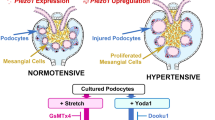
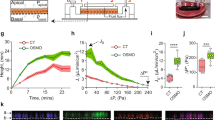
Abstract
Podocytes form the backbone of the glomerular filtration barrier and are exposed to various mechanical forces throughout the lifetime of an individual. The highly dynamic biomechanical environment of the glomerular capillaries greatly influences the cell biology of podocytes and their pathophysiology. Throughout the past two decades, a holistic picture of podocyte cell biology has emerged, highlighting mechanobiological signalling pathways, cytoskeletal dynamics and cellular adhesion as key determinants of biomechanical resilience in podocytes. This biomechanical resilience is essential for the physiological function of podocytes, including the formation and maintenance of the glomerular filtration barrier. Podocytes integrate diverse biomechanical stimuli from their environment and adapt their biophysical properties accordingly. However, perturbations in biomechanical cues or the underlying podocyte mechanobiology can lead to glomerular dysfunction with severe clinical consequences, including proteinuria and glomerulosclerosis. As our mechanistic understanding of podocyte mechanobiology and its role in the pathogenesis of glomerular disease increases, new targets for podocyte-specific therapeutics will emerge. Treating glomerular diseases by targeting podocyte mechanobiology might improve therapeutic precision and efficacy, with potential to reduce the burden of chronic kidney disease on individuals and health-care systems alike.
Key points
-
Podocytes are crucial for maintaining glomerular filtration and must have enough biophysical resilience to adapt to (or balance) mechanical forces in their environment.
-
Loss of biophysical resilience and aberrant mechanobiological signalling is a key feature of the pathophysiology of podocytopathies.
-
Podocytes maintain their biophysical resilience through a complex cytoskeletal signalling network comprising force-sensitive proteins and pathways.
-
Next-generation in vitro models that incorporate organoids, mechanical stimulation and biomimetic substrates offer promising avenues for interrogating podocyte mechanobiology.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Faul, C., Asanuma, K., Yanagida-Asanuma, E., Kim, K. & Mundel, P. Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol. 17, 428–437 (2007).
Ning, L., Suleiman, H. Y. & Miner, J. H. Synaptopodin is dispensable for normal podocyte homeostasis but is protective in the context of acute podocyte injury. J. Am. Soc. Nephrol. 31, 2815–2832 (2020).
Kriz, W. & Lemley, K. V. A potential role for mechanical forces in the detachment of podocytes and the progression of CKD. J. Am. Soc. Nephrol. 26, 258–269 (2015).
Steinhausen, M., Endlich, K. & Wiegman, D. L. Glomerular blood flow. Kidney Int. 38, 769–784 (1990).
Collard, D. et al. Estimation of intraglomerular pressure using invasive renal arterial pressure and flow velocity measurements in humans. J. Am. Soc. Nephrol. 31, 1905–1914 (2020).
Endlich, N. & Endlich, K. The challenge and response of podocytes to glomerular hypertension. Semin. Nephrol. 32, 327–341 (2012).
Butt, L. et al. A mathematical estimation of the physical forces driving podocyte detachment. Kidney Int. 100, 1054–1062 (2021).
Levey, A. S., Coresh, J., Tighiouart, H., Greene, T. & Inker, L. A. Measured and estimated glomerular filtration rate: current status and future directions. Nat. Rev. Nephrol. 16, 51–64 (2020).
Mammoto, A., Mammoto, T. & Ingber, D. E. Mechanosensitive mechanisms in transcriptional regulation. J. Cell Sci. 125, 3061–3073 (2012).
Forst, A.-L. et al. Podocyte purinergic P2X4 channels are mechanotransducers that mediate cytoskeletal disorganization. J. Am. Soc. Nephrol. 27, 848–862 (2016).
Anderson, M., Kim, E. Y., Hagmann, H., Benzing, T. & Dryer, S. E. Opposing effects of podocin on the gating of podocyte TRPC6 channels evoked by membrane stretch or diacylglycerol. Am. J. Physiol. Cell Physiol. 305, C276–C289 (2013).
Schultz, K. et al. Piezo mediates Rho activation and actin stress fibre formation in Drosophila nephrocytes. Preprint at bioRxiv https://doi.org/10.1101/2021.10.23.465463 (2022).
Dalghi, M. G. et al. Expression and distribution of PIEZO1 in the mouse urinary tract. Am. J. Physiol. Ren. Physiol. 317, F303–F321 (2019).
Ziegler, W. H., Liddington, R. C. & Critchley, D. R. The structure and regulation of vinculin. Trends Cell Biol. 16, 453–460 (2006).
Burridge, K. Talin: a protein designed for mechanotransduction. Emerg. Top. Life Sci. 2, 673–675 (2018).
Eng, D. G. et al. Glomerular parietal epithelial cells contribute to adult podocyte regeneration in experimental focal segmental glomerulosclerosis. Kidney Int. 88, 999–1012 (2015).
Kaverina, N. V., Eng, D. G., Schneider, R. R., Pippin, J. W. & Shankland, S. J. Partial podocyte replenishment in experimental FSGS derives from nonpodocyte sources. Am. J. Physiol. Ren. Physiol. 310, F1397–F1413 (2016).
Kaverina, N. V. et al. Dual lineage tracing shows that glomerular parietal epithelial cells can transdifferentiate toward the adult podocyte fate. Kidney Int. 96, 597–611 (2019).
Melica, M. E. et al. Differentiation of crescent-forming kidney progenitor cells into podocytes attenuates severe glomerulonephritis in mice. Sci. Transl. Med. 14, eabg3277 (2022).
Wharram, B. L. et al. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J. Am. Soc. Nephrol. 16, 2941 (2005).
Wanner, N. et al. Unraveling the role of podocyte turnover in glomerular aging and injury. J. Am. Soc. Nephrol. 25, 707 (2014).
Puelles, V. G. et al. mTOR-mediated podocyte hypertrophy regulates glomerular integrity in mice and humans. JCI Insight 4, e99271 (2019).
Kriz, W., Shirato, I., Nagata, M., LeHir, M. & Lemley, K. V. The podocyte’s response to stress: the enigma of foot process effacement. Am. J. Physiol. Ren. Physiol. 304, F333–F347 (2012).
Basgen, J. M., Wong, J. S., Ray, J., Nicholas, S. B. & Campbell, K. N. Podocyte foot process effacement precedes albuminuria and glomerular hypertrophy in CD2-associated protein deficient mice. Front. Med. 8, 745319 (2021).
Butt, L. et al. A molecular mechanism explaining albuminuria in kidney disease. Nat. Metab. 2, 461–474 (2020).
Jiang, S. et al. An ex vivo culture model of kidney podocyte injury reveals mechanosensitive, synaptopodin-templating, sarcomere-like structures. Sci. Adv. 8, eabn6027 (2022).
Suleiman, H. Y. et al. Injury-induced actin cytoskeleton reorganization in podocytes revealed by super-resolution microscopy. JCI Insight 2, e94137 (2017).
Vivarelli, M., Massella, L., Ruggiero, B. & Emma, F. Minimal change disease. Clin. J. Am. Soc. Nephrol. 12, 332–345 (2017).
Tullus, K., Webb, H. & Bagga, A. Management of steroid-resistant nephrotic syndrome in children and adolescents. Lancet Child. Adolesc. Health 2, 880–890 (2018).
Maas, R. J., Deegens, J. K., Smeets, B., Moeller, M. J. & Wetzels, J. F. Minimal change disease and idiopathic FSGS: manifestations of the same disease. Nat. Rev. Nephrol. 12, 768–776 (2016).
Azeloglu, E. U. et al. Interconnected network motifs control podocyte morphology and kidney function. Sci. Signal. 7, ra12 (2014).
Shiiki, H. et al. Cell proliferation and apoptosis of the glomerular epithelial cells in rats with puromycin aminonucleoside nephrosis. Pathobiology 66, 221–229 (1998).
Fogo, A. B. Animal models of FSGS: lessons for pathogenesis and treatment. Semin. Nephrol. 23, 161–171 (2003).
Calizo, R. C. et al. Disruption of podocyte cytoskeletal biomechanics by dasatinib leads to nephrotoxicity. Nat. Commun. 10, 2061 (2019).
Embry, A. E. et al. Similar biophysical abnormalities in glomeruli and podocytes from two distinct models. J. Am. Soc. Nephrol. 29, 1501–1512 (2018).
Vogelmann, S. U., Nelson, W. J., Myers, B. D. & Lemley, K. V. Urinary excretion of viable podocytes in health and renal disease. Am. J. Physiol. -Ren. Physiol. 285, F40–F48 (2003).
Wozniak, M. A., Modzelewska, K., Kwong, L. & Keely, P. J. Focal adhesion regulation of cell behavior. Biochim. Biophys. Acta Mol. Cell Res. 1692, 103–119 (2004).
Goult, B. T., Yan, J. & Schwartz, M. A. Talin as a mechanosensitive signaling hub. J. Cell Biol. 217, 3776–3784 (2018).
Ichimura, K. et al. Three-dimensional architecture of podocytes revealed by block-face scanning electron microscopy. Sci. Rep. 5, 8993 (2015).
Qu, C. et al. Three-dimensional visualization of the podocyte actin network using integrated membrane extraction, electron microscopy, and machine learning. J. Am. Soc. Nephrol. 33, 155–173 (2022).
Reynolds, P. A. The mechanobiology of kidney podocytes in health and disease. Clin. Sci. 134, 1245–1253 (2020).
Grgic, I. et al. Imaging of podocyte foot processes by fluorescence microscopy. J. Am. Soc. Nephrol. 23, 785–791 (2012).
Siegerist, F. et al. Structured illumination microscopy and automatized image processing as a rapid diagnostic tool for podocyte effacement. Sci. Rep. 7, 11473 (2017).
Endlich, N., Siegerist, F. & Endlich, K. Are podocytes motile? Pflügers Arch. Eur. J. Physiol. 469, 951–957 (2017).
Brähler, S. et al. Intravital and kidney slice imaging of podocyte membrane dynamics. J. Am. Soc. Nephrol. 27, 3285–3290 (2016).
Hansen, J. et al. A reference tissue atlas for the human kidney. Sci. Adv. 8, eabn4965 (2022).
Lake, B. B. et al. An atlas of healthy and injured cell states and niches in the human kidney. Nature 619, 585–594 (2023).
Christov, M. et al. Inducible podocyte-specific deletion of CTCF drives progressive kidney disease and bone abnormalities. JCI Insight 3, e95091 (2018).
Clark, A. R. et al. Single-cell transcriptomics reveal disrupted kidney filter cell-cell interactions after early and selective podocyte injury. Am. J. Pathol. 192, 281–294 (2022).
Pozzi, A. et al. β1 integrin expression by podocytes is required to maintain glomerular structural integrity. Dev. Biol. 316, 288–301 (2008).
Sachs, N. et al. Kidney failure in mice lacking the tetraspanin CD151. J. Cell Biol. 175, 33–39 (2006).
Has, C. et al. Integrin α3 mutations with kidney, lung, and skin disease. N. Engl. J. Med. 366, 1508–1514 (2012).
Shukrun, R. et al. A human integrin-α3 mutation confers major renal developmental defects. PLoS ONE 9, e90879 (2014).
Lausecker, F. et al. Vinculin is required to maintain glomerular barrier integrity. Kidney Int. 93, 643–655 (2018).
Artelt, N. et al. The role of palladin in podocytes. J. Am. Soc. Nephrol. 29, 1662–1678 (2018).
Tian, X. et al. Podocyte-associated talin1 is critical for glomerular filtration barrier maintenance. J. Clin. Invest. 124, 1098–1113 (2014).
Dai, C. et al. Essential role of integrin-linked kinase in podocyte biology: bridging the integrin and slit diaphragm signaling. J. Am. Soc. Nephrol. 17, 2164–2175 (2006).
Ma, H. et al. Inhibition of podocyte FAK protects against proteinuria and foot process effacement. J. Am. Soc. Nephrol. 21, 1145–1156 (2010).
Schlaepfer, D. D., Mitra, S. K. & Ilic, D. Control of motile and invasive cell phenotypes by focal adhesion kinase. Biochim. Biophys. Acta 1692, 77–102 (2004).
Webb, D. J. et al. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat. Cell Biol. 6, 154–161 (2004).
Wu, C. Focal adhesion: a focal point in current cell biology and molecular medicine. Cell Adhes. Migr. 1, 13–18 (2007).
Sever, S. & Schiffer, M. Actin dynamics at focal adhesions: a common endpoint and putative therapeutic target for proteinuric kidney diseases. Kidney Int. 93, 1298–1307 (2018).
Ron, A. et al. Cell shape information is transduced through tension-independent mechanisms. Nat. Commun. 8, 2145 (2017).
Unnersjö-Jess, D. et al. Advanced optical imaging reveals preferred spatial orientation of podocyte processes along the axis of glomerular capillaries. Kidney Int. 104, 1164–1169 (2023).
Schell, C. et al. The FERM protein EPB41L5 regulates actomyosin contractility and focal adhesion formation to maintain the kidney filtration barrier. Proc. Natl Acad. Sci. USA 114, E4621–E4630 (2017).
Maier, J. I. et al. EPB41L5 controls podocyte extracellular matrix assembly by adhesome-dependent force transmission. Cell Rep. 34, 108883 (2021).
Ge, X. et al. LIM-nebulette reinforces podocyte structural integrity by linking actin and vimentin filaments. J. Am. Soc. Nephrol. 31, 2372–2391 (2020).
Rogg, M. et al. α-Parvin defines a specific integrin adhesome to maintain the glomerular filtration barrier. J. Am. Soc. Nephrol. 33, 786–808 (2022).
Greiten, J. K. et al. The role of filamins in mechanically stressed podocytes. FASEB J. 35, e21560 (2021).
Kliewe, F. et al. Studying the role of fascin-1 in mechanically stressed podocytes. Sci. Rep. 7, 9916 (2017).
Lal, M. A. et al. Rhophilin-1 is a key regulator of the podocyte cytoskeleton and is essential for glomerular filtration. J. Am. Soc. Nephrol. 26, 647–662 (2015).
Rogg, M. et al. SRGAP1 controls small rho GTPases to regulate podocyte foot process maintenance. J. Am. Soc. Nephrol. 32, 563–579 (2021).
Pan, Y. et al. Dissection of glomerular transcriptional profile in patients with diabetic nephropathy: SRGAP2a protects podocyte structure and function. Diabetes 67, 717–730 (2018).
Matsuda, J., Maier, M., Aoudjit, L., Baldwin, C. & Takano, T. ARHGEF7 (β-PIX) is required for the maintenance of podocyte architecture and glomerular function. J. Am. Soc. Nephrol. 31, 996–1008 (2020).
Sipkema, P., van der Linden, P. J. W., Westerhof, N. & Yin, F. C. P. Effect of cyclic axial stretch of rat arteries on endothelial cytoskeletal morphology and vascular reactivity. J. Biomech. 36, 653–659 (2003).
Jülicher, F., Kruse, K., Prost, J. & Joanny, J. F. Active behavior of the cytoskeleton. Phys. Rep. 449, 3–28 (2007).
Steward, R. L., Cheng, C.-M., Wang, D. L. & LeDuc, P. R. Probing cell structure responses through a shear and stretching mechanical stimulation technique. Cell Biochem. Biophys. 56, 115–124 (2010).
Osborn, E. A., Rabodzey, A., Dewey, C. F. & Hartwig, J. H. Endothelial actin cytoskeleton remodeling during mechanostimulation with fluid shear stress. Am. J. Physiol. Cell Physiol. 290, C444–C452 (2006).
Endlich, N. & Endlich, K. Stretch, tension and adhesion – adaptive mechanisms of the actin cytoskeleton in podocytes. Eur. J. Cell Biol. 85, 229–234 (2006).
Chen, C.-A., Chang, J.-M., Chang, E.-E., Chen, H.-C. & Yang, Y.-L. TGF-β1 modulates podocyte migration by regulating the expression of integrin-β1 and -β3 through different signaling pathways. Biomed. Pharmacother. 105, 974–980 (2018).
Dessapt, C. et al. Mechanical forces and TGFβ1 reduce podocyte adhesion through α3β1 integrin downregulation. Nephrol. Dial. Transplant. 24, 2645–2655 (2009).
Wei, C. et al. Modification of kidney barrier function by the urokinase receptor. Nat. Med. 14, 55–63 (2008).
Reiser, J. Circulating permeability factor suPAR: from concept to discovery to clinic. Trans. Am. Clin. Climatol. Assoc. 124, 133–138 (2013).
Hayek, S. S. et al. A tripartite complex of suPAR, APOL1 risk variants and αvβ3 integrin on podocytes mediates chronic kidney disease. Nat. Med. 23, 945–953 (2017).
Zhang, B. et al. The calcineurin–NFAT pathway allows for urokinase receptor-mediated beta3 integrin signaling to cause podocyte injury. J. Mol. Med. 90, 1407–1420 (2012).
Liu, Z. et al. Control of podocyte and glomerular capillary wall structure and elasticity by WNK1 kinase. Front. Cell Dev. Biol. 8, 618898 (2021).
Puklin-Faucher, E. & Sheetz, M. P. The mechanical integrin cycle. J. Cell Sci. 122, 179–186 (2009).
Discher, D. E., Janmey, P. & Wang, Y. L. Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139–1143 (2005).
Hu, M. et al. A biomimetic gelatin-based platform elicits a pro-differentiation effect on podocytes through mechanotransduction. Sci. Rep. 7, 43934 (2017).
Wyss, H. M. et al. Biophysical properties of normal and diseased renal glomeruli. Am. J. Physiol. Cell Physiol. 300, C397–C405 (2011).
Anderson, S. & Brenner, B. M. Effects of aging on the renal glomerulus. Am. J. Med. 80, 435–442 (1986).
Lv, T. et al. uPAR: an essential factor for tumor development. J. Cancer 12, 7026–7040 (2021).
Melica, M. E. et al. Substrate stiffness modulates renal progenitor cell properties via a ROCK-mediated mechanotransduction mechanism. Cells 8, 1561 (2019).
Treacy, N. J. et al. Growth and differentiation of human induced pluripotent stem cell (hiPSC)-derived kidney organoids using fully synthetic peptide hydrogels. Bioact. Mater. 21, 142–156 (2023).
Naylor, R. W., Morais, M. R. P. T. & Lennon, R. Complexities of the glomerular basement membrane. Nat. Rev. Nephrol. 17, 112–127 (2021).
Randles, M. J. et al. Identification of an altered matrix signature in kidney aging and disease. J. Am. Soc. Nephrol. 32, 1713–1732 (2021).
Nozu, K. et al. A review of clinical characteristics and genetic backgrounds in Alport syndrome. Clin. Exp. Nephrol. 23, 158–168 (2019).
Funk, S. D., Lin, M.-H. & Miner, J. H. Alport syndrome and Pierson syndrome: diseases of the glomerular basement membrane. Matrix Biol. 71-72, 250–261 (2018).
Gyarmati, G. et al. Intravital imaging reveals glomerular capillary distension and endothelial and immune cell activation early in Alport syndrome. JCI Insight 7, e152676 (2022).
Zieman, S. J. & Kass, D. A. Advanced glycation endproduct crosslinking in the cardiovascular system. Drugs 64, 459–470 (2004).
Kliewe, F. et al. Fibronectin is up-regulated in podocytes by mechanical stress. FASEB J. 33, 14450–14460 (2019).
Qu, H. et al. Kindlin-2 regulates podocyte adhesion and fibronectin matrix deposition through interactions with phosphoinositides and integrins. J. Cell Sci. 124, 879–891 (2011).
Fissell, W. H. & Miner, J. H. What is the glomerular ultrafiltration barrier? J. Am. Soc. Nephrol. 29, 2262–2264 (2018).
Pippin, J. W. et al. Upregulated PD-1 signaling antagonizes glomerular health in aged kidneys and disease. J. Clin. Invest. 132, e156250 (2022).
Nardone, G. et al. YAP regulates cell mechanics by controlling focal adhesion assembly. Nat. Commun. 8, 15321 (2017).
Elbediwy, A. et al. Enigma proteins regulate YAP mechanotransduction. J. Cell Sci. 131, jcs221788 (2018).
Rausch, V. & Hansen, C. G. The hippo pathway, YAP/TAZ, and the plasma membrane. Trends Cell Biol. 30, 32–48 (2020).
Chen, J. et al. YAP activation in renal proximal tubule cells drives diabetic renal interstitial fibrogenesis. Diabetes 69, 2446–2457 (2020).
Xu, D. et al. KLF4 initiates sustained YAP activation to promote renal fibrosis in mice after ischemia-reperfusion kidney injury. Acta Pharmacol. Sin. 42, 436–450 (2021).
Qian, X. et al. YAP mediates the interaction between the Hippo and PI3K/Akt pathways in mesangial cell proliferation in diabetic nephropathy. Acta Diabetol. 58, 47–62 (2021).
Liu, F. et al. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 308, L344–L357 (2014).
Marshall, C. B. Rethinking glomerular basement membrane thickening in diabetic nephropathy: adaptive or pathogenic? Am. J. Physiol. Ren. Physiol. 311, F831–F843 (2016).
Schwartzman, M. et al. Podocyte-specific deletion of Yes-associated protein causes FSGS and progressive renal failure. J. Am. Soc. Nephrol. 27, 216–226 (2016).
Chen, J., Wang, X., He, Q. & Harris, R. C. TAZ is important for maintenance of the integrity of podocytes. Am. J. Physiol. Ren. Physiol. 322, F419–F428 (2022).
Meliambro, K. et al. The Hippo pathway regulator KIBRA promotes podocyte injury by inhibiting YAP signaling and disrupting actin cytoskeletal dynamics. J. Biol. Chem. 292, 21137–21148 (2017).
Meliambro, K. et al. KIBRA upregulation increases susceptibility to glomerular injury and correlates with kidney function decline. JCI Insight 8, e165002 (2023).
Zhuang, Q. et al. Nuclear exclusion of YAP exacerbates podocyte apoptosis and disease progression in Adriamycin-induced focal segmental glomerulosclerosis. Lab. Invest. 101, 258–270 (2021).
Haley, K. E. et al. YAP translocation precedes cytoskeletal rearrangement in podocyte stress response: a podometric investigation of diabetic nephropathy. Front. Physiol. 12, 625762 (2021).
Adegbite, B. O. et al. Patient-specific pharmacokinetics and dasatinib nephrotoxicity. Clin. J. Am. Soc. Nephrol. 18, 1175–1185 (2023).
Rinschen, M. M. et al. YAP-mediated mechanotransduction determines the podocyte’s response to damage. Sci. Signal. 10, eaaf8165 (2017).
Koehler, S., Huber, T. B. & Denholm, B. A protective role for Drosophila filamin in nephrocytes via Yorkie mediated hypertrophy. Life Sci. Alliance 5, e202101281 (2022).
La, T. M. et al. Dynamin 1 is important for microtubule organization and stabilization in glomerular podocytes. FASEB J. 34, 16449–16463 (2020).
Gu, C. et al. Dynamin autonomously regulates podocyte focal adhesion maturation. J. Am. Soc. Nephrol. 28, 446–451 (2017).
Schiffer, M. et al. Pharmacological targeting of actin-dependent dynamin oligomerization ameliorates chronic kidney disease in diverse animal models. Nat. Med. 21, 601–609 (2015).
Nobes, C. D. & Hall, A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81, 53–62 (1995).
Falkenberg, C. V. et al. Fragility of foot process morphology in kidney podocytes arises from chaotic spatial propagation of cytoskeletal instability. PLoS Comput. Biol. 13, e1005433 (2017).
Babelova, A. et al. Activation of Rac-1 and RhoA contributes to podocyte injury in chronic kidney disease. PLoS ONE 8, e80328 (2013).
Shen, J. et al. NMDA receptors participate in the progression of diabetic kidney disease by decreasing Cdc42-GTP activation in podocytes. J. Pathol. 240, 149–160 (2016).
Szrejder, M. et al. Metformin reduces TRPC6 expression through AMPK activation and modulates cytoskeleton dynamics in podocytes under diabetic conditions. Biochim. Biophys. Acta Mol. Basis Dis. 1866, 165610 (2020).
Li, S.-Y. et al. FHL2 mediates podocyte Rac1 activation and foot process effacement in hypertensive nephropathy. Sci. Rep. 9, 15552 (2019).
Robins, R. et al. Rac1 activation in podocytes induces the spectrum of nephrotic syndrome. Kidney Int. 92, 349–364 (2017).
Scott, R. P. et al. Podocyte-specific loss of Cdc42 leads to congenital nephropathy. J. Am. Soc. Nephrol. 23, 1149–1154 (2012).
Asao, R. et al. Rac1 in podocytes promotes glomerular repair and limits the formation of sclerosis. Sci. Rep. 8, 5061 (2018).
Wang, L. et al. Mechanisms of the proteinuria induced by Rho GTPases. Kidney Int. 81, 1075–1085 (2012).
Ashraf, S. et al. Mutations in six nephrosis genes delineate a pathogenic pathway amenable to treatment. Nat. Commun. 9, 1960 (2018).
Vivante, A. & Hildebrandt, F. Exploring the genetic basis of early-onset chronic kidney disease. Nat. Rev. Nephrol. 12, 133–146 (2016).
Ilatovskaya, D. V. & Staruschenko, A. TRPC6 channel as an emerging determinant of the podocyte injury susceptibility in kidney diseases. Am. J. Physiol. Ren. Physiol. 309, F393–F397 (2015).
Zhou, Y. et al. A small-molecule inhibitor of TRPC5 ion channels suppresses progressive kidney disease in animal models. Science 358, 1332–1336 (2017).
Greka, A. & Mundel, P. Balancing calcium signals through TRPC5 and TRPC6 in podocytes. J. Am. Soc. Nephrol. 22, 1969–1980 (2011).
Tian, D. et al. Antagonistic regulation of actin dynamics and cell motility by TRPC5 and TRPC6 channels. Sci. Signal. 3, ra77 (2010).
Matsuda, J., Asano-Matsuda, K., Kitzler, T. M. & Takano, T. Rho GTPase regulatory proteins in podocytes. Kidney Int. 99, 336–345 (2021).
Fan, X. et al. SLIT2/ROBO2 signaling pathway inhibits nonmuscle myosin IIA activity and destabilizes kidney podocyte adhesion. JCI Insight 1, e86934 (2016).
Hwang, D.-Y. et al. Mutations of the SLIT2–ROBO2 pathway genes SLIT2 and SRGAP1 confer risk for congenital anomalies of the kidney and urinary tract. Hum. Genet. 134, 905–916 (2015).
Daehn, I. S. & Duffield, J. S. The glomerular filtration barrier: a structural target for novel kidney therapies. Nat. Rev. Drug. Discov. 20, 770–788 (2021).
Reidy, K. & Tufro, A. Semaphorins in kidney development and disease: modulators of ureteric bud branching, vascular morphogenesis, and podocyte-endothelial crosstalk. Pediatr. Nephrol. 26, 1407–1412 (2011).
Sang, Y. et al. Semaphorin3A-inhibitor ameliorates doxorubicin-induced podocyte injury. Int. J. Mol. Sci. 21, 4099 (2020).
Meng, Z. et al. The Hippo pathway mediates Semaphorin signaling. Sci. Adv. 8, eabl9806 (2022).
Regué, L., Mou, F. & Avruch, J. G protein‐coupled receptors engage the mammalian Hippo pathway through F‐actin: F‐actin, assembled in response to Galpha12/13 induced RhoA‐GTP, promotes dephosphorylation and activation of the YAP oncogene. Bioessays 35, 430–435 (2013).
Ma, S. & Guan, K.-L. Polycystic kidney disease: a Hippo connection. Genes Dev. 32, 737–739 (2018).
Ma, S., Meng, Z., Chen, R. & Guan, K.-L. The Hippo pathway: biology and pathophysiology. Annu. Rev. Biochem. 88, 577–604 (2019).
Rogg, M. et al. A YAP/TAZ–ARHGAP29–RhoA signaling axis regulates podocyte protrusions and integrin adhesions. Cells 12, 1795 (2023).
Chen, R., Xie, R., Meng, Z., Ma, S. & Guan, K.-L. STRIPAK integrates upstream signals to initiate the Hippo kinase cascade. Nat. Cell Biol. 21, 1565–1577 (2019).
Abdallah, M. et al. Influence of hydrolyzed polyacrylamide hydrogel stiffness on podocyte morphology, phenotype, and mechanical properties. ACS Appl. Mater. Interfaces 11, 32623–32632 (2019).
Dorison, A. et al. Kidney organoids generated using an allelic series of NPHS2 point variants reveal distinct intracellular podocin mistrafficking. J. Am. Soc. Nephrol. 34, 88–109 (2023).
Chang, S.-Y. et al. Human liver-kidney model elucidates the mechanisms of aristolochic acid nephrotoxicity. JCI Insight 2, e95978 (2017).
Wang, L. et al. A disease model of diabetic nephropathy in a glomerulus-on-a-chip microdevice. Lab. Chip 17, 1749–1760 (2017).
Musah, S. et al. Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip. Nat. Biomed. Eng. 1, 10069 (2017).
Homan, K. A. et al. Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat. Methods 16, 255–262 (2019).
Roye, Y. et al. A personalized glomerulus chip engineered from stem cell-derived epithelium and vascular endothelium. Micromachines 12, 967 (2021).
Anandakrishnan, N. & Azeloglu, E. U. Kidney tissue engineering for precision medicine. Nat. Rev. Nephrol. 16, 623–624 (2020).
Takasato, M. & Little, M. H. A strategy for generating kidney organoids: recapitulating the development in human pluripotent stem cells. Dev. Biol. 420, 210–220 (2016).
Ungricht, R. et al. Genome-wide screening in human kidney organoids identifies developmental and disease-related aspects of nephrogenesis. Cell Stem Cell 29, 160–175.e7 (2022).
Hale, L. J. et al. 3D organoid-derived human glomeruli for personalised podocyte disease modelling and drug screening. Nat. Commun. 9, 5167 (2018).
Li, S. R. et al. Glucose absorption drives cystogenesis in a human organoid-on-chip model of polycystic kidney disease. Nat. Commun. 13, 7918 (2022).
Hiratsuka, K. et al. Organoid-on-a-chip model of human ARPKD reveals mechanosensing pathomechanisms for drug discovery. Sci. Adv. 8, eabq0866 (2022).
Taguchi, A. et al. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell 14, 53–67 (2014).
Lawlor, K. T. et al. Cellular extrusion bioprinting improves kidney organoid reproducibility and conformation. Nat. Mater. 20, 260–271 (2021).
Bas-Cristobal Menendez, A. et al. Creating a kidney organoid-vasculature interaction model using a novel organ-on-chip system. Sci. Rep. 12, 20699 (2022).
Tuffin, J. et al. GlomSpheres as a 3D co-culture spheroid model of the kidney glomerulus for rapid drug-screening. Commun. Biol. 4, 1351 (2021).
Azeloglu, E. U. & Costa, K. D. Atomic force microscopy in mechanobiology: measuring microelastic heterogeneity of living cells. Methods Mol. Biol. 736, 303–329 (2011).
Artelt, N. et al. Comparative analysis of podocyte foot process morphology in three species by 3D super-resolution microscopy. Front. Med. 5, 292 (2018).
Musah, S., Dimitrakakis, N., Camacho, D. M., Church, G. M. & Ingber, D. E. Directed differentiation of human induced pluripotent stem cells into mature kidney podocytes and establishment of a glomerulus chip. Nat. Protoc. 13, 1662–1685 (2018).
Yasuda-Yamahara, M. et al. AIF1L regulates actomyosin contractility and filopodial extensions in human podocytes. PLoS ONE 13, e0200487 (2018).
Gbadegesin, R. A. et al. Mutations in the gene that encodes the F-actin binding protein anillin cause FSGS. J. Am. Soc. Nephrol. 25, 1991–2002 (2014).
Acknowledgements
We would like to acknowledge Chiara Mariottini for critical reading of the manuscript before submission and NIH for funding (R25 DK124917 to EUA). J.H. was partly funded by T32 HD075735.
Author information
Authors and Affiliations
Contributions
Both authors researched data for the article, made substantial contributions to discussions of the content and wrote, reviewed, and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks Thomas Benzing, Richard Miller and Hani Suleiman for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Buttress force
-
Reaction force exerted by podocytes countering dilation of the glomerular capillary.
- Filopodia
-
Thin, spike-like projections found beyond the lamellipodia.
- Foot process effacement
-
Morphological change in podocytes caused by cytoskeletal rearrangement in which the interdigitating foot processes become simplified and broadened, resulting in proteinuria.
- Lamellipodia
-
Membrane protrusions found at the leading edge of cells.
- Shear stress
-
Stress resulting from deformation (for example, due to fluid flow) parallel to the face of a material.
- Stress fibres
-
Actomyosin bundles that serve as the primary source of contractile force in non-muscle cells.
- Tensile stress
-
Stress resulting from a force that is stretching or elongating a material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Haydak, J., Azeloglu, E.U. Role of biophysics and mechanobiology in podocyte physiology. Nat Rev Nephrol (2024). https://doi.org/10.1038/s41581-024-00815-3
Accepted:
Published:
DOI: https://doi.org/10.1038/s41581-024-00815-3