Abstract
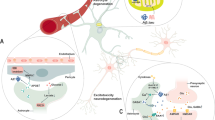
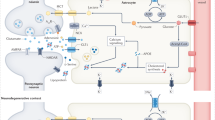
Despite advances in uncovering the mechanisms that underlie neuroinflammation and neurodegenerative disease, therapies that prevent neuronal loss remain elusive. Targeting of disease-defining markers in conditions such as Alzheimer disease (amyloid-β and tau) or Parkinson disease (α-synuclein) has been met with limited success, suggesting that these proteins do not act in isolation but form part of a pathological network. This network could involve phenotypic alteration of multiple cell types in the CNS, including astrocytes, which have a major neurosupportive, homeostatic role in the healthy CNS but adopt reactive states under acute or chronic adverse conditions. Transcriptomic studies in human patients and disease models have revealed the co-existence of many putative reactive sub-states of astrocytes. Inter-disease and even intra-disease heterogeneity of reactive astrocytic sub-states are well established, but the extent to which specific sub-states are shared across different diseases is unclear. In this Review, we highlight how single-cell and single-nuclei RNA sequencing and other ‘omics’ technologies can enable the functional characterization of defined reactive astrocyte states in various pathological scenarios. We provide an integrated perspective, advocating cross-modal validation of key findings to define functionally important sub-states of astrocytes and their triggers as tractable therapeutic targets with cross-disease relevance.
Key points
-
Neurodegenerative diseases are a group of serious and incurable conditions in which astrocytes both cause and respond to neuroinflammation.
-
A better understanding of how neuroinflammation and astrocyte function are linked in neurodegeneration is likely to lead to new therapeutic strategies.
-
To gain this understanding, researchers are using a range of techniques and data sets, including transcriptomic studies at the single-cell and single-nuclei levels, and the findings are being validated using both human and animal models across different stages of neurodegenerative diseases.
-
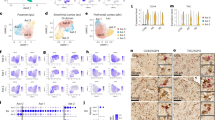
Transcriptomic studies of reactive astrocytes in human and animal models of disease have revealed the co-existence of many pathology-related reactive sub-states of astrocytes.
-
Future priorities for this research field include the determination of functional changes in transcriptomically defined reactive astrocyte sub-states.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Virchow, R. & Chance, F. Cellular Pathology as Based Upon Physiological and Pathological Histology: Twenty Lectures Delivered in the Pathological Institute of Berlin During the Months of February, March and April, 1858 (John Churchill, 1860).
Han, R. T., Kim, R. D., Molofsky, A. V. & Liddelow, S. A. Astrocyte-immune cell interactions in physiology and pathology. Immunity 54, 211–224 (2021).
Ransohoff, R. M., Schafer, D., Vincent, A., Blachere, N. E. & Bar-Or, A. Neuroinflammation: ways in which the immune system affects the brain. Neurotherapeutics 12, 896–909 (2015).
Goverman, J. Autoimmune T cell responses in the central nervous system. Nat. Rev. Immunol. 9, 393–407 (2009).
Guttenplan, K. A. & Liddelow, S. A. Astrocytes and microglia: models and tools. J. Exp. Med. 216, 71–83 (2019).
Liddelow, S. A. & Barres, B. A. Reactive astrocytes: production, function, and therapeutic potential. Immunity 46, 957–967 (2017).
Linnerbauer, M., Wheeler, M. A. & Quintana, F. J. Astrocyte crosstalk in CNS inflammation. Neuron 108, 608–622 (2020).
Liddelow, S. A. et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 (2017). The first transcriptomic and functional characterization of a reactive astrocyte sub-state in a cell-based in vitro assay. Validation in human cells was reported by Barbar et al. in 2020 (ref. 36).
Guttenplan, K. A. et al. Neurotoxic reactive astrocytes drive neuronal death after retinal injury. Cell Rep. 31, 107776 (2020).
Yun, S. P. et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat. Med. 24, 931–938 (2018). The first description of the therapeutic benefits of targeting a specific sub-state of reactive astrocytes. This study used the glucagon-like 1 peptide receptor agonist NYL01 in a mouse model of PD. Clinical trials in humans are ongoing.
Di Giorgio, F. P., Carrasco, M. A., Siao, M. C., Maniatis, T. & Eggan, K. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat. Neurosci. 10, 608–614 (2007).
Nagai, M. et al. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat. Neurosci. 10, 615–622 (2007).
Sun, S. et al. Translational profiling identifies a cascade of damage initiated in motor neurons and spreading to glia in mutant SOD1-mediated ALS. Proc. Natl Acad. Sci. USA 112, E6993–E7002 (2015).
Rothhammer, V. et al. Microglial control of astrocytes in response to microbial metabolites. Nature 557, 724–728 (2018).
Matyash, V. & Kettenmann, H. Heterogeneity in astrocyte morphology and physiology. Brain Res. Rev. 63, 2–10 (2010).
Kohler, S., Winkler, U. & Hirrlinger, J. Heterogeneity of astrocytes in grey and white matter. Neurochem. Res. 46, 3–14 (2021).
Uyeda, C. T., Eng, L. F. & Bignami, A. Immunological study of the glial fibrillary acidic protein. Brain Res. 37, 81–89 (1972).
Bignami, A., Eng, L. F., Dahl, D. & Uyeda, C. T. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res. 43, 429–435 (1972).
Jessen, K. R., Thorpe, R. & Mirsky, R. Molecular identity, distribution and heterogeneity of glial fibrillary acidic protein: an immunoblotting and immunohistochemical study of Schwann cells, satellite cells, enteric glia and astrocytes. J. Neurocytol. 13, 187–200 (1984).
McDermott, K. W. & Lantos, P. L. The distribution of glial fibrillary acidic protein and vimentin in postnatal marmoset (Callithrix jacchus) brain. Brain Res. Dev. Brain Res. 45, 169–177 (1989).
Rasia-Filho, A. A., Xavier, L. L., dos Santos, P., Gehlen, G. & Achaval, M. Glial fibrillary acidic protein immunodetection and immunoreactivity in the anterior and posterior medial amygdala of male and female rats. Brain Res. Bull. 58, 67–75 (2002).
Torres-Platas, S. G., Nagy, C., Wakid, M., Turecki, G. & Mechawar, N. Glial fibrillary acidic protein is differentially expressed across cortical and subcortical regions in healthy brains and downregulated in the thalamus and caudate nucleus of depressed suicides. Mol. Psychiatry 21, 509–515 (2016).
Bayraktar, O. A. et al. Astrocyte layers in the mammalian cerebral cortex revealed by a single-cell in situ transcriptomic map. Nat. Neurosci. 23, 500–509 (2020). The researchers screened 46 astrocyte candidate genes in situ to provide the first description of cortical layer-specific astrocyte subtypes in the mouse brain.
Pang, Y., Cai, Z. & Rhodes, P. G. Analysis of genes differentially expressed in astrocytes stimulated with lipopolysaccharide using cDNA arrays. Brain Res. 914, 15–22 (2001).
Bouton, C. M., Hossain, M. A., Frelin, L. P., Laterra, J. & Pevsner, J. Microarray analysis of differential gene expression in lead-exposed astrocytes. Toxicol. Appl. Pharmacol. 176, 34–53 (2001).
Cahoy, J. D. et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J. Neurosci. 28, 264–278 (2008). The first unbiased cell type-specific expression profiling in the brain. The findings were validated using RNA-seq by Zhang et al. in 2014 (ref. 28) and 2016 (ref. 29) and subsequently in numerous scRNA-seq and snRNA-seq studies.
Zamanian, J. L. et al. Genomic analysis of reactive astrogliosis. J. Neurosci. 32, 6391–6410 (2012). The first sub-state-specific sequencing of reactive astrocytes. Formed the basis of ongoing gene expression profiling of reactive sub-states and functional characterization of reactive astrocytes.
Zhang, Y. et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 34, 11929–11947 (2014).
Zhang, Y. et al. Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron 89, 37–53 (2016).
Guttenplan, K. A. et al. Knockout of reactive astrocyte activating factors slows disease progression in an ALS mouse model. Nat. Commun. 11, 3753 (2020).
Chai, H. et al. Neural circuit-specialized astrocytes: transcriptomic, proteomic, morphological, and functional evidence. Neuron 95, 531–549.e9 (2017). Combined RNA-seq, proteomic and functional (calcium transient) characterization of astrocytes in different brain regions.
Heintz, N. Gene expression nervous system atlas (GENSAT). Nat. Neurosci. 7, 483 (2004).
Doyle, J. P. et al. Application of a translational profiling approach for the comparative analysis of CNS cell types. Cell 135, 749–762 (2008).
Heiman, M. et al. A translational profiling approach for the molecular characterization of CNS cell types. Cell 135, 738–748 (2008).
Foo, L. C. et al. Development of a method for the purification and culture of rodent astrocytes. Neuron 71, 799–811 (2011). The first report of serum-free astrocyte culture to study specific reactive astrocyte functions in vitro.
Barbar, L. et al. CD49f is a novel marker of functional and reactive human iPSC-derived astrocytes. Neuron 107, 436–453 (2020).
Jungblut, M. et al. Isolation and characterization of living primary astroglial cells using the new GLAST-specific monoclonal antibody ACSA-1. Glia 60, 894–907 (2012).
Batiuk, M. Y. et al. Identification of region-specific astrocyte subtypes at single cell resolution. Nat. Commun. 11, 1220 (2020).
Kantzer, C. G. et al. Anti-ACSA-2 defines a novel monoclonal antibody for prospective isolation of living neonatal and adult astrocytes. Glia 65, 990–1004 (2017).
Zhou, Y. et al. Human and mouse single-nucleus transcriptomics reveal TREM2-dependent and TREM2-independent cellular responses in Alzheimer’s disease. Nat. Med. 26, 131–142 (2020).
Wu, T. et al. Complement C3 is activated in human AD brain and is required for neurodegeneration in mouse models of amyloidosis and tauopathy. Cell Rep. 28, 2111–2123.e6 (2019).
Ruffinatti, F. et al. Transcriptional remodeling in primary hippocampal astrocytes from an Alzheimer’s disease mouse model. Curr. Alzheimer Res. 15, 986–1004 (2018).
Ziff, O. J. et al. Meta-analysis of human and mouse ALS astrocytes reveals multi-omic signatures of inflammatory reactive states. Genome Res. 32, 71–84 (2022). This study shows that ALS-associated mutations alter inflammatory responses in astrocytes, highlighting genetics as well as secondary insults as drivers of astrocyte reactivity.
Peng, A. Y. T. et al. Loss of TDP-43 in astrocytes leads to motor deficits by triggering A1-like reactive phenotype and triglial dysfunction. Proc. Natl Acad. Sci. USA 117, 29101–29112 (2020).
Jiang, L. L. et al. Membralin deficiency dysregulates astrocytic glutamate homeostasis leading to ALS-like impairment. J. Clin. Invest. 129, 3103–3120 (2019).
Wheeler, M. A. et al. MAFG-driven astrocytes promote CNS inflammation. Nature 578, 593–599 (2020).
Diaz-Castro, B., Gangwani, M. R., Yu, X., Coppola, G. & Khakh, B. S. Astrocyte molecular signatures in Huntington’s disease. Sci. Transl Med. 11, eaaw8546 (2019).
Song, S. et al. Activation of endothelial Wnt/β-catenin signaling by protective astrocytes repairs BBB damage in ischemic stroke. Prog. Neurobiol. 199, 101963 (2021).
Boghdadi, A. G. et al. NogoA-expressing astrocytes limit peripheral macrophage infiltration after ischemic brain injury in primates. Nat. Commun. 12, 6906 (2021).
Hasel, P., Rose, I. V. L., Sadick, J. S., Kim, R. D. & Liddelow, S. A. Neuroinflammatory astrocyte subtypes in the mouse brain. Nat. Neurosci. 24, 1475–1487 (2021).
Anderson, M. A. et al. Astrocyte scar formation aids central nervous system axon regeneration. Nature 532, 195–200 (2016).
Boisvert, M. M., Erikson, G. A., Shokhirev, M. N. & Allen, N. J. The aging astrocyte transcriptome from multiple regions of the mouse brain. Cell Rep. 22, 269–285 (2018).
Clarke, L. E. et al. Normal aging induces A1-like astrocyte reactivity. Proc. Natl Acad. Sci. USA 115, E1896–E1905 (2018).
Zeisel, A. et al. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science 347, 1138–1142 (2015).
Saunders, A. et al. Molecular diversity and specializations among the cells of the adult mouse brain. Cell 174, 1015–1030.e6 (2018).
Sadick, J. S. et al. Astrocytes and oligodendrocytes undergo subtype-specific transcriptional changes in Alzheimer’s disease. Neuron 110, 1788–1805 (2022).
Diaz-Castro, B., Bernstein, A. M., Coppola, G., Sofroniew, M. V. & Khakh, B. S. Molecular and functional properties of cortical astrocytes during peripherally induced neuroinflammation. Cell Rep. 36, 109508 (2021).
Wheeler, M. A. et al. Environmental control of astrocyte pathogenic activities in CNS inflammation. Cell 176, 581–596.e18 (2019).
Habib, N. et al. Disease-associated astrocytes in Alzheimer’s disease and aging. Nat. Neurosci. 23, 701–706 (2020).
Clark, I. C. et al. Barcoded viral tracing of single-cell interactions in central nervous system inflammation. Science 372, eabf1230 (2021).
Sanmarco, L. M. et al. Gut-licensed IFNγ+ NK cells drive LAMP1+TRAIL+ anti-inflammatory astrocytes. Nature 590, 473–479 (2021).
Mathys, H. et al. Single-cell transcriptomic analysis of Alzheimer’s disease. Nature 570, 332–337 (2019).
Grubman, A. et al. A single-cell atlas of entorhinal cortex from individuals with Alzheimer’s disease reveals cell-type-specific gene expression regulation. Nat. Neurosci. 22, 2087–2097 (2019).
Absinta, M. et al. A lymphocyte-microglia-astrocyte axis in chronic active multiple sclerosis. Nature 597, 709–714 (2021).
Khrameeva, E. et al. Single-cell-resolution transcriptome map of human, chimpanzee, bonobo, and macaque brains. Genome Res. 30, 776–789 (2020).
Sheng, L. et al. Social reprogramming in ants induces longevity-associated glia remodeling. Sci. Adv. 6, eaba9869 (2020).
Vicidomini, R., Nguyen, T. H., Choudhury, S. D., Brody, T. & Serpe, M. Assembly and exploration of a single cell atlas of the Drosophila larval ventral cord. identification of rare cell types. Curr. Protoc. 1, e37 (2021).
Chen, J., Poskanzer, K. E., Freeman, M. R. & Monk, K. R. Live-imaging of astrocyte morphogenesis and function in zebrafish neural circuits. Nat. Neurosci. 23, 1297–1306 (2020).
Wilkinson, M. D. et al. The FAIR Guiding Principles for scientific data management and stewardship. Sci. Data 3, 160018 (2016).
FAIR. FAIR principles. GO FAIR https://www.go-fair.org/fair-principles/ (2016).
Liddelow, S. A., Marsh, S. E. & Stevens, B. Microglia and astrocytes in disease: dynamic duo or partners in crime? Trends Immunol. 41, 820–835 (2020).
Burda, J. E. et al. Divergent transcriptional regulation of astrocyte reactivity across disorders. Nature 606, 557–564 (2022).
de Paiva Lopes, K. et al. Genetic analysis of the human microglial transcriptome across brain regions, aging and disease pathologies. Nat. Genet. 54, 4–17 (2022).
Kenigsbuch, M. et al. A shared disease-associated oligodendrocyte signature among multiple CNS pathologies. Nat. Neurosci. 25, 876–886 (2022).
Chen, W.-T. et al. Spatial transcriptomics and in situ sequencing to study Alzheimer’s disease. Cell 182, 976–991.e19 (2020).
Castranio, E. et al. INPP5D limits plaque formation and glial reactivity in the APP/PS1 mouse model of Alzheimer’s disease. Alzheimers Dement. https://doi.org/10.1002/alz.12821 (2022).
Oberheim, N. A. et al. Uniquely hominid features of adult human astrocytes. J. Neurosci. 29, 3276–3287 (2009).
Han, X. et al. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell 12, 342–353 (2013).
Frazel, P. et al. Early stage differentiation of glia in human and mouse. Preprint at bioRxiv https://doi.org/10.1101/2021.12.07.471509 (2021).
Li, J. et al. Conservation and divergence of vulnerability and responses to stressors between human and mouse astrocytes. Nat. Commun. 12, 3958 (2021).
Hertz, L., Lovatt, D., Goldman, S. A. & Nedergaard, M. Adrenoceptors in brain: cellular gene expression and effects on astrocytic metabolism and [Ca2+]i. Neurochem. Int. 57, 411–420 (2010).
Nolle, A. et al. Enrichment of glial cells from human post-mortem tissue for transcriptome and proteome analysis using immunopanning. Front. Cell Neurosci. 15, 772011 (2021).
Patani, R. et al. Investigating the utility of human embryonic stem cell-derived neurons to model ageing and neurodegenerative disease using whole-genome gene expression and splicing analysis. J. Neurochem. 122, 738–751 (2012).
Miller, J. D. et al. Human iPSC-based modeling of late-onset disease via progerin-induced aging. Cell Stem Cell 13, 691–705 (2013).
Gatto, N. et al. Directly converted astrocytes retain the ageing features of the donor fibroblasts and elucidate the astrocytic contribution to human CNS health and disease. Aging Cell 20, e13281 (2021).
Sloan, S. A. et al. Human astrocyte maturation captured in 3D cerebral cortical spheroids derived from pluripotent stem cells. Neuron 95, 779–790.e776 (2017).
McCarthy, K. D. & de Vellis, J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J. Cell Biol. 85, 890–902 (1980). The first purification and culture methods for astrocytes. Most physiological astrocyte functions were discovered using these methods.
Stogsdill, J. A. et al. Astrocytic neuroligins control astrocyte morphogenesis and synaptogenesis. Nature 551, 192–197 (2017).
Allen, N. J. & Eroglu, C. Cell biology of astrocyte-synapse interactions. Neuron 96, 697–708 (2017).
Qiu, J. et al. Mixed-species RNA-seq for elucidation of non-cell-autonomous control of gene transcription. Nat. Protoc. 13, 2176–2199 (2018).
Hasel, P. et al. Neurons and neuronal activity control gene expression in astrocytes to regulate their development and metabolism. Nat. Commun. 8, 15132 (2017). Mixed-species culture and neuron–astrocyte interactions demonstrated in a dish. Among the first studies to show that cell–cell contact, in addition to secreted molecules, is important for astrocyte morphology, gene expression and function.
Hansson, E. Co-cultivation of astroglial and neuronal primary cultures from rat brain. Brain Res. 366, 159–168 (1986).
Swanson, R. A. et al. Neuronal regulation of glutamate transporter subtype expression in astrocytes. J. Neurosci. 17, 932–940 (1997).
Baxter, P. S. et al. Microglial identity and inflammatory responses are controlled by the combined effects of neurons and astrocytes. Cell Rep. 34, 108882 (2021).
Guttenplan, K. A. et al. Neurotoxic reactive astrocytes induce cell death via saturated lipids. Nature 599, 102–107 (2021). Definition of a neurotoxic lipid secreted by the inflammation-induced reactive astrocyte sub-state defined by this group in 2017. This study brought together in vitro, in vivo and multiple omics approaches to define a specific toxic molecule released by astrocytes.
Leng, K. et al. Molecular characterization of selectively vulnerable neurons in Alzheimer’s disease. Nat. Neurosci. 24, 276–287 (2021).
Leng, K. et al. CRISPRi screens in human iPSC-derived astrocytes elucidate regulators of distinct inflammatory reactive states. Nat. Neurosci. 25, 1528–1542 (2022).
Rothstein, J. D. et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 16, 675–686 (1996).
Shi, Y. et al. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature 549, 523–527 (2017).
Park, J. et al. A 3D human triculture system modeling neurodegeneration and neuroinflammation in Alzheimer’s disease. Nat. Neurosci. 21, 941–951 (2018).
Liang, Y. et al. Upregulation of Alzheimer’s disease amyloid-β protein precursor in astrocytes both in vitro and in vivo. J. Alzheimers Dis. 76, 1071–1082 (2020).
Liu, X. et al. Stat3 inhibition attenuates mechanical allodynia through transcriptional regulation of chemokine expression in spinal astrocytes. PLoS ONE 8, e75804 (2013).
Kucukdereli, H. et al. Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins Hevin and SPARC. Proc. Natl Acad. Sci. USA 108, E440–E449 (2011).
Christopherson, K. S. et al. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 120, 421–433 (2005).
Allen, N. J. et al. Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature 486, 410–414 (2012).
Chung, W. S. et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 504, 394–400 (2013).
Hong, S. et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352, 712–716 (2016).
Maragakis, N. J. & Rothstein, J. D. Glutamate transporters: animal models to neurologic disease. Neurobiol. Dis. 15, 461–473 (2004).
Sasaki, S., Komori, T. & Iwata, M. Excitatory amino acid transporter 1 and 2 immunoreactivity in the spinal cord in amyotrophic lateral sclerosis. Acta Neuropathol. 100, 138–144 (2000).
D’Erchia, A. M. et al. Massive transcriptome sequencing of human spinal cord tissues provides new insights into motor neuron degeneration in ALS. Sci. Rep. 7, 10046 (2017).
Lee, D. H. et al. Fingolimod effects in neuroinflammation: regulation of astroglial glutamate transporters? PLoS ONE 12, e0171552 (2017).
Volterra, A., Liaudet, N. & Savtchouk, I. Astrocyte Ca2+ signalling: an unexpected complexity. Nat. Rev. Neurosci. 15, 327–335 (2014).
Nagai, J. et al. Behaviorally consequential astrocytic regulation of neural circuits. Neuron 109, 576–596 (2021).
Yu, X., Nagai, J. & Khakh, B. S. Improved tools to study astrocytes. Nat. Rev. Neurosci. 21, 121–138 (2020).
Panattoni, G. et al. Diverse inflammatory threats modulate astrocytes Ca2+ signaling via connexin43 hemichannels in organotypic spinal slices. Mol. Brain 14, 159 (2021).
Dong, Q. et al. Mechanism and consequence of abnormal calcium homeostasis in Rett syndrome astrocytes. eLife 7, e33417 (2018).
Heuser, K. et al. Ca2+ signals in astrocytes facilitate spread of epileptiform activity. Cereb. Cortex 28, 4036–4048 (2018).
Jiang, R., Diaz-Castro, B., Looger, L. L. & Khakh, B. S. Dysfunctional calcium and glutamate signaling in striatal astrocytes from Huntington’s disease model mice. J. Neurosci. 36, 3453–3470 (2016).
Shah, D. et al. Astrocyte calcium dysfunction causes early network hyperactivity in Alzheimer’s disease. Cell Rep. 40, 111280 (2022).
Shigetomi, E., Patel, S. & Khakh, B. S. Probing the complexities of astrocyte calcium signaling. Trends Cell Biol. 26, 300–312 (2016).
Dong, Q. P., He, J. Q. & Chai, Z. Astrocytic Ca2+ waves mediate activation of extrasynaptic NMDA receptors in hippocampal neurons to aggravate brain damage during ischemia. Neurobiol. Dis. 58, 68–75 (2013).
Duffy, S. & MacVicar, B. A. In vitro ischemia promotes calcium influx and intracellular calcium release in hippocampal astrocytes. J. Neurosci. 16, 71–81 (1996).
Xu, Q. et al. Astrocytes contribute to pain gating in the spinal cord. Sci. Adv. 7, eabi6287 (2021).
Reichenbach, N. et al. P2Y1 receptor blockade normalizes network dysfunction and cognition in an Alzheimer’s disease model. J. Exp. Med. 215, 1649–1663 (2018).
Stackhouse, T. L. & Mishra, A. Neurovascular coupling in development and disease: focus on astrocytes. Front. Cell Dev. Biol. 9, 702832 (2021).
Cvetkovic, C. et al. Assessing Gq-GPCR-induced human astrocyte reactivity using bioengineered neural organoids. J. Cell Biol. 221, e202107135 (2022).
Zhou, Z., Ikegaya, Y. & Koyama, R. The astrocytic cAMP pathway in health and disease. Int. J. Mol. Sci. 20, 779 (2019).
Hertz, L. et al. Astrocytic glycogenolysis: mechanisms and functions. Metab. Brain Dis. 30, 317–333 (2015).
Gavrilyuk, V. et al. Norepinephrine increases IκBα expression in astrocytes. J. Biol. Chem. 277, 29662–29668 (2002).
Ceyzeriat, K., Abjean, L., Carrillo-de Sauvage, M. A., Ben Haim, L. & Escartin, C. The complex STATes of astrocyte reactivity: how are they controlled by the JAK-STAT3 pathway? Neuroscience 330, 205–218 (2016).
LeComte, M. D., Shimada, I. S., Sherwin, C. & Spees, J. L. Notch1-STAT3-ETBR signaling axis controls reactive astrocyte proliferation after brain injury. Proc. Natl Acad. Sci. USA 112, 8726–8731 (2015).
Hung, C. C. et al. Soluble epoxide hydrolase modulates immune responses in activated astrocytes involving regulation of STAT3 activity. J. Neuroinflammation 16, 123 (2019).
Zheng, J. Y. et al. Selective deletion of apolipoprotein E in astrocytes ameliorates the spatial learning and memory deficits in Alzheimer’s disease (APP/PS1) mice by inhibiting TGF-β/Smad2/STAT3 signaling. Neurobiol. Aging 54, 112–132 (2017).
Wang, J. et al. Astrocyte-derived estrogen regulates reactive astrogliosis and is neuroprotective following ischemic brain injury. J. Neurosci. 40, 9751–9771 (2020).
Lee, S. H. et al. TREM2-independent oligodendrocyte, astrocyte, and T cell responses to tau and amyloid pathology in mouse models of Alzheimer disease. Cell Rep. 37, 110158 (2021).
Kim, H. et al. Reactive astrocytes transduce inflammation in a blood-brain barrier model through a TNFα-STAT3 signaling axis and secretion of alpha 1-antichymotrypsin. Nat. Commun. 3, 6581 (2022).
Lu, T. Y. et al. Axon degeneration induces glial responses through Draper-TRAF4-JNK signalling. Nat. Commun. 8, 14355 (2017).
Ceyzeriat, K. et al. Modulation of astrocyte reactivity improves functional deficits in mouse models of Alzheimer’s disease. Acta Neuropathol. Commun. 6, 104 (2018).
Guillemaud, O. et al. Complex roles for reactive astrocytes in the triple transgenic mouse model of Alzheimer disease. Neurobiol. Aging 90, 135–146 (2020).
Abjean, L. et al. Reactive astrocytes promote proteostasis in Huntington’s disease through the JAK2-STAT3 pathway. Brain 146, 149–166 (2023).
Zamboni, M., Llorens-Bobadilla, E., Magnusson, J. P. & Frisen, J. A widespread neurogenic potential of neocortical astrocytes is induced by injury. Cell Stem Cell 27, 605–617.e5 (2020).
Iyer, A. et al. MicroRNA-146a: a key regulator of astrocyte-mediated inflammatory response. PLoS ONE 7, e44789 (2012).
Wang, C. Y., Yang, S. H. & Tzeng, S. F. MicroRNA-145 as one negative regulator of astrogliosis. Glia 63, 194–205 (2015).
Pogue, A. I. et al. Micro RNA-125b (miRNA-125b) function in astrogliosis and glial cell proliferation. Neurosci. Lett. 476, 18–22 (2010).
Jiwaji, Z. et al. Reactive astrocytes acquire neuroprotective as well as deleterious signatures in response to Tau and Ass pathology. Nat. Commun. 13, 135 (2022).
Koffie, R. M. et al. Oligomeric amyloid β associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc. Natl Acad. Sci. USA 106, 4012–4017 (2009).
Dong, H., Martin, M. V., Chambers, S. & Csernansky, J. G. Spatial relationship between synapse loss and β-amyloid deposition in Tg2576 mice. J. Comp. Neurol. 500, 311–321 (2007).
Hammond, T. R. et al. Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity 50, 253–271.e6 (2019).
Xia, M. Q., Bacskai, B. J., Knowles, R. B., Qin, S. X. & Hyman, B. T. Expression of the chemokine receptor CXCR3 on neurons and the elevated expression of its ligand IP-10 in reactive astrocytes: in vitro ERK1/2 activation and role in Alzheimer’s disease. J. Neuroimmunol. 108, 227–235 (2000).
Sorensen, T. L. et al. Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J. Clin. Invest. 103, 807–815 (1999).
La Manno, G. et al. RNA velocity of single cells. Nature 560, 494–498 (2018).
Bergen, V., Lange, M., Peidli, S., Wolf, F. A. & Theis, F. J. Generalizing RNA velocity to transient cell states through dynamical modeling. Nat. Biotechnol. 38, 1408–1414 (2020).
Bergen, V., Soldatov, R. A., Kharchenko, P. V. & Theis, F. J. RNA velocity-current challenges and future perspectives. Mol. Syst. Biol. 17, e10282 (2021).
Brenner, M. et al. Mutations in GFAP, encoding glial fibrillary acidic protein, are associated with Alexander disease. Nat. Genet. 27, 117–120 (2001).
Serio, A. et al. Astrocyte pathology and the absence of non-cell autonomy in an induced pluripotent stem cell model of TDP-43 proteinopathy. Proc. Natl Acad. Sci. USA 110, 4697–4702 (2013).
Hall, C. E. et al. Progressive motor neuron pathology and the role of astrocytes in a human stem cell model of VCP-related ALS. Cell Rep. 19, 1739–1749 (2017).
Ziff, O. J. et al. Reactive astrocytes in ALS display diminished intron retention. Nucleic Acids Res. 49, 3168–3184 (2021).
Taha, D. M. et al. Astrocytes display cell autonomous and diverse early reactive states in familial amyotrophic lateral sclerosis. Brain 145, 481–489 (2022). The first study to show that ALS-associated mutations are sufficient to induce cell-autonomous reactive phenotypes in astrocytes (defined transcriptionally, at the protein level and functionally by an aberrant secretome).
Zhao, C. et al. Mutant C9orf72 human iPSC-derived astrocytes cause non-cell autonomous motor neuron pathophysiology. Glia 68, 1046–1064 (2020).
Selvaraj, B. T. et al. C9ORF72 repeat expansion causes vulnerability of motor neurons to Ca2+-permeable AMPA receptor-mediated excitotoxicity. Nat. Commun. 9, 347 (2018).
Mehta, A. R. et al. Mitochondrial bioenergetic deficits in C9orf72 amyotrophic lateral sclerosis motor neurons cause dysfunctional axonal homeostasis. Acta Neuropathol. 141, 257–279 (2021).
Smethurst, P. et al. Distinct responses of neurons and astrocytes to TDP-43 proteinopathy in amyotrophic lateral sclerosis. Brain 143, 430–440 (2020).
Smethurst, P., Franklin, H., Clarke, B. E., Sidle, K. & Patani, R. The role of astrocytes in prion-like mechanisms of neurodegeneration. Brain 145, 17–26 (2022).
Tcw, J. et al. Cholesterol and matrisome pathways dysregulated in astrocytes and microglia. Cell 185, 2213–2233.e25 (2022).
Jones, V. C., Atkinson-Dell, R., Verkhratsky, A. & Mohamet, L. Aberrant iPSC-derived human astrocytes in Alzheimer’s disease. Cell Death Dis. 8, e2696 (2017).
Oksanen, M. et al. PSEN1 Mutant iPSC-derived model reveals severe astrocyte pathology in Alzheimer’s disease. Stem Cell Rep. 9, 1885–1897 (2017).
Kondo, T. et al. Modeling Alzheimer’s disease with iPSCs reveals stress phenotypes associated with intracellular Aβ and differential drug responsiveness. Cell Stem Cell 12, 487–496 (2013).
Lin, Y. T. et al. APOE4 causes widespread molecular and cellular alterations associated with Alzheimer’s disease phenotypes in human iPSC-derived brain cell types. Neuron 98, 1141–1154.e7 (2018).
Booth, H. D. E. et al. RNA sequencing reveals MMP2 and TGFB1 downregulation in LRRK2 G2019S Parkinson’s iPSC-derived astrocytes. Neurobiol. Dis. 129, 56–66 (2019).
di Domenico, A. et al. Patient-specific iPSC-derived astrocytes contribute to non-cell-autonomous neurodegeneration in Parkinson’s disease. Stem Cell Rep. 12, 213–229 (2019).
Garcia, V. J. et al. Huntington’s disease patient-derived astrocytes display electrophysiological impairments and reduced neuronal support. Front. Neurosci. 13, 669 (2019).
Juopperi, T. A. et al. Astrocytes generated from patient induced pluripotent stem cells recapitulate features of Huntington’s disease patient cells. Mol. Brain 5, 17 (2012).
Franklin, H., Clarke, B. E. & Patani, R. Astrocytes and microglia in neurodegenerative diseases: lessons from human in vitro models. Prog. Neurobiol. 200, 101973 (2021).
Brosh, R. et al. A versatile platform for locus-scale genome rewriting and verification. Proc. Natl Acad. Sci. USA 118, e2023952118 (2021).
Zhang, W. et al. Mouse genomic rewriting and tailoring: synthetic Trp53 and humanized ACE2. Preprint at bioRxiv https://doi.org/10.1101/2022.06.22.495814 (2022).
Agnew-Svoboda, W. et al. A genetic tool for the longitudinal study of a subset of post-inflammatory reactive astrocytes. Cell Rep. Methods 2, 100276 (2022).
Yang, Z. et al. Ultrasensitive single extracellular vesicle detection using high throughput droplet digital enzyme-linked immunosorbent assay. Nano Lett. 22, 4315–4324 (2022). Technical advances in labelling and collection enable RNA sequencing of individual cellular components such as single vesicles or mitochondria.
Murdock, M. H. & Tsai, L. H. Insights into Alzheimer’s disease from single-cell genomic approaches. Nat. Neurosci. 26, 181–195 (2023).
Gibson, E. M. et al. Methotrexate chemotherapy induces persistent tri-glial dysregulation that underlies chemotherapy-related cognitive impairment. Cell 176, 43–55.e13 (2019).
Hartmann, K. et al. Complement 3+-astrocytes are highly abundant in prion diseases, but their abolishment led to an accelerated disease course and early dysregulation of microglia. Acta Neuropathol. Commun. 7, 83 (2019).
Sterling, J. K. et al. GLP-1 receptor agonist NLY01 reduces retinal inflammation and neuron death secondary to ocular hypertension. Cell Rep. 33, 108271 (2020).
Kim, J. H. et al. Gamma subunit of complement component 8 is a neuroinflammation inhibitor. Brain 144, 528–552 (2021).
Matloubian, M. et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427, 355–360 (2004).
Mandala, S. et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 296, 346–349 (2002).
Foster, C. A. et al. Brain penetration of the oral immunomodulatory drug FTY720 and its phosphorylation in the central nervous system during experimental autoimmune encephalomyelitis: consequences for mode of action in multiple sclerosis. J. Pharmacol. Exp. Ther. 323, 469–475 (2007).
Bigaud, M. et al. Siponimod (BAF312) penetrates, distributes, and acts in the central nervous system: preclinical insights. Mult. Scler. J. Exp. Transl. Clin. 7, 20552173211049168 (2021).
Bhusal, A. et al. Cathelicidin-related antimicrobial peptide promotes neuroinflammation through astrocyte-microglia communication in experimental autoimmune encephalomyelitis. Glia 70, 1902–1926 (2022).
Kim, J. H. et al. Soluble ANPEP released from human astrocytes as a positive regulator of microglial activation and neuroinflammation: brain renin-angiotensin system in astrocyte-microglia crosstalk. Mol. Cell Proteom. 21, 100424 (2022).
Sun, W. et al. Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science 339, 197–200 (2013).
Zhu, Y. et al. Hematogenous macrophage depletion reduces the fibrotic scar and increases axonal growth after spinal cord injury. Neurobiol. Dis. 74, 114–125 (2015).
Zhu, Y., Soderblom, C., Trojanowsky, M., Lee, D. H. & Lee, J. K. Fibronectin matrix assembly after spinal cord injury. J. Neurotrauma 32, 1158–1167 (2015).
Wu, W. et al. Axonal and glial responses to a mid-thoracic spinal cord hemisection in the Macaca fascicularis monkey. J. Neurotrauma 30, 826–839 (2013).
Ma, Z. et al. A controlled spinal cord contusion for the rhesus macaque monkey. Exp. Neurol. 279, 261–273 (2016).
Ghosh, S. & Hui, S. P. Axonal regeneration in zebrafish spinal cord. Regeneration 5, 43–60 (2018).
Labib, D. et al. Proteomic alterations and novel markers of neurotoxic reactive astrocytes in human induced pluripotent stem cell models. Front. Mol. Neurosci. 15, 870085 (2022).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
R.P., G.E.H. and S.A.L. maintain a financial interest in AstronauTx.
Peer review
Peer review information
Nature Reviews Neurology thanks K. Suk, who co-reviewed with A. Bhusal, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Patani, R., Hardingham, G.E. & Liddelow, S.A. Functional roles of reactive astrocytes in neuroinflammation and neurodegeneration. Nat Rev Neurol 19, 395–409 (2023). https://doi.org/10.1038/s41582-023-00822-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-023-00822-1
This article is cited by
-
Preclinical translational platform of neuroinflammatory disease biology relevant to neurodegenerative disease
Journal of Neuroinflammation (2024)
-
TYROBP/DAP12 knockout in Huntington’s disease Q175 mice cell-autonomously decreases microglial expression of disease-associated genes and non-cell-autonomously mitigates astrogliosis and motor deterioration
Journal of Neuroinflammation (2024)
-
Overlaps and divergences between tauopathies and synucleinopathies: a duet of neurodegeneration
Translational Neurodegeneration (2024)
-
Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases
Signal Transduction and Targeted Therapy (2024)
-
APOE3ch alleviates Aβ and tau pathology and neurodegeneration in the human APPNL-G-F cerebral organoid model of Alzheimer’s disease
Cell Research (2024)