, L. J. et al. Blocking FcRn in humans reduces circulating IgG levels and inhibits IgG immune complex-mediated immune responses. Sci. Adv. 5, eaax9586 (2019).
Baker, K., Rath, T., Pyzik, M. & Blumberg, R. S. The role of FcRn in antigen presentation. Front. Immunol. 5, 408 (2014).
Jongbloed, S. L. et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J. Exp. Med. 207, 1247–1260 (2010).
Ulrichts, P. et al. Neonatal Fc receptor antagonist efgartigimod safely and sustainably reduces IgGs in humans. J. Clin. Invest. 128, 4372–4386 (2018).
Howard, J. F. Jr. et al. Safety, efficacy, and tolerability of efgartigimod in patients with generalised myasthenia gravis (ADAPT): a multicentre, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 20, 526–536 (2021).
Hoy, S. M. Rozanolixizumab: first approval. Drugs 83, 1341–1347 (2023).
Bril, V. et al. Safety and efficacy of rozanolixizumab in patients with generalised myasthenia gravis (MycarinG): a randomised, double-blind, placebo-controlled, adaptive phase 3 study. Lancet Neurol. 22, 383–394 (2023).
Kiessling, P. et al. The FcRn inhibitor rozanolixizumab reduces human serum IgG concentration: a randomized phase 1 study. Sci. Transl. Med. 9, eaan1208 (2017).
Guptill, J. A. et al. Vivacity-MG: a phase 2, multicenter, randomized, double-blind, placebo-controlled study to evaluate the safety, tolerability, efficacy, pharmacokinetics, pharmacodynamics, and immunogenicity of nipocalimab administered to adults with generalized myasthenia gravis [abstract]. Neurology https://doi.org/10.1212/WNL.96.15_supplement.2157 (2021).
Yap, D. Y. H. et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of HBM9161, a novel FcRn inhibitor, in a phase I study for healthy Chinese volunteers. Clin. Transl. Sci. 14, 1769–1779 (2021).
Yan, C. et al. Therapeutic effects of batoclimab in Chinese patients with generalized myasthenia gravis: a double-blinded, randomized, placebo-controlled phase II study. Neurol. Ther. 11, 815–834 (2022).
Robinson, M. J. et al. Long-lived plasma cells accumulate in the bone marrow at a constant rate from early in an immune response. Sci. Immunol. 7, eabm8389 (2022).
Lightman, S. M., Utley, A. & Lee, K. P. Survival of long-lived plasma cells (LLPC): piecing together the puzzle. Front. Immunol. 10, 965 (2019).
Mahevas, M., Michel, M., Weill, J. C. & Reynaud, C. A. Long-lived plasma cells in autoimmunity: lessons from B-cell depleting therapy. Front. Immunol. 4, 494 (2013).
Robinson, M. J., Webster, R. H. & Tarlinton, D. M. How intrinsic and extrinsic regulators of plasma cell survival might intersect for durable humoral immunity. Immunol. Rev. 296, 87–103 (2020).
Manasanch, E. E. & Orlowski, R. Z. Proteasome inhibitors in cancer therapy. Nat. Rev. Clin. Oncol. 14, 417–433 (2017).
San Miguel, J. F. et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N. Engl. J. Med. 359, 906–917 (2008).
Zhang, S. et al. Bortezomib-based consolidation or maintenance therapy for multiple myeloma: a meta-analysis. Blood Cancer J. 10, 33 (2020).
Gomez, A. M. et al. Proteasome inhibition with bortezomib depletes plasma cells and autoantibodies in experimental autoimmune myasthenia gravis. J. Immunol. 186, 2503–2513 (2011).
Field-Smith, A., Morgan, G. J. & Davies, F. E. Bortezomib (Velcadetrade) in the treatment of multiple myeloma. Ther. Clin. Risk Manag. 2, 271–279 (2006).
Siegel, D. et al. Integrated safety profile of single-agent carfilzomib: experience from 526 patients enrolled in 4 phase II clinical studies. Haematologica 98, 1753–1761 (2013).
Zeng, G. et al. Recent advances and future perspectives of noncompetitive proteasome inhibitors. Bioorg. Chem. 135, 106507 (2023).
Yang, J. C. & Rosenberg, S. A. Adoptive T-cell therapy for cancer. Adv. Immunol. 130, 279–294 (2016).
Sterner, R. C. & Sterner, R. M. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 11, 69 (2021).
Sermer, D. & Brentjens, R. CAR T-cell therapy: full speed ahead. Hematol. Oncol. 37, 95–100 (2019).
Alnefaie, A. et al. Chimeric antigen receptor T-cells: an overview of concepts, applications, limitations, and proposed solutions. Front. Bioeng. Biotechnol. 10, 797440 (2022).
Chatenoud, L. Precision medicine for autoimmune disease. Nat. Biotechnol. 34, 930–932 (2016).
Granit, V. et al. Safety and clinical activity of autologous RNA chimeric antigen receptor T-cell therapy in myasthenia gravis (MG-001): a prospective, multicentre, open-label, non-randomised phase 1b/2a study. Lancet Neurol. 22, 578–590 (2023).
Ellebrecht, C. T. et al. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science 353, 179–184 (2016).
Oh, S. et al. Precision targeting of autoantigen-specific B cells in muscle-specific tyrosine kinase myasthenia gravis with chimeric autoantibody receptor T cells. Nat. Biotechnol. 353, 1229–1238 (2023).
Bonifant, C. L., Jackson, H. J., Brentjens, R. J. & Curran, K. J. Toxicity and management in CAR T-cell therapy. Mol. Ther. Oncolytics 3, 16011 (2016).
Baysoy, A., Bai, Z., Satija, R. & Fan, R. The technological landscape and applications of single-cell multi-omics. Nat. Rev. Mol. Cell Biol. 24, 695–713 (2023).
Velez-Santamaria, V. et al. Eculizumab as a promising treatment in thymoma-associated myasthenia gravis. Ther. Adv. Neurol. Disord. 13, 1756286420932035 (2020).
Strano, C. M. M. et al. Eculizumab as a fast-acting rescue therapy in a refractory myasthenic crisis: a case report. J. Neurol. 269, 6152–6154 (2022).
Vinciguerra, C. et al. Starting eculizumab as rescue therapy in refractory myasthenic crisis. Neurol. Sci. 44, 3707–3709 (2023).
Lin, C. Y. et al. iPSC-derived functional human neuromuscular junctions model the pathophysiology of neuromuscular diseases. JCI Insight 4, e124299 (2019).
Tice, J. A. et al. The effectiveness and value of eculizumab and efgartigimod for generalized myasthenia gravis. J. Manag. Care Spec. Pharm. 28, 119–124 (2022).
Bubuioc, A. M., Kudebayeva, A., Turuspekova, S., Lisnic, V. & Leone, M. A. The epidemiology of myasthenia gravis. J. Med. Life 14, 7–16 (2021).
Guptill, J. T. et al. Addressing outcome measure variability in myasthenia gravis clinical trials. Neurology 101, 442–451 (2023).
Jaretzki, A. 3rd et al. Myasthenia gravis: recommendations for clinical research standards. Task force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America. Neurology 55, 16–23 (2000).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author has received consultancy fees and speaker honoraria from Alexion, Argenx and UCB.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Iorio, R. Myasthenia gravis: the changing treatment landscape in the era of molecular therapies. Nat Rev Neurol 20, 84–98 (2024). https://doi.org/10.1038/s41582-023-00916-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-023-00916-w
This article is cited by
-
Integrative multi-omics analysis identifies genetically supported druggable targets and immune cell specificity for myasthenia gravis
Journal of Translational Medicine (2024)
-
Bispecific BCMA/CD19 targeted CAR-T cell therapy forces sustained disappearance of symptoms and anti-acetylcholine receptor antibodies in refractory myasthenia gravis: a case report
Journal of Neurology (2024)