Abstract
Clinical symptom worsening in patients with multiple sclerosis (MS) is driven by inflammation compartmentalized within the CNS, which results in chronic neuronal damage owing to insufficient repair mechanisms. The term ‘smouldering inflammation’ summarizes the biological aspects underlying this chronic, non-relapsing and immune-mediated mechanism of disease progression. Smouldering inflammation is likely to be shaped and sustained by local factors in the CNS that account for the persistence of this inflammatory response and explain why current treatments for MS do not sufficiently target this process. Local factors that affect the metabolic properties of glial cells and neurons include cytokines, pH value, lactate levels and nutrient availability. This Review summarizes current knowledge of the local inflammatory microenvironment in smouldering inflammation and how it interacts with the metabolism of tissue-resident immune cells, thereby promoting inflammatory niches within the CNS. The discussion highlights environmental and lifestyle factors that are increasingly recognized as capable of altering immune cell metabolism and potentially responsible for smouldering pathology in the CNS. Currently approved MS therapies that target metabolic pathways are also discussed, along with their potential for preventing the processes that contribute to smouldering inflammation and thereby to progressive neurodegenerative damage in MS.
Key points
-
The term ‘smouldering inflammation’ summarizes the biological aspects that underlie compartmentalized CNS inflammation and chronic neuronal damage, which are insufficiently targeted by currently approved therapies.
-
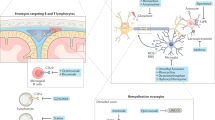
The chronically inflamed CNS provides a unique tissue microenvironment characterized by alterations in nutrient availability, pH value, lactate levels and cytokine profiles.
-
Tissue-resident memory T cells, microglia and astrocytes are key immune cells in smouldering inflammation that can adapt their metabolic profiles in response to the inflamed microenvironment.
-
Environmental and lifestyle factors are increasingly recognized as modulators of immune cell metabolism.
-
Modulation of immune cell metabolism and the inflammatory microenvironment might foster novel treatment approaches in smouldering inflammation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Reich, D. S., Lucchinetti, C. F. & Calabresi, P. A. Multiple sclerosis. N. Engl. J. Med. 378, 169–180 (2018).
Larochelle, C., Uphaus, T., Prat, A. & Zipp, F. Secondary progression in multiple sclerosis: neuronal exhaustion or distinct pathology? Trends Neurosci. 39, 325–339 (2016).
Tintore, M., Vidal-Jordana, A. & Sastre-Garriga, J. Treatment of multiple sclerosis — success from bench to bedside. Nat. Rev. Neurol. 15, 53–58 (2019).
Bittner, S. & Zipp, F. Progression in multiple sclerosis — a long-term problem. Curr. Opin. Neurol. 35, 293–298 (2022).
Frischer, J. M. et al. Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann. Neurol. 78, 710–721 (2015).
Lassmann, H. The contribution of neuropathology to multiple sclerosis research. Eur. J. Neurol. 29, 2869–2877 (2022).
Geltink, R. I. K., Kyle, R. L. & Pearce, E. L. Unraveling the complex interplay between T cell metabolism and function. Annu. Rev. Immunol. 36, 461–488 (2018).
O’Neill, L. A., Kishton, R. J. & Rathmell, J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 16, 553–565 (2016).
Attfield, K. E., Jensen, L. T., Kaufmann, M., Friese, M. A. & Fugger, L. The immunology of multiple sclerosis. Nat. Rev. Immunol. 22, 734–750 (2022).
Kuhlmann, T. et al. Multiple sclerosis progression: time for a new mechanism-driven framework. Lancet Neurol. 22, 78–88 (2023).
Bittner, S. & Zipp, F. AAN unveils new guidelines for MS disease-modifying therapy. Nat. Rev. Neurol. 14, 384–386 (2018).
Giovannoni, G. et al. Smouldering multiple sclerosis: the ‘real MS’. Ther. Adv. Neurol. Disord. 15, 17562864211066751 (2022).
Kappos, L. et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 77, 1132–1140 (2020).
Gartner, J. et al. Efficacy and safety of ofatumumab in recently diagnosed, treatment-naive patients with multiple sclerosis: results from ASCLEPIOS I and II. Mult. Scler. 28, 1562–1575 (2022).
von Wyl, V. et al. Disability progression in relapse-free multiple sclerosis patients on fingolimod versus interferon-β/glatiramer acetate. Mult. Scler. 27, 439–448 (2021).
Uphaus, T. et al. NfL predicts relapse-free progression in a longitudinal multiple sclerosis cohort study. EBioMedicine 72, 103590 (2021).
Kuhlmann, T. et al. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 133, 13–24 (2017).
Absinta, M. et al. Persistent 7-Tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J. Clin. Invest. 126, 2597–2609 (2016).
Maggi, P. et al. Chronic white matter inflammation and serum neurofilament levels in multiple sclerosis. Neurology 97, e543–e553 (2021).
Kaufmann, M. et al. Identification of early neurodegenerative pathways in progressive multiple sclerosis. Nat. Neurosci. 25, 944–955 (2022).
Elliott, C. et al. Chronic white matter lesion activity predicts clinical progression in primary progressive multiple sclerosis. Brain 142, 2787–2799 (2019).
Ng Kee Kwong, K. C. et al. The prevalence of paramagnetic rim lesions in multiple sclerosis: a systematic review and meta-analysis. PLoS One 16, e0256845 (2021).
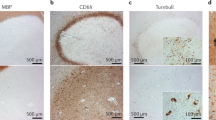
Absinta, M. et al. A lymphocyte-microglia-astrocyte axis in chronic active multiple sclerosis. Nature 597, 709–714 (2021). This MRI-informed RNA sequencing study characterizes the immunological landscape and interactome in the rim of smouldering lesions.
Calvi, A. et al. Association of slowly expanding lesions on MRI with disability in people with secondary progressive multiple sclerosis. Neurology 98, e1783–e1793 (2022).
Bittner, S. & Zipp, F. A lymphocyte–glia connection sets the pace for smoldering inflammation. Cell 184, 5696–5698 (2021).
Calvi, A. et al. Slowly expanding lesions relate to persisting black-holes and clinical outcomes in relapse-onset multiple sclerosis. Neuroimage Clin. 35, 103048 (2022).
Preziosa, P. et al. Slowly expanding lesions predict 9-year multiple sclerosis disease progression. Neurol. Neuroimmunol. Neuroinflamm. https://doi.org/10.1212/NXI.0000000000001139 (2022).
Elliott, C. et al. Patterning chronic active demyelination in slowly expanding/evolving white matter MS lesions. AJNR Am. J. Neuroradiol. 41, 1584–1591 (2020).
Gubser, P. M. et al. Rapid effector function of memory CD8+ T cells requires an immediate-early glycolytic switch. Nat. Immunol. 14, 1064–1072 (2013).
Shi, L. Z. et al. HIF1α-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J. Exp. Med. 208, 1367–1376 (2011).
Dufort, F. J. et al. Glucose-dependent de novo lipogenesis in B lymphocytes: a requirement for ATP-citrate lyase in lipopolysaccharide-induced differentiation. J. Biol. Chem. 289, 7011–7024 (2014).
O’Sullivan, D. et al. Memory CD8+ T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity 41, 75–88 (2014).
Gerriets, V. A. et al. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J. Clin. Invest. 125, 194–207 (2015).
Chang, C. H. et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 153, 1239–1251 (2013). This landmark study directly links metabolic pathways in lymphocytes to the production of pro-inflammatory cytokines.
Moon, J. S. et al. mTORC1-induced HK1-dependent glycolysis regulates NLRP3 inflammasome activation. Cell Rep. 12, 102–115 (2015).
Berod, L. et al. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat. Med. 20, 1327–1333 (2014).
Nakaya, M. et al. Inflammatory T cell responses rely on amino acid transporter ASCT2 facilitation of glutamine uptake and mTORC1 kinase activation. Immunity 40, 692–705 (2014). This study shows that inhibition of amino acid metabolism reduces T cell inflammation and suppresses EAE symptoms.
Bantug, G. R., Galluzzi, L., Kroemer, G. & Hess, C. The spectrum of T cell metabolism in health and disease. Nat. Rev. Immunol. 18, 19–34 (2018).
Birkner, K. et al. β1-Integrin- and Kv1.3 channel-dependent signaling stimulates glutamate release from Th17 cells. J. Clin. Invest 130, 715–732 (2020).
Prinz, M., Masuda, T., Wheeler, M. A. & Quintana, F. J. Microglia and central nervous system-associated macrophages — from origin to disease modulation. Annu. Rev. Immunol. 39, 251–277 (2021).
Bottcher, C. et al. Single-cell mass cytometry reveals complex myeloid cell composition in active lesions of progressive multiple sclerosis. Acta Neuropathol. Commun. 8, 136 (2020).
Yong, V. W. Microglia in multiple sclerosis: protectors turn destroyers. Neuron 110, 3534–3548 (2022).
Zrzavy, T. et al. Loss of ‘homeostatic’ microglia and patterns of their activation in active multiple sclerosis. Brain 140, 1900–1913 (2017).
Masuda, T. et al. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 566, 388–392 (2019).
Bernier, L. P. et al. Microglial metabolic flexibility supports immune surveillance of the brain parenchyma. Nat. Commun. 11, 1559 (2020).
Wang, L. et al. Glucose transporter 1 critically controls microglial activation through facilitating glycolysis. Mol. Neurodegener. 14, 2 (2019).
van der Poel, M. et al. Transcriptional profiling of human microglia reveals grey–white matter heterogeneity and multiple sclerosis-associated changes. Nat. Commun. 10, 1139 (2019). This in-depth profiling study reveals the metabolic specialization of microglia in different anatomical locations of the brain.
Holland, R. et al. Inflammatory microglia are glycolytic and iron retentive and typify the microglia in APP/PS1 mice. Brain Behav. Immun. 68, 183–196 (2018).
Costa, I. et al. Molecular mechanisms of ferroptosis and their involvement in brain diseases. Pharmacol. Ther. 244, 108373 (2023).
Ryan, S. K. et al. Microglia ferroptosis is regulated by SEC24B and contributes to neurodegeneration. Nat. Neurosci. 26, 12–26 (2023).
Rothammer, N. et al. G9a dictates neuronal vulnerability to inflammatory stress via transcriptional control of ferroptosis. Sci. Adv. 8, eabm5500 (2022).
Dong, Y. et al. Oxidized phosphatidylcholines found in multiple sclerosis lesions mediate neurodegeneration and are neutralized by microglia. Nat. Neurosci. 24, 489–503 (2021).
Wasser, B. et al. CNS-localized myeloid cells capture living invading T cells during neuroinflammation. J. Exp. Med. https://doi.org/10.1084/jem.20190812 (2020).
Brandt, A. U. et al. Association of a marker of N-acetylglucosamine with progressive multiple sclerosis and neurodegeneration. JAMA Neurol. 78, 842–852 (2021).
Magistretti, P. J. & Allaman, I. Lactate in the brain: from metabolic end-product to signalling molecule. Nat. Rev. Neurosci. 19, 235–249 (2018).
Chao, C. C. et al. Metabolic control of astrocyte pathogenic activity via cPLA2-MAVS. Cell 179, 1483–1498.e22 (2019). This paper links dysfunction of sphingolipid metabolism to astrocyte inflammation driving progressive CNS inflammation.
Polyzos, A. et al. Mitochondrial targeting of XJB-5-131 attenuates or improves pathophysiology in HdhQ150 animals with well-developed disease phenotypes. Hum. Mol. Genet. 25, 1792–1802 (2016).
Mahad, D. H., Trapp, B. D. & Lassmann, H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 14, 183–193 (2015).
Tullius, S. G. et al. NAD+ protects against EAE by regulating CD4+ T-cell differentiation. Nat. Commun. 5, 5101 (2014).
Cameron, A. M. et al. Inflammatory macrophage dependence on NAD+ salvage is a consequence of reactive oxygen species-mediated DNA damage. Nat. Immunol. 20, 420–432 (2019).
Meyer, T. et al. NAD+ metabolism drives astrocyte proinflammatory reprogramming in central nervous system autoimmunity. Proc. Natl Acad. Sci. USA 119, e2211310119 (2022).
Schirmer, L. et al. Neuronal vulnerability and multilineage diversity in multiple sclerosis. Nature 573, 75–82 (2019).
Dienel, G. A. Brain glucose metabolism: integration of energetics with function. Physiol. Rev. 99, 949–1045 (2019).
Jarius, S. et al. Cerebrospinal fluid findings in patients with myelin oligodendrocyte glycoprotein (MOG) antibodies. Part 2: results from 108 lumbar punctures in 80 pediatric patients. J. Neuroinflamm. 17, 262 (2020).
Jarius, S. et al. Cerebrospinal fluid findings in aquaporin-4 antibody positive neuromyelitis optica: results from 211 lumbar punctures. J. Neurol. Sci. 306, 82–90 (2011).
Stampanoni Bassi, M. et al. Cerebrospinal fluid levels of L-glutamate signal central inflammatory neurodegeneration in multiple sclerosis. J. Neurochem. 159, 857–866 (2021).
Simone, I. L. et al. High resolution proton MR spectroscopy of cerebrospinal fluid in MS patients. Comparison with biochemical changes in demyelinating plaques. J. Neurol. Sci. 144, 182–190 (1996).
Bitsch, A. et al. Inflammatory CNS demyelination: histopathologic correlation with in vivo quantitative proton MR spectroscopy. AJNR Am. J. Neuroradiol. 20, 1619–1627 (1999).
Friese, M. A. et al. Acid-sensing ion channel-1 contributes to axonal degeneration in autoimmune inflammation of the central nervous system. Nat. Med. 13, 1483–1489 (2007).
Albanese, M. et al. Cerebrospinal fluid lactate is associated with multiple sclerosis disease progression. J. Neuroinflamm. 13, 36 (2016).
Bittner, S., Oh, J., Havrdova, E. K., Tintore, M. & Zipp, F. The potential of serum neurofilament as biomarker for multiple sclerosis. Brain 144, 2954–2963 (2021).
Regenold, W. T., Phatak, P., Makley, M. J., Stone, R. D. & Kling, M. A. Cerebrospinal fluid evidence of increased extra-mitochondrial glucose metabolism implicates mitochondrial dysfunction in multiple sclerosis disease progression. J. Neurol. Sci. 275, 106–112 (2008).
Friese, M. A., Schattling, B. & Fugger, L. Mechanisms of neurodegeneration and axonal dysfunction in multiple sclerosis. Nat. Rev. Neurol. 10, 225–238 (2014).
Lee, Y. S., Wollam, J. & Olefsky, J. M. An integrated view of immunometabolism. Cell 172, 22–40 (2018).
Huppke, B. et al. Association of obesity with multiple sclerosis risk and response to first-line disease modifying drugs in children. JAMA Neurol. 76, 1157–1165 (2019).
Maffei, M. et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat. Med. 1, 1155–1161 (1995).
Matarese, G. et al. Leptin increase in multiple sclerosis associates with reduced number of CD4+CD25+ regulatory T cells. Proc. Natl Acad. Sci. USA 102, 5150–5155 (2005).
Gerriets, V. A. et al. Leptin directly promotes T-cell glycolytic metabolism to drive effector T-cell differentiation in a mouse model of autoimmunity. Eur. J. Immunol. 46, 1970–1983 (2016).
Lehmann, R. et al. Medium chain acylcarnitines dominate the metabolite pattern in humans under moderate intensity exercise and support lipid oxidation. PLoS One 5, e11519 (2010).
Lewis, G. D. et al. Metabolic signatures of exercise in human plasma. Sci. Transl. Med. 2, 33ra37 (2010).
Christ, A., Lauterbach, M. & Latz, E. Western diet and the immune system: an inflammatory connection. Immunity 51, 794–811 (2019).
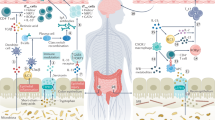
Ghezzi, L., Cantoni, C., Pinget, G. V., Zhou, Y. & Piccio, L. Targeting the gut to treat multiple sclerosis. J. Clin. Invest. 131, e143774 (2021).
Luu, M. et al. The short-chain fatty acid pentanoate suppresses autoimmunity by modulating the metabolic-epigenetic crosstalk in lymphocytes. Nat. Commun. 10, 760 (2019).
Duscha, A. et al. Propionic acid shapes the multiple sclerosis disease course by an immunomodulatory mechanism. Cell 180, 1067–1080.e16 (2020).
Sonner, J. K. et al. Dietary tryptophan links encephalogenicity of autoreactive T cells with gut microbial ecology. Nat. Commun. 10, 4877 (2019).
Rothhammer, V. et al. Microglial control of astrocytes in response to microbial metabolites. Nature 557, 724–728 (2018).
Cirac, A. et al. The aryl hydrocarbon receptor-dependent TGF-α/VEGF-B ratio correlates with disease subtype and prognosis in multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 8, e1043 (2021).
Tsaktanis, T. et al. Aryl hydrocarbon receptor plasma agonist activity correlates with disease activity in progressive MS. Neurol. Neuroimmunol. Neuroinflamm. 8, e933 (2021).
Scott, L. J. Teriflunomide: a review in relapsing–remitting multiple sclerosis. Drugs 79, 875–886 (2019).
Ambrosius, B. et al. Teriflunomide and monomethylfumarate target HIV-induced neuroinflammation and neurotoxicity. J. Neuroinflamm. 14, 51 (2017).
Wostradowski, T. et al. In vitro evaluation of physiologically relevant concentrations of teriflunomide on activation and proliferation of primary rodent microglia. J. Neuroinflamm. 13, 250 (2016).
Groh, J., Horner, M. & Martini, R. Teriflunomide attenuates neuroinflammation-related neural damage in mice carrying human PLP1 mutations. J. Neuroinflamm. 15, 194 (2018).
Schimrigk, S. et al. Oral fumaric acid esters for the treatment of active multiple sclerosis: an open-label, baseline-controlled pilot study. Eur. J. Neurol. 13, 604–610 (2006).
Liebmann, M. et al. Dimethyl fumarate treatment restrains the antioxidative capacity of T cells to control autoimmunity. Brain 144, 3126–3141 (2021). This work shows that dimethyl fumarate reduces glutathione levels, thereby impairing mitochondrial function and inducing apoptosis in activated T lymphocytes.
Fleischer, V. et al. Treatment response to dimethyl fumarate is characterized by disproportionate CD8+ T cell reduction in MS. Mult. Scler. 24, 632–641 (2018).
Ghadiri, M. et al. Dimethyl fumarate-induced lymphopenia in MS due to differential T-cell subset apoptosis. Neurol. Neuroimmunol. Neuroinflamm. 4, e340 (2017).
Luckel, C. et al. IL-17+ CD8+ T cell suppression by dimethyl fumarate associates with clinical response in multiple sclerosis. Nat. Commun. 10, 5722 (2019).
Carlstrom, K. E. et al. Therapeutic efficacy of dimethyl fumarate in relapsing-remitting multiple sclerosis associates with ROS pathway in monocytes. Nat. Commun. 10, 3081 (2019).
Kornberg, M. D. et al. Dimethyl fumarate targets GAPDH and aerobic glycolysis to modulate immunity. Science 360, 449–453 (2018).
Shestov, A. A. et al. Quantitative determinants of aerobic glycolysis identify flux through the enzyme GAPDH as a limiting step. eLife https://doi.org/10.7554/eLife.03342 (2014).
Xia, L. et al. Inhibition of gasdermin D-mediated pyroptosis attenuates the severity of seizures and astroglial damage in kainic acid-induced epileptic mice. Front. Pharmacol. 12, 751644 (2021).
Zhang, J. et al. Gasdermin D-mediated microglial pyroptosis exacerbates neurotoxicity of aflatoxins B1 and M1 in mouse primary microglia and neuronal cultures. Neurotoxicology 91, 305–320 (2022).
Gil, A. et al. Neuronal metabolism and neuroprotection: neuroprotective effect of fingolimod on menadione-induced mitochondrial damage. Cells https://doi.org/10.3390/cells10010034 (2020).
Feng, Y., Feng, F., Pan, S., Zhang, J. & Li, W. Fingolimod ameliorates chronic experimental autoimmune neuritis by modulating inflammatory cytokines and Akt/mTOR/NF-κB signaling. Brain Behav. 13, e2965 (2023).
Makled, M. N., Serrya, M. S. & El-Sheakh, A. R. Fingolimod ameliorates acetic acid-induced ulcerative colitis: an insight into its modulatory impact on pro/anti-inflammatory cytokines and AKT/mTOR signalling. Basic Clin. Pharmacol. Toxicol. 130, 569–580 (2022).
Cui, L. et al. FTY720 inhibits the activation of pancreatic stellate cells by promoting apoptosis and suppressing autophagy via the AMPK/mTOR pathway. Life Sci. 217, 243–250 (2019).
Galvan-Pena, S. & O’Neill, L. A. Metabolic reprograming in macrophage polarization. Front. Immunol. 5, 420 (2014).
Perl, A. Activation of mTOR (mechanistic target of rapamycin) in rheumatic diseases. Nat. Rev. Rheumatol. 12, 169–182 (2016).
Huang, Z. et al. mTORC1 links pathology in experimental models of Still’s disease and macrophage activation syndrome. Nat. Commun. 13, 6915 (2022).
Li, Z., Nie, L., Chen, L., Sun, Y. & Li, G. Rapamycin relieves inflammation of experimental autoimmune encephalomyelitis by altering the balance of Treg/Th17 in a mouse model. Neurosci. Lett. 705, 39–45 (2019).
Lisi, L. et al. Rapamycin reduces clinical signs and neuropathic pain in a chronic model of experimental autoimmune encephalomyelitis. J. Neuroimmunol. 243, 43–51 (2012).
Bagherpour, B. et al. Promising effect of rapamycin on multiple sclerosis. Mult. Scler. Relat. Disord. 26, 40–45 (2018).
O’Neill, L. A. & Hardie, D. G. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature 493, 346–355 (2013).
Mangalam, A. K. et al. AMP-activated protein kinase suppresses autoimmune central nervous system disease by regulating M1-type macrophage-Th17 axis. J. Immunol. 197, 747–760 (2016).
Zhou, G. et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 108, 1167–1174 (2001).
Nath, N. et al. Metformin attenuated the autoimmune disease of the central nervous system in animal models of multiple sclerosis. J. Immunol. 182, 8005–8014 (2009).
Phair, I. R. et al. AMPK integrates metabolite and kinase-based immunometabolic control in macrophages. Mol. Metab. 68, 101661 (2023).
Negrotto, L., Farez, M. F. & Correale, J. Immunologic effects of metformin and pioglitazone treatment on metabolic syndrome and multiple sclerosis. JAMA Neurol. 73, 520–528 (2016).
Rhoads, J. P., Major, A. S. & Rathmell, J. C. Fine tuning of immunometabolism for the treatment of rheumatic diseases. Nat. Rev. Rheumatol. 13, 313–320 (2017).
Sundrud, M. S. et al. Halofuginone inhibits TH17 cell differentiation by activating the amino acid starvation response. Science 324, 1334–1338 (2009).
Faissner, S., Plemel, J. R., Gold, R. & Yong, V. W. Progressive multiple sclerosis: from pathophysiology to therapeutic strategies. Nat. Rev. Drug Discov. 18, 905–922 (2019).
Schweitzer, F. et al. Age and the risks of high-efficacy disease modifying drugs in multiple sclerosis. Curr. Opin. Neurol. 32, 305–312 (2019).
Irani, S. R., Nath, A. & Zipp, F. The neuroinflammation collection: a vision for expanding neuro-immune crosstalk in brain. Brain 144, e59 (2021).
Vigo, T. et al. IFNβ enhances mesenchymal stromal (stem) cells immunomodulatory function through STAT1–3 activation and mTOR-associated promotion of glucose metabolism. Cell Death Dis. 10, 85 (2019).
La Rocca, C. et al. Immunometabolic profiling of T cells from patients with relapsing–remitting multiple sclerosis reveals an impairment in glycolysis and mitochondrial respiration. Metabolism 77, 39–46 (2017).
Haghikia, A. et al. Interferon-β affects mitochondrial activity in CD4+ lymphocytes: implications for mechanism of action in multiple sclerosis. Mult. Scler. 21, 1262–1270 (2015).
Di Filippo, M. et al. Interferon-β1a protects neurons against mitochondrial toxicity via modulation of STAT1 signaling: electrophysiological evidence. Neurobiol. Dis. 62, 387–393 (2014).
Bustamante, M. F., Nurtdinov, R. N., Rio, J., Montalban, X. & Comabella, M. Baseline gene expression signatures in monocytes from multiple sclerosis patients treated with interferon-β. PLoS One 8, e60994 (2013).
Lorefice, L. et al. Assessing the metabolomic profile of multiple sclerosis patients treated with interferon β1a by 1H-NMR spectroscopy. Neurotherapeutics 16, 797–807 (2019).
De Riccardis, L. et al. Metabolic response to glatiramer acetate therapy in multiple sclerosis patients. BBA Clin. 6, 131–137 (2016).
Signoriello, E. et al. 12-months prospective pentraxin-3 and metabolomic evaluation in multiple sclerosis patients treated with glatiramer acetate. J. Neuroimmunol. 348, 577385 (2020).
Ruggieri, M. et al. Glatiramer acetate induces pro-apoptotic mechanisms involving Bcl-2, Bax and Cyt-c in peripheral lymphocytes from multiple sclerosis patients. J. Neurol. 253, 231–236 (2006).
Ntranos, A. et al. Bacterial neurotoxic metabolites in multiple sclerosis cerebrospinal fluid and plasma. Brain 145, 569–583 (2022).
Carlstrom, K. E. et al. Gsta4 controls apoptosis of differentiating adult oligodendrocytes during homeostasis and remyelination via the mitochondria-associated Fas-Casp8-Bid-axis. Nat. Commun. 11, 4071 (2020).
Hayashi, G. et al. Dimethyl fumarate mediates Nrf2-dependent mitochondrial biogenesis in mice and humans. Hum. Mol. Genet. 26, 2864–2873 (2017).
Humphries, F. et al. Succination inactivates gasdermin D and blocks pyroptosis. Science 369, 1633–1637 (2020).
Diebold, M. et al. Dimethyl fumarate influences innate and adaptive immunity in multiple sclerosis. J. Autoimmun. 86, 39–50 (2018).
Cheng, J. et al. Fumarate suppresses B-cell activation and function through direct inactivation of LYN. Nat. Chem. Biol. 18, 954–962 (2022).
Mouton, A. J. et al. Dimethyl fumarate preserves left ventricular infarct integrity following myocardial infarction via modulation of cardiac macrophage and fibroblast oxidative metabolism. J. Mol. Cell Cardiol. 158, 38–48 (2021).
Schmitt, A. et al. Dimethyl fumarate induces ferroptosis and impairs NF-κB/STAT3 signaling in DLBCL. Blood 138, 871–884 (2021).
Kabiraj, P. et al. Teriflunomide shifts the astrocytic bioenergetic profile from oxidative metabolism to glycolysis and attenuates TNFα-induced inflammatory responses. Sci. Rep. 12, 3049 (2022).
Malla, B. et al. Teriflunomide preserves neuronal activity and protects mitochondria in brain slices exposed to oxidative stress. Int. J. Mol. Sci. https://doi.org/10.3390/ijms23031538 (2022).
Malla, B. et al. Teriflunomide preserves peripheral nerve mitochondria from oxidative stress-mediated alterations. Ther. Adv. Chronic Dis. 11, 2040622320944773 (2020).
Klotz, L. et al. Teriflunomide treatment for multiple sclerosis modulates T cell mitochondrial respiration with affinity-dependent effects. Sci. Transl. Med. 11, eaao5563 (2019). This study of immunometabolism demonstrates that high-affinity T cell clones in patients with MS are metabolically distinct and are targeted by teriflunomide.
Bajwa, A. et al. Sphingosine 1-phosphate receptor-1 enhances mitochondrial function and reduces cisplatin-induced tubule injury. J. Am. Soc. Nephrol. 26, 908–925 (2015).
Tian, L. et al. S1P/S1PR1 signaling differentially regulates the allogeneic response of CD4 and CD8 T cells by modulating mitochondrial fission. Cell Mol. Immunol. 19, 1235–1250 (2022).
Squillace, S. et al. Sphingosine-1-phosphate receptor 1 activation in the central nervous system drives cisplatin-induced cognitive impairment. J. Clin. Invest. https://doi.org/10.1172/JCI157738 (2022).
Rousselle, T. V. et al. FTY720 regulates mitochondria biogenesis in dendritic cells to prevent kidney ischemic reperfusion injury. Front. Immunol. 11, 1278 (2020).
O’Sullivan, S. A., Velasco-Estevez, M. & Dev, K. K. Demyelination induced by oxidative stress is regulated by sphingosine 1-phosphate receptors. Glia 65, 1119–1136 (2017).
Conrad, D. M. et al. 2-Chloro-2′-deoxyadenosine-induced apoptosis in T leukemia cells is mediated via a caspase-3-dependent mitochondrial feedback amplification loop. Int. J. Oncol. 32, 1325–1333 (2008).
Janoschka, C. et al. Enhanced pathogenicity of Th17 cells due to natalizumab treatment: implications for MS disease rebound. Proc. Natl Acad. Sci. USA 120, e2209944120 (2023).
Zeng, Q. H. et al. B cells polarize pathogenic inflammatory T helper subsets through ICOSL-dependent glycolysis. Sci. Adv. https://doi.org/10.1126/sciadv.abb6296 (2020).
Kaushik, D. K. & Yong, V. W. Metabolic needs of brain-infiltrating leukocytes and microglia in multiple sclerosis. J. Neurochem. 158, 14–24 (2021).
Acknowledgements
The authors’ research work is supported by grants from the German Research Council (DFG): CRC-TR-128 to F.Z. and S.B.; CRC-TR-355 to S.B.; CRC1080 and SFB1292 to F.Z.; the Progressive Multiple Sclerosis Alliance (PMSA): BRAVEinMS PA-1604–08492 to F.Z.; and the Herman and Lilly Schilling Foundation to S.B. The authors thank C. Ernest for proofreading and editing the manuscript.
Author information
Authors and Affiliations
Contributions
S.B., K.P. and F.Z. wrote the manuscript. S.B., K.P. and L.K. researched data for the article. All authors contributed substantially to discussions of the content and to review and/or editing of the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
S.B. declares that he has received honoraria from Biogen Idec, Bristol Meyers Squibb, Merck Healthcare, Novartis, Sanofi, Roche and Teva. F.Z. declares that she has received research grants and/or consultation funds from Biogen, Ministry of Education and Research (BMBF), Bristol Meyers Squibb, Celgene, German Research Foundation (DFG), Janssen, the Max Planck Society (MPG), Merck Serono, Novartis, Progressive MS Alliance (PMSA), Roche, Sandoz and Sanofi Genzyme. K.P. and L.K. declare no competing interests.
Peer review
Peer review information
Nature Reviews Neurology thanks Lars Fugger, Bianca Weinstock-Guttman and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Aerobic glycolysis
-
The pathway that converts pyruvate into lactate in an aerobic environment and generates NAD+ molecules.
- Experimental autoimmune encephalomyelitis
-
(EAE). The most common experimental animal model of multiple sclerosis; inflammatory demyelinating brain disease can be induced in various animal species and strains by different methods depending on the experimental question.
- Fatty acid oxidation
-
The metabolic pathway that converts fatty acids to acetyl coenzyme A to fuel the tricarboxylic acid cycle.
- Glutaminolysis
-
The metabolic pathway that converts glutamine to α-ketoglutarate to fuel the tricarboxylic acid cycle.
- Glycolysis
-
An oxygen-independent metabolic pathway that rapidly converts glucose into pyruvate to produce ATP and several metabolic intermediates used in further pathways.
- Immunometabolism
-
The interplay between metabolism and immunology in both health and disease.
- Oxidative phosphorylation
-
The metabolic pathway in which substrates are oxidized in the tricarboxylic acid cycle to generate ATP via the mitochondrial electron transport chain.
- Smouldering inflammation
-
An umbrella term that includes all CNS-intrinsic, non-relapsing inflammatory processes in patients with multiple sclerosis such as chronically active lesions, diffuse alterations of the grey matter and normal-appearing white matter, meningeal inflammation, and cortical inflammation.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bittner, S., Pape, K., Klotz, L. et al. Implications of immunometabolism for smouldering MS pathology and therapy. Nat Rev Neurol 19, 477–488 (2023). https://doi.org/10.1038/s41582-023-00839-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-023-00839-6