Abstract
The risk of Alzheimer disease (AD) increases with age, family history and informative genetic variants. Sadly, there is still no cure or means of prevention. As in other complex diseases, uncovering genetic causes of AD could identify underlying pathological mechanisms and lead to potential treatments. Rare, autosomal dominant forms of AD occur in middle age as a result of highly penetrant genetic mutations, but the most common form of AD occurs later in life. Large-scale, genome-wide analyses indicate that 70 or more genes or loci contribute to AD. One of the major factors limiting progress is that most genetic data have been obtained from non-Hispanic white individuals in Europe and North America, preventing the development of personalized approaches to AD in individuals of other ethnicities. Fortunately, emerging genetic data from other regions — including Africa, Asia, India and South America — are now providing information on the disease from a broader range of ethnicities. Here, we summarize the current knowledge on AD genetics in populations across the world. We predominantly focus on replicated genetic discoveries but also include studies in ethnic groups where replication might not be feasible. We attempt to identify gaps that need to be addressed to achieve a complete picture of the genetic and molecular factors that drive AD in individuals across the globe.
Key points
-
The genetic variation underlying Alzheimer disease (AD) differs across ethnic groups.
-
Large-scale genomic studies have identified over 70 genes or genetic loci associated with AD risk, but these data have largely been obtained from populations in Europe and North America, which hinders our understanding of the molecular mechanism(s) underlying the disease in under-represented populations and the development of a personalized therapeutic approach.
-
Expansion of efforts to sequence and analyse the genomes of people from under-studied areas of the world, combined with acquisition of additional multiomics data and appropriate development of infrastructure, resources, training and ethical guidelines, will be essential to improve our understanding of global genetic variation profiles underlying dementia.
-
Pathological heterogeneity is the norm in AD and efforts to incorporate this information into genetic studies is underway.
-
Incorporation of improved biomarkers that can be obtained in low-resource countries will be critical to increase diagnostic accuracy in these efforts.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 18, 700–789 (2022).
Gatz, M. et al. Role of genes and environments for explaining Alzheimer disease. Arch. Gen. Psychiatry 63, 168–174 (2006).
Chartier-Harlin, M. C. et al. Early-onset Alzheimer’s disease caused by mutations at codon 717 of the β-amyloid precursor protein gene. Nature 353, 844–846 (1991).
Goate, A. et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature 349, 704–706 (1991).
Sherrington, R. et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature 375, 754–760 (1995).
Sherrington, R. et al. Alzheimer’s disease associated with mutations in presenilin 2 is rare and variably penetrant. Hum. Mol. Genet. 5, 985–988 (1996).
Cacace, R., Sleegers, K. & Van Broeckhoven, C. Molecular genetics of early-onset Alzheimer’s disease revisited. Alzheimers Dement. 12, 733–748 (2016).
Jarmolowicz, A. I., Chen, H. Y. & Panegyres, P. K. The patterns of inheritance in early-onset dementia: Alzheimer’s disease and frontotemporal dementia. Am. J. Alzheimers Dis. Other Demen 30, 299–306 (2015).
Wallon, D. et al. The French series of autosomal dominant early onset Alzheimer’s disease cases: mutation spectrum and cerebrospinal fluid biomarkers. J. Alzheimers Dis. 30, 847–856 (2012).
Cruchaga, C. et al. Rare variants in APP, PSEN1 and PSEN2 increase risk for AD in late-onset Alzheimer’s disease families. PLoS One 7, e31039 (2012).
Arboleda-Velasquez, J. F. et al. Resistance to autosomal dominant Alzheimer’s disease in an APOE3 Christchurch homozygote: a case report. Nat. Med. 25, 1680–1683 (2019).
Pericak-Vance, M. A. et al. Linkage studies in familial Alzheimer disease: evidence for chromosome 19 linkage. Am. J. Hum. Genet. 48, 1034–1050 (1991).
Wingo, T. S., Lah, J. J., Levey, A. I. & Cutler, D. J. Autosomal recessive causes likely in early-onset Alzheimer disease. Arch. Neurol. 69, 59–64 (2012).
Wightman, D. P. et al. A genome-wide association study with 1,126,563 individuals identifies new risk loci for Alzheimer’s disease. Nat. Genet. 53, 1276–1282 (2021).
Kunkle, B. W. et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat. Genet. 51, 414–430 (2019).
Bellenguez, C. et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat. Genet. 54, 412–436 (2022).
Jansen, I. E. et al. Genome-wide meta-analysis for Alzheimer’s disease cerebrospinal fluid biomarkers. Acta Neuropathol. 144, 821–842 (2022).
Rubinski, A. et al. Polygenic effect on tau pathology progression in Alzheimer’s disease. Ann. Neurol. https://doi.org/10.1002/ana.26588 (2022).
Vigilant, L., Stoneking, M., Harpending, H., Hawkes, K. & Wilson, A. C. African populations and the evolution of human mitochondrial DNA. Science 253, 1503–1507 (1991).
Schlebusch, C. M. et al. Genomic variation in seven Khoe-San groups reveals adaptation and complex African history. Science 338, 374–379 (2012).
Gronau, I., Hubisz, M. J., Gulko, B., Danko, C. G. & Siepel, A. Bayesian inference of ancient human demography from individual genome sequences. Nat. Genet. 43, 1031–1034 (2011).
Rasmussen, M. et al. Ancient human genome sequence of an extinct Palaeo-Eskimo. Nature 463, 757–762 (2010).
Green, R. E. et al. A draft sequence of the Neandertal genome. Science 328, 710–722 (2010).
Ramachandran, S. et al. Support from the relationship of genetic and geographic distance in human populations for a serial founder effect originating in Africa. Proc. Natl Acad. Sci. USA 102, 15942–15947 (2005).
Jakobsson, M. et al. Genotype, haplotype and copy-number variation in worldwide human populations. Nature 451, 998–1003 (2008).
Li, J. Z. et al. Worldwide human relationships inferred from genome-wide patterns of variation. Science 319, 1100–1104 (2008).
Rosenberg, N. A. et al. Genetic structure of human populations. Science 298, 2381–2385 (2002).
Gurdasani, D. et al. The African Genome Variation Project shapes medical genetics in Africa. Nature 517, 327–332 (2015).
Beltrame, M. H., Rubel, M. A. & Tishkoff, S. A. Inferences of African evolutionary history from genomic data. Curr. Opin. Genet. Dev. 41, 159–166 (2016).
Tishkoff, S. A. et al. The genetic structure and history of Africans and African Americans. Science 324, 1035–1044 (2009).
Duncan, L. et al. Analysis of polygenic risk score usage and performance in diverse human populations. Nat. Commun. 10, 3328 (2019).
The 1000 Genomes Project Consortium. A global reference for human genetic variation. Nature 526, 68–74 (2015).
Rotimi, C. N. & Adeyemo, A. A. From one human genome to a complex tapestry of ancestry. Nature 590, 220–221 (2021).
Hong, S. et al. TMEM106B and CPOX are genetic determinants of cerebrospinal fluid Alzheimer’s disease biomarker levels. Alzheimers Dement. 17, 1628–1640 (2021).
Rotimi, C. N. et al. The genomic landscape of African populations in health and disease. Hum. Mol. Genet. 26, R225–R236 (2017).
Kunkle, B. W. et al. Novel Alzheimer disease risk loci and pathways in African American individuals using the African genome resources panel: a meta-analysis. JAMA Neurol. 78, 102–113 (2021).
Hohman, T. J. et al. Global and local ancestry in African-Americans: implications for Alzheimer’s disease risk. Alzheimers Dement. 12, 233–243 (2016).
Reitz, C. et al. Variants in the ATP-binding cassette transporter (ABCA7), apolipoprotein E ε4, and the risk of late-onset Alzheimer disease in African Americans. JAMA 309, 1483–1492 (2013).
Bussies, P. L. et al. Use of local genetic ancestry to assess TOMM40-523’ and risk for Alzheimer disease. Neurol. Genet. 6, e404 (2020).
Vardarajan, B. N. et al. Whole genome sequencing of Caribbean Hispanic families with late-onset Alzheimer’s disease. Ann. Clin. Transl. Neurol. 5, 406–417 (2018).
Vardarajan, B. N. et al. Ultra-rare mutations in SRCAP segregate in Caribbean Hispanic families with Alzheimer disease. Neurol. Genet. 3, e178 (2017).
Feliciano-Astacio, B. E. et al. The Puerto Rico Alzheimer Disease Initiative (PRADI): a multisource ascertainment approach. Front. Genet. 10, 538 (2019).
Tosto, G. et al. F-box/LRR-repeat protein 7 is genetically associated with Alzheimer’s disease. Ann. Clin. Transl. Neurol. 2, 810–820 (2015).
Tosto, G. et al. Association of variants in PINX1 and TREM2 with late-onset Alzheimer disease. JAMA Neurol. 76, 942–948 (2019).
Farrer, L. A. et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 278, 1349–1356 (1997).
Maestre, G. et al. Apolipoprotein E and Alzheimer’s disease: ethnic variation in genotypic risks. Ann. Neurol. 37, 254–259 (1995).
Tang, M. X. et al. The APOE-ε4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA 279, 751–755 (1998).
Jonsson, T. et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. N. Engl. J. Med. 368, 107–116 (2013).
Raghavan, N. S. et al. Whole-exome sequencing in 20,197 persons for rare variants in Alzheimer’s disease. Ann. Clin. Transl. Neurol. 5, 832–842 (2018).
Griswold, A. J. et al. Increased APOE ε4 expression is associated with the difference in Alzheimer’s disease risk from diverse ancestral backgrounds. Alzheimers Dement. 17, 1179–1188 (2021).
Jun, G. R. et al. Transethnic genome-wide scan identifies novel Alzheimer’s disease loci. Alzheimers Dement. 13, 727–738 (2017).
Cukier, H. N. et al. ABCA7 frameshift deletion associated with Alzheimer disease in African Americans. Neurol. Genet. 2, e79 (2016).
Bryc, K. et al. Genome-wide patterns of population structure and admixture in West Africans and African Americans. Proc. Natl Acad. Sci. USA 107, 786–791 (2010).
Kunkle, B. W. et al. Targeted sequencing of ABCA7 identifies splicing, stop-gain and intronic risk variants for Alzheimer disease. Neurosci. Lett. 649, 124–129 (2017).
De Roeck, A. et al. Deleterious ABCA7 mutations and transcript rescue mechanisms in early onset Alzheimer’s disease. Acta Neuropathol. 134, 475–487 (2017).
Cuyvers, E. et al. Mutations in ABCA7 in a Belgian cohort of Alzheimer’s disease patients: a targeted resequencing study. Lancet Neurol. 14, 814–822 (2015).
Luukkainen, L. et al. Mutation analysis of the genes linked to early onset Alzheimer’s disease and frontotemporal lobar degeneration. J. Alzheimers Dis. 69, 775–782 (2019).
Thordardottir, S. & Graff, C. Findings from the Swedish study on familial Alzheimer’s disease including the APP Swedish double mutation. J. Alzheimers Dis. 64, S491–S496 (2018).
Al-Thani, H. F., Ahmad, M. N., Younes, S. & Zayed, H. Genetic variants associated with Alzheimer disease in the 22 Arab countries: a systematic review. Alzheimer Dis. Assoc. Disord. 35, 178–186 (2021).
Eryilmaz, I. E. et al. Evaluation of the clinical features accompanied by the gene mutations: the 2 novel PSEN1 variants in a Turkish early-onset Alzheimer disease cohort. Alzheimer Dis. Assoc. Disord. 35, 214–222 (2021).
Lee, J. H. et al. Disease-related mutations among Caribbean Hispanics with familial dementia. Mol. Genet. Genom. Med. 2, 430–437 (2014).
Athan, E. S. et al. A founder mutation in presenilin 1 causing early-onset Alzheimer disease in unrelated Caribbean Hispanic families. JAMA 286, 2257–2263 (2001).
Bertoli Avella, A. M. et al. A novel presenilin 1 mutation (L174 M) in a large Cuban family with early onset Alzheimer disease. Neurogenetics 4, 97–104 (2002).
Arnold, S. E. et al. Frequency and clinicopathological characteristics of presenilin 1 Gly206Ala mutation in Puerto Rican Hispanics with dementia. J. Alzheimers Dis. 33, 1089–1095 (2013).
Ramos, C., Aguillon, D., Cordano, C. & Lopera, F. Genetics of dementia: insights from Latin America. Dement. Neuropsychol. 14, 223–236 (2020).
Itzcovich, T. et al. A novel mutation in PSEN1 (p.T119I) in an Argentine family with early- and late-onset Alzheimer’s disease. Neurobiol. Aging 85, 155.e9–155.e12 (2020).
Abdala, B. B. et al. Influence of low frequency PSEN1 variants on familial Alzheimer’s disease risk in Brazil. Neurosci. Lett. 653, 341–345 (2017).
El Kadmiri, N. et al. Novel mutations in the amyloid precursor protein gene within Moroccan patients with Alzheimer’s disease. J. Mol. Neurosci. 53, 189–195 (2014).
El Kadmiri, N. et al. Novel presenilin mutations within Moroccan patients with early-onset Alzheimer’s disease. Neuroscience 269, 215–222 (2014).
Heckmann, J. M. et al. Novel presenilin 1 mutation with profound neurofibrillary pathology in an indigenous Southern African family with early-onset Alzheimer’s disease. Brain 127, 133–142 (2004).
Guerreiro, R. J. et al. Genetic screening of Alzheimer’s disease genes in Iberian and African samples yields novel mutations in presenilins and APP. Neurobiol. Aging 31, 725–731 (2010).
Joshi, P., Gardner, M., Lintott, C. & Anderson, T. Novel presenilin-1 mutation (Ala275Ser) associated with clinical features of dementia with Lewy bodies. Alzheimer Dis. Assoc. Disord. 35, 350–352 (2021).
Bechara, J. A., Brooks, W. S., Kwok, J. B., Halliday, G. M. & Schofield, P. R. Familial Alzheimer’s disease in Australia. Alzheimer’s Dement. 16, e040062 (2020).
Syama, A. et al. Mutation burden profile in familial Alzheimer’s disease cases from India. Neurobiol. Aging 64, 158.e7–158.e13 (2018).
Li, H. et al. The identification of PSEN1 p.Tyr159Ser mutation in a non-canonic early-onset Alzheimer’s disease family. Mol. Cell Neurosci. 120, 103715 (2022).
Qin, Q. et al. Gene mutations associated with early onset familial Alzheimer’s disease in China: an overview and current status. Mol. Genet. Genom. Med. 8, e1443 (2020).
Bagyinszky, E., Youn, Y. C., An, S. S. & Kim, S. Mutations, associated with early-onset Alzheimer’s disease, discovered in Asian countries. Clin. Interv. Aging 11, 1467–1488 (2016).
Ikeuchi, T. et al. Mutational analysis in early-onset familial dementia in the Japanese population. The role of PSEN1 and MAPT R406W mutations. Dement. Geriatr. Cogn. Disord. 26, 43–49 (2008).
Hsu, J. L., Lin, C. H., Chen, P. L., Lin, K. J. & Chen, T. F. Genetic study of young-onset dementia using targeted gene panel sequencing in Taiwan. Am. J. Med. Genet. B Neuropsychiatr. Genet. 186, 67–76 (2021).
Bagyinszky, E., Ch’ng, G. S., Chan, M. Y., An, S. S. A. & Kim, S. A pathogenic presenilin-1 Val96Phe mutation from a Malaysian family. Brain Sci. 11, 1328 (2021).
Alzheimer’s Disease International. Dementia in Sub-Saharan Africa: Challenges and Opportunities (Alzheimer’s Disease International, 2017).
Landoulsi, Z. et al. Genetic analysis of TREM2 variants in Tunisian patients with Alzheimer’s disease. Med. Princ. Pract. 27, 317–322 (2018).
Hendrie, H. C. et al. APOE ε4 and the risk for Alzheimer disease and cognitive decline in African Americans and Yoruba. Int. Psychogeriatr. 26, 977–985 (2014).
Hall, K. et al. Cholesterol, APOE genotype, and Alzheimer disease: an epidemiologic study of Nigerian Yoruba. Neurology 66, 223–227 (2006).
Chen, C. H. et al. A comparative study to screen dementia and APOE genotypes in an ageing East African population. Neurobiol. Aging 31, 732–740 (2010).
Gureje, O. et al. APOE ε4 is not associated with Alzheimer’s disease in elderly Nigerians. Ann. Neurol. 59, 182–185 (2006).
Haithem, H. et al. Association between dementia and vascular disease-associated polymorphisms in a Tunisian population. Int. J. Neurosci. 128, 32–41 (2018).
Corder, E. H. et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261, 921–923 (1993).
Mayeux, R. et al. The apolipoprotein epsilon 4 allele in patients with Alzheimer’s disease. Ann. Neurol. 34, 752–754 (1993).
McKhann, G. et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services task force on Alzheimer’s disease. Neurology 34, 939–944 (1984).
World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines (WHO, 1992).
Blue, E. E., Horimoto, A., Mukherjee, S., Wijsman, E. M. & Thornton, T. A. Local ancestry at APOE modifies Alzheimer’s disease risk in Caribbean Hispanics. Alzheimers Dement. 15, 1524–1532 (2019).
Rajabli, F. et al. Ancestral origin of ApoE ε4 Alzheimer disease risk in Puerto Rican and African American populations. PLoS Genet. 14, e1007791 (2018).
Rajabli, F. et al. A locus at 19q13.31 significantly reduces the ApoE ε4 risk for Alzheimer’s disease in African ancestry. PLoS Genet. 18, e1009977 (2022).
Hendrie, H. C. et al. Incidence of dementia and Alzheimer disease in 2 communities: Yoruba residing in Ibadan, Nigeria, and African Americans residing in Indianapolis, Indiana. JAMA 285, 739–747 (2001).
Adhikari, K., Chacon-Duque, J. C., Mendoza-Revilla, J., Fuentes-Guajardo, M. & Ruiz-Linares, A. The genetic diversity of the Americas. Annu. Rev. Genomics Hum. Genet. 18, 277–296 (2017).
Adhikari, K., Mendoza-Revilla, J., Chacon-Duque, J. C., Fuentes-Guajardo, M. & Ruiz-Linares, A. Admixture in Latin America. Curr. Opin. Genet. Dev. 41, 106–114 (2016).
Salzano, F. M. & Bortolini, M. C. The Evolution and Genetics of Latin American Populations (Cambridge Univ. Press, 2001).
Prince, M. et al. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 9, 63–75.e2 (2013).
Ferri, C. P. et al. Global prevalence of dementia: a Delphi consensus study. Lancet 366, 2112–2117 (2005).
Ramirez Aguilar, L. et al. Genetic origin of a large family with a novel PSEN1 mutation (Ile416Thr). Alzheimers Dement. 15, 709–719 (2019).
Lopera, F. et al. Clinical features of early-onset Alzheimer disease in a large kindred with an E280A presenilin-1 mutation. JAMA 277, 793–799 (1997).
Tariot, P. N. et al. The Alzheimer’s Prevention Initiative Autosomal-Dominant Alzheimer’s Disease Trial: a study of crenezumab versus placebo in preclinical PSEN1 E280A mutation carriers to evaluate efficacy and safety in the treatment of autosomal-dominant Alzheimer’s disease, including a placebo-treated noncarrier cohort. Alzheimers Dement. 4, 150–160 (2018).
Guerreiro, R. et al. TREM2 variants in Alzheimer’s disease. N. Engl. J. Med. 368, 117–127 (2013).
Vardarajan, B. N. et al. Rare coding mutations identified by sequencing of Alzheimer disease genome-wide association studies loci. Ann. Neurol. 78, 487–498 (2015).
Vardarajan, B. N. et al. Inbreeding among Caribbean Hispanics from the Dominican Republic and its effects on risk of Alzheimer disease. Genet. Med. 17, 639–643 (2015).
An, F. et al. MiR-124 acts as a target for Alzheimer’s disease by regulating BACE1. Oncotarget 8, 114065–114071 (2017).
Sims, R. et al. Rare coding variants in PLCG2, ABI3, and TREM2 implicate microglial-mediated innate immunity in Alzheimer’s disease. Nat. Genet. 49, 1373–1384 (2017).
Kizil, C. et al. Admixture mapping of Alzheimer’s disease in Caribbean Hispanics identifies a new locus on 22q13.1. Mol. Psychiatry 27, 2813–2820 (2022).
Toral-Rios, D. et al. SORL1 polymorphisms in Mexican patients with Alzheimer’s disease. Genes 13, 587 (2022).
Jia, L. et al. Dementia in China: epidemiology, clinical management, and research advances. Lancet Neurol. 19, 81–92 (2020).
Gan, C. L., Zhang, T. & Lee, T. H. The genetics of Alzheimer’s disease in the Chinese population. Int. J. Mol. Sci. 21, 2381 (2020).
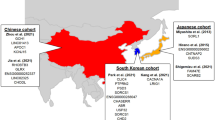
Miyashita, A., Kikuchi, M., Hara, N. & Ikeuchi, T. Genetics of Alzheimer’s disease: an East Asian perspective. J. Hum. Genet. 68, 115–124 (2022).
Jiang, T. et al. A rare coding variant in TREM2 increases risk for Alzheimer’s disease in Han Chinese. Neurobiol. Aging 42, 217 (2016).
Jiang, T. et al. TREM2 p.H157Y variant and the risk of Alzheimer’s disease: a meta-analysis involving 14,510 subjects. Curr. Neurovasc Res. 13, 318–320 (2016).
Hirano, A. et al. A genome-wide association study of late-onset Alzheimer’s disease in a Japanese population. Psychiatr. Genet. 25, 139–146 (2015).
Jia, L. et al. Prediction of Alzheimer’s disease using multi-variants from a Chinese genome-wide association study. Brain 144, 924–937 (2021).
Kang, S. et al. Potential novel genes for late-onset Alzheimer’s disease in East-Asian descent identified by APOE-stratified genome-wide association study. J. Alzheimers Dis. 82, 1451–1460 (2021).
Park, J. H. et al. Novel Alzheimer’s disease risk variants identified based on whole-genome sequencing of APOE ε4 carriers. Transl. Psychiatry 11, 296 (2021).
Shigemizu, D. et al. Ethnic and trans-ethnic genome-wide association studies identify new loci influencing Japanese Alzheimer’s disease risk. Transl. Psychiatry 11, 151 (2021).
Zhou, X. et al. Identification of genetic risk factors in the Chinese population implicates a role of immune system in Alzheimer’s disease pathogenesis. Proc. Natl Acad. Sci. USA 115, 1697–1706 (2018).
Wang, Y., Lu, D., Chung, Y. J. & Xu, S. Genetic structure, divergence and admixture of Han Chinese, Japanese and Korean populations. Hereditas 155, 19 (2018).
Dogan, M. et al. Clinical and molecular findings in a Turkish family who had a (c.869-1G>A) splicing variant in PSEN1 gene with a rare condition: the variant Alzheimer’s disease with spastic paraparesis. Curr. Alzheimer Res. 19, 223–235 (2022).
El Bitar, F. et al. Genetic study of Alzheimer’s disease in Saudi population. J. Alzheimers Dis. 67, 231–242 (2019).
Guven, G. et al. A novel PSEN2 p.Ser175Phe variant in a family with Alzheimer’s disease. Neurol. Sci. 42, 2497–2504 (2021).
Kalfon, L. et al. Familial early-onset Alzheimer’s caused by novel genetic variant and APP duplication: a cross-sectional study. Curr. Alzheimer Res. 19, 694–707 (2022).
Lohmann, E. et al. Identification of PSEN1 and PSEN2 gene mutations and variants in Turkish dementia patients. Neurobiol. Aging 33, 1850.e17–1850.e27 (2012).
Bazrgar, M. et al. Apolipoprotein E polymorphism in Southern Iran: E4 allele in the lowest reported amounts. Mol. Biol. Rep. 35, 495–499 (2008).
Abdi, S. et al. Association of Alzheimer’s disease with genetic variants of apolipoprotein E, clusterin, TNF-α, and IL-6 among elderly Saudis. Curr. Pharm. Biotechnol. 23, 1893–1902 (2022).
Abyadeh, M., Djafarian, K., Heydarinejad, F., Alizadeh, S. & Shab-Bidar, S. Association between apolipoprotein E gene polymorphism and Alzheimer’s disease in an Iranian population: a meta-analysis. J. Mol. Neurosci. 69, 557–562 (2019).
Amer, M. S. et al. Interaction between apolipoprotein E genotyping, serum inflammatory biomarkers and cognitive functions in Egyptian elderly. Egypt. J. Immunol. 28, 1–11 (2021).
Durmaz, A. et al. Genetic factors associated with the predisposition to late onset Alzheimer’s disease. Gene 707, 212–215 (2019).
El Shamieh, S., Costanian, C., Kassir, R., Visvkis-Siest, S. & Bissar-Tadmouri, N. APOE genotypes in Lebanon: distribution and association with hypercholesterolemia and Alzheimer’s disease. Per Med. 16, 15–23 (2019).
Isbir, T. et al. Interaction between apolipoprotein-E and angiotensin-converting enzyme genotype in Alzheimer’s disease. Am. J. Alzheimers Dis. Other Demen. 16, 205–210 (2001).
Gharesouran, J., Rezazadeh, M., Khorrami, A., Ghojazadeh, M. & Talebi, M. Genetic evidence for the involvement of variants at APOE, BIN1, CR1, and PICALM loci in risk of late-onset Alzheimer’s disease and evaluation for interactions with APOE genotypes. J. Mol. Neurosci. 54, 780–786 (2014).
Kleinberger, G. et al. TREM2 mutations implicated in neurodegeneration impair cell surface transport and phagocytosis. Sci. Transl. Med. 6, 243ra286 (2014).
Manzali, S. B. et al. Association of the CD2AP locus with cognitive functioning among middle-aged individuals with a family history of Alzheimer’s disease. Neurobiol. Aging 101, 50–56 (2021).
Mehdizadeh, E. et al. Association of MS4A6A, CD33, and TREM2 gene polymorphisms with the late-onset Alzheimer’s disease. Bioimpacts 9, 219–225 (2019).
Mehrjoo, Z. et al. Association study of the TREM2 gene and identification of a novel variant in exon 2 in Iranian patients with late-onset Alzheimer’s disease. Med. Princ. Pract. 24, 351–354 (2015).
Rezazadeh, M. et al. Genetic factors affecting late-onset Alzheimer’s disease susceptibility. Neuromolecular Med. 18, 37–49 (2016).
Talebi, M. et al. ABCA7 and EphA1 genes polymorphisms in late-onset Alzheimer’s disease. J. Mol. Neurosci. 70, 167–173 (2020).
Masri, I., Salami, A., El Shamieh, S. & Bissar-Tadmouri, N. rs3851179G>A in PICALM is protective against Alzheimer’s disease in five different countries surrounding the Mediterranean. Curr. Aging Sci. 13, 162–168 (2020).
Akinyemi, R. O. et al. Dementia in Africa: current evidence, knowledge gaps, and future directions. Alzheimers Dement. 18, 790–809 (2022).
Bis, J. C. et al. Whole exome sequencing study identifies novel rare and common Alzheimer’s-associated variants involved in immune response and transcriptional regulation. Mol. Psychiatry 25, 1859–1875 (2020).
Mena, P. R. et al. The Alzheimer’s disease sequencing project–follow up study (ADSP-FUS): increasing ethnic diversity in Alzheimer’s genetics research with the addition of potential new cohorts. Alzheimer’s Dement. 16, e046400 (2020).
John P. Hussman Institute to lead international genetic study of Alzheimer’s disease in people of Hispanic and African ancestry. Inventum https://physician-news.umiamihealth.org/john-p-hussman-institute-to-lead-international-genetic-study-of-alzheimers-disease-in-people-of-hispanic-and-african-ancestry/ (2022).
Weiner, M. W. et al. Increasing participant diversity in AD research: plans for digital screening, blood testing, and a community-engaged approach in the Alzheimer’s Disease Neuroimaging Initiative 4. Alzheimers Dement. 19, 307–317 (2022).
McKhann, G. M. et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 263–269 (2011).
Ayodele, T., Rogaeva, E., Kurup, J. T., Beecham, G. & Reitz, C. Early-onset Alzheimer’s disease: what is missing in research? Curr. Neurol. Neurosci. Rep. 21, 4 (2021).
Reitz, C., Rogaeva, E. & Beecham, G. W. Late-onset vs nonmendelian early-onset Alzheimer disease: a distinction without a difference? Neurol. Genet. 6, e512 (2020).
Bateman, R. J. et al. Autosomal-dominant Alzheimer’s disease: a review and proposal for the prevention of Alzheimer’s disease. Alzheimers Res. Ther. 3, 1 (2011).
van der Flier, W. M., Pijnenburg, Y. A., Fox, N. C. & Scheltens, P. Early-onset versus late-onset Alzheimer’s disease: the case of the missing APOE ε4 allele. Lancet Neurol. 10, 280–288 (2011).
Lee, S. et al. White matter hyperintensities are a core feature of Alzheimer’s disease: evidence from the dominantly inherited Alzheimer network. Ann. Neurol. 79, 929–939 (2016).
Moller, C. et al. Different patterns of gray matter atrophy in early- and late-onset Alzheimer’s disease. Neurobiol. Aging 34, 2014–2022 (2013).
Marshall, G. A., Fairbanks, L. A., Tekin, S., Vinters, H. V. & Cummings, J. L. Early-onset Alzheimer’s disease is associated with greater pathologic burden. J. Geriatr. Psychiatry Neurol. 20, 29–33 (2007).
Lowe, V. J. et al. Widespread brain tau and its association with ageing, Braak stage and Alzheimer’s dementia. Brain 141, 271–287 (2018).
Scholl, M. et al. Distinct 18F-AV-1451 tau PET retention patterns in early- and late-onset Alzheimer’s disease. Brain 140, 2286–2294 (2017).
Josephs, K. A. et al. Rates of hippocampal atrophy and presence of post-mortem TDP-43 in patients with Alzheimer’s disease: a longitudinal retrospective study. Lancet Neurol. 16, 917–924 (2017).
Attems, J. & Jellinger, K. A. The overlap between vascular disease and Alzheimer’s disease – lessons from pathology. BMC Med. 12, 206 (2014).
Kapasi, A., DeCarli, C. & Schneider, J. A. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol. 134, 171–186 (2017).
Schneider, J. A., Arvanitakis, Z., Bang, W. & Bennett, D. A. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 69, 2197–2204 (2007).
DeTure, M. A. & Dickson, D. W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 14, 32 (2019).
Schneider, J. A., Wilson, R. S., Bienias, J. L., Evans, D. A. & Bennett, D. A. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology 62, 1148–1155 (2004).
Barnes, D. E. & Yaffe, K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 10, 819–828 (2011).
Barker, W. W. et al. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis. Assoc. Disord. 16, 203–212 (2002).
Galvin, J. E., Pollack, J. & Morris, J. C. Clinical phenotype of Parkinson disease dementia. Neurology 67, 1605–1611 (2006).
Lippa, C. F. et al. Lewy bodies contain altered α-synuclein in brains of many familial Alzheimer’s disease patients with mutations in presenilin and amyloid precursor protein genes. Am. J. Pathol. 153, 1365–1370 (1998).
Chung, E. J. et al. Clinical features of Alzheimer disease with and without Lewy bodies. JAMA Neurol. 72, 789–796 (2015).
Azar, M. et al. Cognitive tests aid in clinical differentiation of Alzheimer’s disease versus Alzheimer’s disease with Lewy body disease: evidence from a pathological study. Alzheimers Dement. 16, 1173–1181 (2020).
Kraybill, M. L. et al. Cognitive differences in dementia patients with autopsy-verified AD, Lewy body pathology, or both. Neurology 64, 2069–2073 (2005).
Forstl, H., Burns, A., Luthert, P., Cairns, N. & Levy, R. The Lewy-body variant of Alzheimer’s disease. Clinical and pathological findings. Br. J. Psychiatry 162, 385–392 (1993).
Merdes, A. R. et al. Influence of Alzheimer pathology on clinical diagnostic accuracy in dementia with Lewy bodies. Neurology 60, 1586–1590 (2003).
Ferreira, D. et al. Distinct subtypes of Alzheimer’s disease based on patterns of brain atrophy: longitudinal trajectories and clinical applications. Sci. Rep. 7, 46263 (2017).
Moreno-Grau, S. et al. Genome-wide association analysis of dementia and its clinical endophenotypes reveal novel loci associated with Alzheimer’s disease and three causality networks: the GR@ACE project. Alzheimers Dement. 15, 1333–1347 (2019).
Lee, A. J. et al. FMNL2 regulates gliovascular interactions and is associated with vascular risk factors and cerebrovascular pathology in Alzheimer’s disease. Acta Neuropathol. 144, 59–79 (2022).
Jack, C. R. Jr. et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 14, 535–562 (2018).
Jack, C. R. Jr. et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 87, 539–547 (2016).
Balogun, W. G., Zetterberg, H., Blennow, K. & Karikari, T. K. Plasma biomarkers for neurodegenerative disorders: ready for prime time? Curr. Opin. Psychiatry 36, 112–118 (2023).
Apostolova, L. G. et al. Associations of the top 20 Alzheimer disease risk variants with brain amyloidosis. JAMA Neurol. 75, 328–341 (2018).
Shi, Z. et al. Amyloid PET in dementia syndromes: a Chinese multicenter study. J. Nucl. Med. 61, 1814–1819 (2020).
Raghavan, N. S. et al. Association between common variants in RBFOX1, an RNA-binding protein, and brain amyloidosis in early and preclinical Alzheimer disease. JAMA Neurol. 77, 1288–1298 (2020).
Kim, S. et al. Genome-wide association study of CSF biomarkers Aβ1-42, t-tau, and p-tau181p in the ADNI cohort. Neurology 76, 69–79 (2011).
Babapour Mofrad, R. et al. Sex differences in CSF biomarkers vary by Alzheimer disease stage and APOE ε4 genotype. Neurology 95, e2378–e2388 (2020).
Deming, Y. et al. Genome-wide association study identifies four novel loci associated with Alzheimer’s endophenotypes and disease modifiers. Acta Neuropathol. 133, 839–856 (2017).
Deming, Y. et al. The MS4A gene cluster is a key modulator of soluble TREM2 and Alzheimer’s disease risk. Sci. Transl. Med. 11, eaau2291 (2019).
Hong, S. et al. Genome-wide association study of Alzheimer’s disease CSF biomarkers in the EMIF-AD multimodal biomarker discovery dataset. Transl. Psychiatry 10, 403 (2020).
Li, W. W. et al. Association of polygenic risk score with age at onset and cerebrospinal fluid biomarkers of Alzheimer’s disease in a Chinese cohort. Neurosci. Bull. 36, 696–704 (2020).
Zhao, B. et al. Common genetic variation influencing human white matter microstructure. Science 372, eabf3736 (2021).
Zhao, B. et al. Large-scale GWAS reveals genetic architecture of brain white matter microstructure and genetic overlap with cognitive and mental health traits (n = 17,706). Mol. Psychiatry 26, 3943–3955 (2021).
Homann, J. et al. Genome-wide association study of Alzheimer’s disease brain imaging biomarkers and neuropsychological phenotypes in the European medical information framework for Alzheimer’s disease multimodal biomarker discovery dataset. Front. Aging Neurosci. 14, 840651 (2022).
Li, J. Q. et al. GWAS-linked loci and neuroimaging measures in Alzheimer’s disease. Mol. Neurobiol. 54, 146–153 (2017).
Squillario, M. et al. A telescope GWAS analysis strategy, based on SNPs-genes-pathways ensamble and on multivariate algorithms, to characterize late onset Alzheimer’s disease. Sci. Rep. 10, 12063 (2020).
Park, J. Y. et al. A missense variant in SHARPIN mediates Alzheimer’s disease-specific brain damages. Transl. Psychiatry 11, 590 (2021).
Janelidze, S. et al. Plasma P-tau181 in Alzheimer’s disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat. Med. 26, 379–386 (2020).
Thijssen, E. H. et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat. Med. 26, 387–397 (2020).
Stevenson-Hoare, J. et al. Plasma biomarkers and genetics in the diagnosis and prediction of Alzheimer’s disease. Brain https://doi.org/10.1093/brain/awac128 (2022).
Wang, Z. T. et al. Genome-wide association study identifies CD1A associated with rate of increase in plasma neurofilament light in non-demented elders. Aging 11, 4521–4535 (2019).
Jiao, B. et al. Associations of risk genes with onset age and plasma biomarkers of Alzheimer’s disease: a large case-control study in mainland China. Neuropsychopharmacology 47, 1121–1127 (2022).
Kim, J. et al. miR-27a and miR-27b regulate autophagic clearance of damaged mitochondria by targeting PTEN-induced putative kinase 1 (PINK1). Mol. Neurodegener. 11, 55 (2016).
Garcia-Aranda, M., Serrano, A. & Redondo, M. Regulation of clusterin gene expression. Curr. Protein Pept. Sci. 19, 612–622 (2018).
Kumar, P. et al. Circulating miRNA biomarkers for Alzheimer’s disease. PLoS One 8, e69807 (2013).
Hampel, H. et al. Omics sciences for systems biology in Alzheimer’s disease: state-of-the-art of the evidence. Ageing Res. Rev. 69, 101346 (2021).
Joyce, A. R. & Palsson, B. O. The model organism as a system: integrating ‘omics’ data sets. Nat. Rev. Mol. Cell Biol. 7, 198–210 (2006).
Ritchie, M. D., Holzinger, E. R., Li, R., Pendergrass, S. A. & Kim, D. Methods of integrating data to uncover genotype-phenotype interactions. Nat. Rev. Genet. 16, 85–97 (2015).
Choudhury, A. et al. High-depth African genomes inform human migration and health. Nature 586, 741–748 (2020).
Mez, J. et al. Two novel loci, COBL and SLC10A2, for Alzheimer’s disease in African Americans. Alzheimers Dement. 13, 119–129 (2017).
Miyashita, A. et al. SORL1 is genetically associated with late-onset Alzheimer’s disease in Japanese, Koreans and Caucasians. PLoS One 8, e58618 (2013).
Nielsen, R. et al. Tracing the peopling of the world through genomics. Nature 541, 302–310 (2017).
Tosto, G. et al. Polygenic risk scores in familial Alzheimer disease. Neurology 88, 1180–1186 (2017).
DeMichele-Sweet, M. A. A. et al. Genome-wide association identifies the first risk loci for psychosis in Alzheimer disease. Mol. Psychiatry 26, 5797–5811 (2021).
Kunkle, B. W. et al. Genome-wide linkage analyses of non-Hispanic white families identify novel loci for familial late-onset Alzheimer’s disease. Alzheimers Dement. 12, 2–10 (2016).
Barral, S. et al. Linkage analyses in Caribbean Hispanic families identify novel loci associated with familial late-onset Alzheimer’s disease. Alzheimers Dement. 11, 1397–1406 (2015).
Lanoiselee, H. M. et al. APP, PSEN1, and PSEN2 mutations in early-onset Alzheimer disease: a genetic screening study of familial and sporadic cases. PLoS Med. 14, e1002270 (2017).
Golan, M. P. et al. Early-onset Alzheimer’s disease with a de novo mutation in the presenilin 1 gene. Exp. Neurol. 208, 264–268 (2007).
Kukull, W. A. et al. Dementia and Alzheimer disease incidence: a prospective cohort study. Arch. Neurol. 59, 1737–1746 (2002).
Frenzel, S. et al. A biomarker for Alzheimer’s disease based on patterns of regional brain atrophy. Front. Psychiatry 10, 953 (2019).
Dickerson, B. C. et al. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb. Cortex 19, 497–510 (2009).
Killiany, R. J. et al. MRI measures of entorhinal cortex vs hippocampus in preclinical AD. Neurology 58, 1188–1196 (2002).
Delaby, C. et al. Clinical reporting following the quantification of cerebrospinal fluid biomarkers in Alzheimer’s disease: an international overview. Alzheimers Dement. 18, 1868–1879 (2021).
Morris, J. C. et al. Assessment of racial disparities in biomarkers for Alzheimer disease. JAMA Neurol. 76, 264–273 (2019).
Chaudhry, A. & Rizig, M. Comparing fluid biomarkers of Alzheimer’s disease between African American or Black African and white groups: a systematic review and meta-analysis. J. Neurol. Sci. 421, 117270 (2021).
Leuzy, A. et al. Blood-based biomarkers for Alzheimer’s disease. EMBO Mol. Med. 14, e14408 (2022).
Zetterberg, H. et al. Hypoxia due to cardiac arrest induces a time-dependent increase in serum amyloid β levels in humans. PLoS One 6, e28263 (2011).
Gaiottino, J. et al. Increased neurofilament light chain blood levels in neurodegenerative neurological diseases. PLoS One 8, e75091 (2013).
Khalil, M. et al. Neurofilaments as biomarkers in neurological disorders. Nat. Rev. Neurol. 14, 577–589 (2018).
Ashton, N. J. et al. Increased plasma neurofilament light chain concentration correlates with severity of post-mortem neurofibrillary tangle pathology and neurodegeneration. Acta Neuropathol. Commun. 7, 5 (2019).
Feinberg, A. P. Phenotypic plasticity and the epigenetics of human disease. Nature 447, 433–440 (2007).
Fransquet, P. D. & Ryan, J. Micro RNA as a potential blood-based epigenetic biomarker for Alzheimer’s disease. Clin. Biochem. 58, 5–14 (2018).
Lister, R. et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature 462, 315–322 (2009).
Gertz, J. et al. Analysis of DNA methylation in a three-generation family reveals widespread genetic influence on epigenetic regulation. PLoS Genet. 7, e1002228 (2011).
Shoemaker, R., Deng, J., Wang, W. & Zhang, K. Allele-specific methylation is prevalent and is contributed by CpG-SNPs in the human genome. Genome Res. 20, 883–889 (2010).
Zhi, D. et al. SNPs located at CpG sites modulate genome-epigenome interaction. Epigenetics 8, 802–806 (2013).
Dehghani, R., Rahmani, F. & Rezaei, N. MicroRNA in Alzheimer’s disease revisited: implications for major neuropathological mechanisms. Rev. Neurosci. 29, 161–182 (2018).
Newgard, C. B. Metabolomics and metabolic diseases: where do we stand? Cell Metab. 25, 43–56 (2017).
Mittelstrass, K. et al. Discovery of sexual dimorphisms in metabolic and genetic biomarkers. PLoS Genet. 7, e1002215 (2011).
Shah, S. H. et al. Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia 55, 321–330 (2012).
Shah, S. H., Kraus, W. E. & Newgard, C. B. Metabolomic profiling for the identification of novel biomarkers and mechanisms related to common cardiovascular diseases: form and function. Circulation 126, 1110–1120 (2012).
Vardarajan, B. et al. Differences in plasma metabolites related to Alzheimer’s disease, APOE epsilon4 status, and ethnicity. Alzheimers Dement. 6, e12025 (2020).
Wang, T. J. et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 17, 448–453 (2011).
Heath, L. et al. Manifestations of Alzheimer’s disease genetic risk in the blood are evident in a multiomic analysis in healthy adults aged 18 to 90. Sci. Rep. 12, 6117 (2022).
Walker, D. I. et al. High-resolution metabolomics of occupational exposure to trichloroethylene. Int. J. Epidemiol. 45, 1517–1527 (2016).
Acknowledgements
The authors acknowledge support from the National Institute on Aging of the National Institutes of Health in the USA. C.R. (U24AG056270, P30AG066462, U19AG074865, RO1AG064614); M.A.P.-V. (R56AG072547, RO1AG070864, UO1AG057659, UO1AG062943, UO1AG076482, U19AG074865); T.F. (U24AG021886, P30AG072976, U24AG056270), R.M. (U24AG056270, R01AG072474, RF1AG066107, R01AG067501). The authors also thank R. Akinyemi for his help with the review of genetics in Africa and M. Miller for continued encouragement and support of the investigation of AD worldwide.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article. All authors contributed substantially to discussion of the content. All authors wrote the article, and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neurology thanks Ricardo Nitrini, who co-reviewed with Leonel Takada; and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Alzheimer’s Disease Neuroimaging Initiative: https://adni.loni.usc.edu/
Alzheimer’s Disease Sequencing Project-Follow-Up Study (ADSP-FUS): https://adsp.niagads.org/
Alzheimer’s Disease Sequencing Project-Follow-Up Study: https://www.nia.nih.gov/research/ad-genetics
Asian Cohort for Alzheimer’s Disease: https://acadstudy.org/
Estudio Familiar de Influencia Genetica en Alzheimer (Puerto Rican Alzheimer Disease Initiative; EFIGA): https://dss.niagads.org/cohorts/estudio-familiar-de-influencia-genetica-en-alzheimer-efiga/
Gwangju Alzheimer’s and Related Dementias (GARD) Study: https://dss.niagads.org/cohorts/gwangju-alzheimers-and-related-dementia-gard/
Longitudinal Aging Study in India: https://lasi-india.org/
Mexican Health and Aging Study: https://www.mhasweb.org/Home/index.aspx
Research in African American Alzheimer’s Disease Initiative (REAAADI): https://med.miami.edu/centers-and-institutes/hihg/research-programs/alzheimers-disease-and-related-dementias/research-in-african-american-alzheimer-disease-initiative
WHO dementia fact sheet: https://www.who.int/news-room/fact-sheets/detail/dementia
Glossary
- Kindred
-
An aggregate of genetically related individuals.
- Principal components
-
Principal components analysis is a statistical method commonly used in population genetics to identify substructure in the distribution of genetic variation within populations.
- Quantitative trait loci
-
Regions of DNA each associated with a particular quantitative phenotypic trait.
- Recombination
-
Genetic recombination is the exchange of genetic material between different individuals which leads to offspring with combinations of traits that differ from those in either parent.
- Variants of uncertain significance
-
Genetic variants for which association with a specific trait is unclear.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Reitz, C., Pericak-Vance, M.A., Foroud, T. et al. A global view of the genetic basis of Alzheimer disease. Nat Rev Neurol 19, 261–277 (2023). https://doi.org/10.1038/s41582-023-00789-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-023-00789-z
This article is cited by
-
Immunological aspects of central neurodegeneration
Cell Discovery (2024)
-
Alzheimer’s disease risk reduction in clinical practice: a priority in the emerging field of preventive neurology
Nature Mental Health (2024)
-
Rare genetic variation in fibronectin 1 (FN1) protects against APOEε4 in Alzheimer’s disease
Acta Neuropathologica (2024)
-
Nerve growth factor receptor (Ngfr) induces neurogenic plasticity by suppressing reactive astroglial Lcn2/Slc22a17 signaling in Alzheimer’s disease
npj Regenerative Medicine (2023)