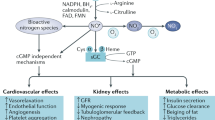
Abstract
Chronic kidney disease (CKD) is a major public health concern, underscoring a need to identify pathogenic mechanisms and potential therapeutic targets. Reactive oxygen species (ROS) are derivatives of oxygen molecules that are generated during aerobic metabolism and are involved in a variety of cellular functions that are governed by redox conditions. Low levels of ROS are required for diverse processes, including intracellular signal transduction, metabolism, immune and hypoxic responses, and transcriptional regulation. However, excess ROS can be pathological, and contribute to the development and progression of chronic diseases. Despite evidence linking elevated levels of ROS to CKD development and progression, the use of low-molecular-weight antioxidants to remove ROS has not been successful in preventing or slowing disease progression. More recent advances have enabled evaluation of the molecular interactions between specific ROS and their targets in redox signalling pathways. Such studies may pave the way for the development of sophisticated treatments that allow the selective control of specific ROS-mediated signalling pathways.
Key points
-
Oxidative stress occurs when the generation of oxidants exceeds the metabolizing or degradative capacity of antioxidants.
-
Reactive oxygen species (ROS) are radical or molecular species that contain oxygen and are produced by cellular organelles; they can cause damage to cells and tissues when present in excessive amounts.
-
Although potentially harmful in excess, ROS also contribute to cell survival and can act as signalling molecules at appropriate intracellular concentrations.
-
Within the kidney, ROS are produced by a variety of organelles and enzyme systems, and contribute to a range of physiological and pathological processes.
-
The involvement of ROS in pathological processes suggests that therapeutic targeting of ROS may be beneficial; a number of therapeutic approaches are under investigation, including targeting of Nrf2.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Sies, H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 4, 180–183 (2015).
Harman, D. Origin and evolution of the free radical theory of aging: a brief personal history, 1954–2009. Biogerontology 10, 773–781 (2009).
Sundaresan, M., Yu, Z. X., Ferrans, V. J., Irani, K. & Finkel, T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 270, 296–299 (1995).
Rhee, S. G. Cell signaling. H2O2, a necessary evil for cell signaling. Science 312, 1882–1883 (2006).
Forrester, S. J., Kikuchi, D. S., Hernandes, M. S., Xu, Q. & Griendling, K. K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 122, 877–902 (2018).
Martínez-Reyes, I. et al. TCA cycle and mitochondrial membrane potential are necessary for diverse biological functions. Mol. Cell 61, 199–209 (2016).
Oliveira-Marques, V., Marinho, H. S., Cyrne, L. & Antunes, F. Role of hydrogen peroxide in NF-κB activation: from inducer to modulator. Antioxid. Redox Signal. 11, 2223–2243 (2009).
Ruiz, S., Pergola, P. E., Zager, R. A. & Vaziri, N. D. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 83, 1029–1041 (2013).
Nishikawa, T. et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404, 787–790 (2000).
Satoh, M. et al. NAD(P)H oxidase and uncoupled nitric oxide synthase are major sources of glomerular superoxide in rats with experimental diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 288, F1144–F1152 (2005).
Sies, H. & Jones, D. P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 21, 363–383 (2020).
Kunsch, C. & Medford, R. M. Oxidative stress as a regulator of gene expression in the vasculature. Circ. Res. 85, 753–766 (1999).
Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Redox Biol. 11, 613–619 (2017).
Rhee, S. G. Redox signaling: hydrogen peroxide as intracellular messenger. Exp. Mol. Med. 31, 53–59 (1999).
Carlström, M. Nitric oxide signalling in kidney regulation and cardiometabolic health. Nat. Rev. Nephrol. 17, 575–590 (2021).
Zorov, D. B., Juhaszova, M. & Sollott, S. J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 94, 909–950 (2014).
Martínez, M. C. & Andriantsitohaina, R. Reactive nitrogen species: molecular mechanisms and potential significance in health and disease. Antioxid. Redox Signal. 11, 669–702 (2009).
Drummond, G. R., Selemidis, S., Griendling, K. K. & Sobey, C. G. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat. Rev. Drug. Discov. 10, 453–471 (2011).
Koppenol, W. H. The Haber–Weiss cycle – 70 years later. Redox Rep. 6, 229–234 (2001).
Nakamura, H., Nakamura, K. & Yodoi, J. Redox regulation of cellular activation. Annu. Rev. Immunol. 15, 351–369 (1997).
Lobo, V., Patil, A., Phatak, A. & Chandra, N. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn. Rev. 4, 118–126 (2010).
Sena, L. A. & Chandel, N. S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 48, 158–167 (2012).
Finkel, T. Signal transduction by mitochondrial oxidants. J. Biol. Chem. 287, 4434–4440 (2012).
Li, Y. et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat. Genet. 11, 376–381 (1995).
Schieber, M. & Chandel, N. S. ROS function in redox signaling and oxidative stress. Curr. Biol. 24, R453–R462 (2014).
Davies, K. J. Adaptive homeostasis. Mol. Asp. Med. 49, 1–7 (2016).
Peris, E. et al. Antioxidant treatment induces reductive stress associated with mitochondrial dysfunction in adipocytes. J. Biol. Chem. 294, 2340–2352 (2019).
Xiao, W. & Loscalzo, J. Metabolic responses to reductive stress. Antioxid. Redox Signal. 32, 1330–1347 (2020).
Scherz-Shouval, R. et al. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 26, 1749–1760 (2007).
Uchida, T. et al. The effect of long-term inorganic iodine on intrathyroidal iodothyronine content and gene expression in mice with Graves’ hyperthyroidism. Thyroid 33, 330–337 (2023).
Guzy, R. D. et al. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 1, 401–408 (2005).
Colman, R. J. et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 325, 201–204 (2009).
Mattison, J. A. et al. Caloric restriction improves health and survival of rhesus monkeys. Nat. Commun. 8, 14063 (2017).
Roth, G. S. et al. Biomarkers of caloric restriction may predict longevity in humans. Science 297, 811 (2002).
López-Lluch, G. et al. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc. Natl Acad. Sci. USA 103, 1768–1773 (2006).
Saunders, L. R. & Verdin, E. Sirtuins: critical regulators at the crossroads between cancer and aging. Oncogene 26, 5489–5504 (2007).
Tracz, M. J., Alam, J. & Nath, K. A. Physiology and pathophysiology of heme: implications for kidney disease. J. Am. Soc. Nephrol. 18, 414–420 (2007).
Nath, M. & Agarwal, A. New insights into the role of heme oxygenase-1 in acute kidney injury. Kidney Res. Clin. Pract. 39, 387–401 (2020).
Gozzelino, R., Jeney, V. & Soares, M. P. Mechanisms of cell protection by heme oxygenase-1. Annu. Rev. Pharmacol. Toxicol. 50, 323–354 (2010).
Blydt-Hansen, T. D. et al. Gene transfer-induced local heme oxygenase-1 overexpression protects rat kidney transplants from ischemia/reperfusion injury. J. Am. Soc. Nephrol. 14, 745–754 (2003).
Shimizu, H. et al. Protective effect of heme oxygenase induction in ischemic acute renal failure. Crit. Care Med. 28, 809–817 (2000).
Nakahira, K. et al. Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS-induced trafficking of TLRs to lipid rafts. J. Exp. Med. 203, 2377–2389 (2006).
Stocker, R., Yamamoto, Y., McDonagh, A. F., Glazer, A. N. & Ames, B. N. Bilirubin is an antioxidant of possible physiological importance. Science 235, 1043–1046 (1987).
Valtin, H. Renal function: mechanisms preserving fluid and solute balance in health. Arch. Surg. 109, 462–463 (1973).
Forbes, J. M. & Thorburn, D. R. Mitochondrial dysfunction in diabetic kidney disease. Nat. Rev. Nephrol. 14, 291–312 (2018).
Li, X. et al. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J. Hematol. Oncol. 6, 19 (2013).
Pizzino, G. et al. Oxidative stress: harms and benefits for human health. Oxid. Med. Cell Longev. 2017, 8416763 (2017).
Zeeshan, H. M., Lee, G. H., Kim, H. R. & Chae, H. J. Endoplasmic reticulum stress and associated ROS. Int. J. Mol. Sci. 17, 327 (2016).
Lee, Y. M., He, W. & Liou, Y. C. The redox language in neurodegenerative diseases: oxidative post-translational modifications by hydrogen peroxide. Cell Death Dis. 12, 58 (2021).
Antonenkov, V. D., Grunau, S., Ohlmeier, S. & Hiltunen, J. K. Peroxisomes are oxidative organelles. Antioxid. Redox Signal. 13, 525–537 (2010).
Litwin, J. A., Völkl, A., Müller-Höcker, J. & Fahimi, H. D. Immunocytochemical demonstration of peroxisomal enzymes in human kidney biopsies. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 54, 207–213 (1988).
Litwin, J. A., Völkl, A., Stachura, J. & Fahimi, H. D. Detection of peroxisomes in human liver and kidney fixed with formalin and embedded in paraffin: the use of catalase and lipid β-oxidation enzymes as immunocytochemical markers. Histochem. J. 20, 165–173 (1988).
Sedeek, M., Nasrallah, R., Touyz, R. M. & Hébert, R. L. NADPH oxidases, reactive oxygen species, and the kidney: friend and foe. J. Am. Soc. Nephrol. 24, 1512–1518 (2013).
Groemping, Y., Lapouge, K., Smerdon, S. J. & Rittinger, K. Molecular basis of phosphorylation-induced activation of the NADPH oxidase. Cell 113, 343–355 (2003).
Muñoz, M. et al. Hydrogen peroxide derived from NADPH oxidase 4- and 2 contributes to the endothelium-dependent vasodilatation of intrarenal arteries. Redox Biol. 19, 92–104 (2018).
Fukuda, M. et al. Potentiation by candesartan of protective effects of pioglitazone against type 2 diabetic cardiovascular and renal complications in obese mice. J. Hypertens. 28, 340–352 (2010).
Oudit, G. Y. et al. Human recombinant ACE2 reduces the progression of diabetic nephropathy. Diabetes 59, 529–538 (2010).
Irazabal, M. V. & Torres, V. E. Reactive oxygen species and redox signaling in chronic kidney disease. Cells 9, 1342 (2020).
Nlandu Khodo, S. et al. NADPH-oxidase 4 protects against kidney fibrosis during chronic renal injury. J. Am. Soc. Nephrol. 23, 1967–1976 (2012).
Hille, R. & Nishino, T. Flavoprotein structure and mechanism. 4. Xanthine oxidase and xanthine dehydrogenase. FASEB J. 9, 995–1003 (1995).
Nishino, T. The conversion of xanthine dehydrogenase to xanthine oxidase and the role of the enzyme in reperfusion injury. J. Biochem. 116, 1–6 (1994).
Li, H., Horke, S. & Förstermann, U. Oxidative stress in vascular disease and its pharmacological prevention. Trends Pharmacol. Sci. 34, 313–319 (2013).
George, J., Carr, E., Davies, J., Belch, J. J. & Struthers, A. High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation 114, 2508–2516 (2006).
Itano, S. et al. Non-purine selective xanthine oxidase inhibitor ameliorates glomerular endothelial injury in Ins(Akita) diabetic mice. Am. J. Physiol. Ren. Physiol. 319, F765–F772 (2020).
Winterbourn, C. C., Kettle, A. J. & Hampton, M. B. Reactive oxygen species and neutrophil function. Annu. Rev. Biochem. 85, 765–792 (2016).
Brinkmann, V. et al. Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 (2004).
Kenny, E. F. et al. Diverse stimuli engage different neutrophil extracellular trap pathways. Elife 6, e24437 (2017).
Stendahl, O., Coble, B. I., Dahlgren, C., Hed, J. & Molin, L. Myeloperoxidase modulates the phagocytic activity of polymorphonuclear neutrophil leukocytes. Studies with cells from a myeloperoxidase-deficient patient. J. Clin. Invest. 73, 366–373 (1984).
Daugherty, A., Dunn, J. L., Rateri, D. L. & Heinecke, J. W. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J. Clin. Invest. 94, 437–444 (1994).
Brennan, M. L. et al. Prognostic value of myeloperoxidase in patients with chest pain. N. Engl. J. Med. 349, 1595–1604 (2003).
Jennette, J. C. et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 65, 1–11 (2013).
Kallenberg, C. G. Antineutrophil cytoplasmic autoantibody-associated small-vessel vasculitis. Curr. Opin. Rheumatol. 19, 17–24 (2007).
Falk, R. J., Terrell, R. S., Charles, L. A. & Jennette, J. C. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc. Natl Acad. Sci. USA 87, 4115–4119 (1990).
Malle, E., Buch, T. & Grone, H. J. Myeloperoxidase in kidney disease. Kidney Int. 64, 1956–1967 (2003).
Zweier, J. L., Wang, P., Samouilov, A. & Kuppusamy, P. Enzyme-independent formation of nitric oxide in biological tissues. Nat. Med. 1, 804–809 (1995).
Benjamin, N. et al. Stomach NO synthesis. Nature 368, 502 (1994).
Oliveira, F. R. M. G., Assreuy, J. & Sordi, R. The role of nitric oxide in sepsis-associated kidney injury. Biosci. Rep. 42, BSR20220093 (2022).
Kosaka, H. et al. Induction of LOX-1 and iNOS expressions by ischemia-reperfusion of rat kidney and the opposing effect of l-arginine. FASEB J. 17, 636–643 (2003).
Sordi, R., Menezes-de-Lima, O., Della-Justina, A. M., Rezende, E. & Assreuy, J. Pneumonia-induced sepsis in mice: temporal study of inflammatory and cardiovascular parameters. Int. J. Exp. Pathol. 94, 144–155 (2013).
Sordi, R., Chiazza, F., Collino, M., Assreuy, J. & Thiemermann, C. Neuronal nitric oxide synthase is involved in vascular hyporeactivity and multiple organ dysfunction associated with hemorrhagic shock. Shock 45, 525–533 (2016).
Baylis, C. Arginine, arginine analogs and nitric oxide production in chronic kidney disease. Nat. Clin. Pract. Nephrol. 2, 209–220 (2006).
Nishimura, K. et al. Dual disruption of eNOS and ApoE gene accelerates kidney fibrosis and senescence after injury. Biochem. Biophys. Res. Commun. 556, 142–148 (2021).
Knowles, J. W. et al. Enhanced atherosclerosis and kidney dysfunction in eNOS−/−Apoe−/− mice are ameliorated by enalapril treatment. J. Clin. Invest. 105, 451–458 (2000).
Carlström, M. et al. Dietary inorganic nitrate reverses features of metabolic syndrome in endothelial nitric oxide synthase-deficient mice. Proc. Natl Acad. Sci. USA 107, 17716–17720 (2010).
Gil, C. L., Hooker, E. & Larrivée, B. Diabetic kidney disease, endothelial damage, and podocyte–endothelial crosstalk. Kidney Med. 3, 105–115 (2021).
Jha, J. C., Banal, C., Chow, B. S., Cooper, M. E. & Jandeleit-Dahm, K. Diabetes and kidney disease: role of oxidative stress. Antioxid. Redox Signal. 25, 657–684 (2016).
Satoh, M. et al. Angiotensin II type 1 receptor blocker ameliorates uncoupled endothelial nitric oxide synthase in rats with experimental diabetic nephropathy. Nephrol. Dial. Transpl. 23, 3806–3813 (2008).
Kidokoro, K. et al. Maintenance of endothelial guanosine triphosphate cyclohydrolase I ameliorates diabetic nephropathy. J. Am. Soc. Nephrol. 24, 1139–1150 (2013).
Liu, R. et al. The role of macula densa nitric oxide synthase 1 beta splice variant in modulating tubuloglomerular feedback. Compr. Physiol. 13, 4215–4229 (2023).
Song, J. et al. Oxidative status in the macula densa modulates tubuloglomerular feedback responsiveness in angiotensin II-induced hypertension. Acta Physiol. 213, 249–258 (2015).
Araujo, M. & Welch, W. J. Tubuloglomerular feedback is decreased in COX-1 knockout mice after chronic angiotensin II infusion. Am. J. Physiol. Ren. Physiol. 298, F1059–F1063 (2010).
Lu, D. et al. Salt-sensitive splice variant of nNOS expressed in the macula densa cells. Am. J. Physiol. Ren. Physiol. 298, F1465–F1471 (2010).
Lu, Y. et al. Macula densa nitric oxide synthase 1β protects against salt-sensitive hypertension. J. Am. Soc. Nephrol. 27, 2346–2356 (2016).
Campese, V. M., Parise, M., Karubian, F. & Bigazzi, R. Abnormal renal hemodynamics in black salt-sensitive patients with hypertension. Hypertension 18, 805–812 (1991).
Wei, J. et al. Macula densa NOS1β modulates renal hemodynamics and blood pressure during pregnancy: role in gestational hypertension. J. Am. Soc. Nephrol. 32, 2485–2500 (2021).
Gielis, J. F. et al. Pathogenetic role of eNOS uncoupling in cardiopulmonary disorders. Free. Radic. Biol. Med. 50, 765–776 (2011).
Münzel, T., Daiber, A., Ullrich, V. & Mülsch, A. Vascular consequences of endothelial nitric oxide synthase uncoupling for the activity and expression of the soluble guanylyl cyclase and the cGMP-dependent protein kinase. Arterioscler. Thromb. Vasc. Biol. 25, 1551–1557 (2005).
Janaszak-Jasiecka, A., Płoska, A., Wierońska, J. M., Dobrucki, L. W. & Kalinowski, L. Endothelial dysfunction due to eNOS uncoupling: molecular mechanisms as potential therapeutic targets. Cell Mol. Biol. Lett. 28, 21 (2023).
Bendall, J. K., Douglas, G., McNeill, E., Channon, K. M. & Crabtree, M. J. Tetrahydrobiopterin in cardiovascular health and disease. Antioxid. Redox Signal. 20, 3040–3077 (2014).
Moens, A. L. & Kass, D. A. Tetrahydrobiopterin and cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 26, 2439–2444 (2006).
Antoniades, C. et al. Altered plasma versus vascular biopterins in human atherosclerosis reveal relationships between endothelial nitric oxide synthase coupling, endothelial function, and inflammation. Circulation 116, 2851–2859 (2007).
Ismaeel, A. et al. The nitric oxide system in peripheral artery disease: connection with oxidative stress and biopterins. Antioxidants 9, 590 (2020).
Heitzer, T., Krohn, K., Albers, S. & Meinertz, T. Tetrahydrobiopterin improves endothelium-dependent vasodilation by increasing nitric oxide activity in patients with type II diabetes mellitus. Diabetologia 43, 1435–1438 (2000).
Higashi, Y. et al. Tetrahydrobiopterin enhances forearm vascular response to acetylcholine in both normotensive and hypertensive individuals. Am. J. Hypertens. 15, 326–332 (2002).
Stroes, E. et al. Tetrahydrobiopterin restores endothelial function in hypercholesterolemia. J. Clin. Invest. 99, 41–46 (1997).
Yamamoto, E. et al. The pivotal role of eNOS uncoupling in vascular endothelial dysfunction in patients with heart failure with preserved ejection fraction. Int. J. Cardiol. 190, 335–337 (2015).
Shinozaki, K. et al. Oral administration of tetrahydrobiopterin prevents endothelial dysfunction and vascular oxidative stress in the aortas of insulin-resistant rats. Circ. Res. 87, 566–573 (2000).
Hong, H. J., Hsiao, G., Cheng, T. H. & Yen, M. H. Supplemention with tetrahydrobiopterin suppresses the development of hypertension in spontaneously hypertensive rats. Hypertension 38, 1044–1048 (2001).
Landmesser, U. et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J. Clin. Invest. 111, 1201–1209 (2003).
Maier, W. et al. Tetrahydrobiopterin improves endothelial function in patients with coronary artery disease. J. Cardiovasc. Pharmacol. 35, 173–178 (2000).
Setoguchi, S., Hirooka, Y., Eshima, K., Shimokawa, H. & Takeshita, A. Tetrahydrobiopterin improves impaired endothelium-dependent forearm vasodilation in patients with heart failure. J. Cardiovasc. Pharmacol. 39, 363–368 (2002).
Cosentino, F. et al. Chronic treatment with tetrahydrobiopterin reverses endothelial dysfunction and oxidative stress in hypercholesterolaemia. Heart 94, 487–492 (2008).
Porkert, M. et al. Tetrahydrobiopterin: a novel antihypertensive therapy. J. Hum. Hypertens. 22, 401–407 (2008).
Mäki-Petäjä, K. M. et al. Tetrahydrobiopterin supplementation improves endothelial function but does not alter aortic stiffness in patients with rheumatoid arthritis. J. Am. Heart Assoc. 5, e002762 (2016).
Cunnington, C. et al. Systemic and vascular oxidation limits the efficacy of oral tetrahydrobiopterin treatment in patients with coronary artery disease. Circulation 125, 1356–1366 (2012).
Heller, R. et al. l-Ascorbic acid potentiates endothelial nitric oxide synthesis via a chemical stabilization of tetrahydrobiopterin. J. Biol. Chem. 276, 40–47 (2001).
Gao, S. Q. et al. Endothelial NOX4 aggravates eNOS uncoupling by decreasing dihydrofolate reductase after subarachnoid hemorrhage. Free. Radic. Biol. Med. 193, 499–510 (2022).
Wu, G. & Meininger, C. J. Arginine nutrition and cardiovascular function. J. Nutr. 130, 2626–2629 (2000).
Cylwik, D., Mogielnicki, A. & Buczko, W. L-arginine and cardiovascular system. Pharmacol. Rep. 57, 14–22 (2005).
Chen, C. A. et al. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature 468, 1115–1118 (2010).
Holmgren, A. Antioxidant function of thioredoxin and glutaredoxin systems. Antioxid. Redox Signal. 2, 811–820 (2000).
Chen, C. A., De Pascali, F., Basye, A., Hemann, C. & Zweier, J. L. Redox modulation of endothelial nitric oxide synthase by glutaredoxin-1 through reversible oxidative post-translational modification. Biochemistry 52, 6712–6723 (2013).
Subramani, J., Kundumani-Sridharan, V., Hilgers, R. H., Owens, C. & Das, K. C. Thioredoxin uses a GSH-independent route to deglutathionylate endothelial nitric-oxide synthase and protect against myocardial infarction. J. Biol. Chem. 291, 23374–23389 (2016).
Lennicke, C. & Cochemé, H. M. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol. Cell 81, 3691–3707 (2021).
Baird, L. & Yamamoto, M. The molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol. Cell Biol. 40, e00090-20 (2020).
Yamamoto, T. et al. Physiological significance of reactive cysteine residues of Keap1 in determining Nrf2 activity. Mol. Cell Biol. 28, 2758–2770 (2008).
Nezu, M., Suzuki, N. & Yamamoto, M. Targeting the KEAP1-NRF2 system to prevent kidney disease progression. Am. J. Nephrol. 45, 473–483 (2017).
Liu, M. et al. Transcription factor Nrf2 is protective during ischemic and nephrotoxic acute kidney injury in mice. Kidney Int. 76, 277–285 (2009).
Jiang, T. et al. The protective role of Nrf2 in streptozotocin-induced diabetic nephropathy. Diabetes 59, 850–860 (2010).
Kim, H. J. & Vaziri, N. D. Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am. J. Physiol. Ren. Physiol. 298, F662–F671 (2010).
Kim, H. J., Sato, T., Rodríguez-Iturbe, B. & Vaziri, N. D. Role of intrarenal angiotensin system activation, oxidative stress, inflammation, and impaired nuclear factor-erythroid-2-related factor 2 activity in the progression of focal glomerulosclerosis. J. Pharmacol. Exp. Ther. 337, 583–590 (2011).
Eijkelenboom, A. & Burgering, B. M. FOXOs: signalling integrators for homeostasis maintenance. Nat. Rev. Mol. Cell Biol. 14, 83–97 (2013).
Putker, M. et al. Redox-dependent control of FOXO/DAF-16 by transportin-1. Mol. Cell 49, 730–742 (2013).
Dansen, T. B. et al. Redox-sensitive cysteines bridge p300/CBP-mediated acetylation and FoxO4 activity. Nat. Chem. Biol. 5, 664–672 (2009).
Wang, Y. & He, W. Improving the dysregulation of FoxO1 activity is a potential therapy for alleviating diabetic kidney disease. Front. Pharmacol. 12, 630617 (2021).
Li, N. & Karin, M. Is NF-κB the sensor of oxidative stress? FASEB J. 13, 1137–1143 (1999).
Sanz, A. B. et al. NF-κB in renal inflammation. J. Am. Soc. Nephrol. 21, 1254–1262 (2010).
Zhang, H. & Sun, S. C. NF-κB in inflammation and renal diseases. Cell Biosci. 5, 63 (2015).
Li, W. et al. Activation of Nrf2-antioxidant signaling attenuates NFκB-inflammatory response and elicits apoptosis. Biochem. Pharmacol. 76, 1485–1489 (2008).
Tanaka, T. & Nangaku, M. Angiogenesis and hypoxia in the kidney. Nat. Rev. Nephrol. 9, 211–222 (2013).
Akhtar, M. Z., Sutherland, A. I., Huang, H., Ploeg, R. J. & Pugh, C. W. The role of hypoxia-inducible factors in organ donation and transplantation: the current perspective and future opportunities. Am. J. Transpl. 14, 1481–1487 (2014).
Bernhardt, W. M. et al. Donor treatment with a PHD-inhibitor activating HIFs prevents graft injury and prolongs survival in an allogenic kidney transplant model. Proc. Natl Acad. Sci. USA 106, 21276–21281 (2009).
Matsumoto, M. et al. Induction of renoprotective gene expression by cobalt ameliorates ischemic injury of the kidney in rats. J. Am. Soc. Nephrol. 14, 1825–1832 (2003).
Sugahara, M., Tanaka, T. & Nangaku, M. Hypoxia-inducible factor and oxygen biology in the kidney. Kidney360 1, 1021–1031 (2020).
Iguchi, M. et al. Acute inactivation of the VHL gene contributes to protective effects of ischemic preconditioning in the mouse kidney. Nephron Exp. Nephrol. 110, e82–e90 (2008).
Hill, P. et al. Inhibition of hypoxia inducible factor hydroxylases protects against renal ischemia-reperfusion injury. J. Am. Soc. Nephrol. 19, 39–46 (2008).
Kojima, I. et al. Protective role of hypoxia-inducible factor-2α against ischemic damage and oxidative stress in the kidney. J. Am. Soc. Nephrol. 18, 1218–1226 (2007).
Ito, M. et al. Prolyl hydroxylase inhibition protects the kidneys from ischemia via upregulation of glycogen storage. Kidney Int. 97, 687–701 (2020).
Kapitsinou, P. P. et al. Preischemic targeting of HIF prolyl hydroxylation inhibits fibrosis associated with acute kidney injury. Am. J. Physiol. Ren. Physiol. 302, F1172–F1179 (2012).
Wang, Z. et al. The protective effect of prolyl-hydroxylase inhibition against renal ischaemia requires application prior to ischaemia but is superior to EPO treatment. Nephrol. Dial. Transpl. 27, 929–936 (2012).
Rosenberger, C. et al. Adaptation to hypoxia in the diabetic rat kidney. Kidney Int. 73, 34–42 (2008).
Yamanaka, S. et al. Adipose tissue-derived mesenchymal stem cells in long-term dialysis patients display downregulation of PCAF expression and poor angiogenesis activation. PLoS One 9, e102311 (2014).
Malhotra, J. D. & Kaufman, R. J. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword. Antioxid. Redox Signal. 9, 2277–2293 (2007).
Cao, S. S. & Kaufman, R. J. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid. Redox Signal. 21, 396–413 (2014).
Cybulsky, A. V. Endoplasmic reticulum stress, the unfolded protein response and autophagy in kidney diseases. Nat. Rev. Nephrol. 13, 681–696 (2017).
Dvela-Levitt, M. et al. Small molecule targets TMED9 and promotes lysosomal degradation to reverse proteinopathy. Cell 178, 521–535.e3 (2019).
Yamashita, K. et al. Mitotic phosphorylation of Pex14p regulates peroxisomal import machinery. J. Cell Biol. 219, e202001003 (2020).
Okumoto, K. et al. The peroxisome counteracts oxidative stresses by suppressing catalase import via Pex14 phosphorylation. Elife 9, e55896 (2020).
Gulati, S. et al. Ischemia-reperfusion injury: biochemical alterations in peroxisomes of rat kidney. Arch. Biochem. Biophys. 295, 90–100 (1992).
Vasko, R. Peroxisomes and kidney injury. Antioxid. Redox Signal. 25, 217–231 (2016).
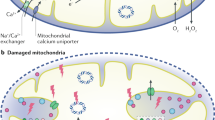
Al-Mehdi, A. B. et al. Perinuclear mitochondrial clustering creates an oxidant-rich nuclear domain required for hypoxia-induced transcription. Sci. Signal. 5, ra47 (2012).
Murphy, M. P. Modulating mitochondrial intracellular location as a redox signal. Sci. Signal. 5, pe39 (2012).
Van Houten, B., Woshner, V. & Santos, J. H. Role of mitochondrial DNA in toxic responses to oxidative stress. DNA Repair 5, 145–152 (2006).
Nadalutti, C. A., Ayala-Peña, S. & Santos, J. H. Mitochondrial DNA damage as driver of cellular outcomes. Am. J. Physiol. Cell Physiol. 322, C136–C150 (2022).
Nissanka, N. & Moraes, C. T. Mitochondrial DNA damage and reactive oxygen species in neurodegenerative disease. FEBS Lett. 592, 728–742 (2018).
Rottenberg, H. & Hoek, J. B. The path from mitochondrial ROS to aging runs through the mitochondrial permeability transition pore. Aging Cell 16, 943–955 (2017).
Lan, R. et al. Mitochondrial pathology and glycolytic shift during proximal tubule atrophy after ischemic AKI. J. Am. Soc. Nephrol. 27, 3356–3367 (2016).
Duann, P. & Lin, P. H. Mitochondria damage and kidney disease. Adv. Exp. Med. Biol. 982, 529–551 (2017).
Gohil, V. M. et al. Nutrient-sensitized screening for drugs that shift energy metabolism from mitochondrial respiration to glycolysis. Nat. Biotechnol. 28, 249–255 (2010).
Kishi, S. et al. Meclizine preconditioning protects the kidney against ischemia-reperfusion injury. EBioMedicine 2, 1090–1101 (2015).
Tang, C. et al. PINK1-PRKN/PARK2 pathway of mitophagy is activated to protect against renal ischemia-reperfusion injury. Autophagy 14, 880–897 (2018).
Hashizume, O. et al. Specific mitochondrial DNA mutation in mice regulates diabetes and lymphoma development. Proc. Natl Acad. Sci. USA 109, 10528–10533 (2012).
Dieter, B. P. et al. Novel therapies for diabetic kidney disease: storied past and forward paths. Diabetes Spectr. 28, 167–174 (2015).
Susztak, K., Raff, A. C., Schiffer, M. & Böttinger, E. P. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 55, 225–233 (2006).
Dugan, L. L. et al. AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. J. Clin. Invest. 123, 4888–4899 (2013).
Maremonti, F., Meyer, C. & Linkermann, A. Mechanisms and models of kidney tubular necrosis and nephron loss. J. Am. Soc. Nephrol. 33, 472–486 (2022).
Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012).
Friedmann Angeli, J. P. et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 16, 1180–1191 (2014).
Martin-Sanchez, D. et al. Ferroptosis, but not necroptosis, is important in nephrotoxic folic acid-induced AKI. J. Am. Soc. Nephrol. 28, 218–229 (2017).
Zeitler, L. et al. Anti-ferroptotic mechanism of IL4i1-mediated amino acid metabolism. Elife 10, e64806 (2021).
Mishima, E. et al. A non-canonical vitamin K cycle is a potent ferroptosis suppressor. Nature 608, 778–783 (2022).
Mishima, E. et al. Drugs repurposed as antiferroptosis agents suppress organ damage, including AKI, by functioning as lipid peroxyl radical scavengers. J. Am. Soc. Nephrol. 31, 280–296 (2020).
Yuan, J., Amin, P. & Ofengeim, D. Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases. Nat. Rev. Neurosci. 20, 19–33 (2019).
Deragon, M. A. et al. Mitochondrial ROS prime the hyperglycemic shift from apoptosis to necroptosis. Cell Death Discov. 6, 132 (2020).
Chen, H. et al. RIPK3-MLKL-mediated necroinflammation contributes to AKI progression to CKD. Cell Death Dis. 9, 878 (2018).
Collins, L. V., Hajizadeh, S., Holme, E., Jonsson, I. M. & Tarkowski, A. Endogenously oxidized mitochondrial DNA induces in vivo and in vitro inflammatory responses. J. Leukoc. Biol. 75, 995–1000 (2004).
Nakahira, K. et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol. 12, 222–230 (2011).
Shimada, K. et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36, 401–414 (2012).
West, A. P. & Shadel, G. S. Mitochondrial DNA in innate immune responses and inflammatory pathology. Nat. Rev. Immunol. 17, 363–375 (2017).
Chung, K. W. et al. Mitochondrial damage and activation of the sting pathway lead to renal inflammation and fibrosis. Cell Metab. 30, 784–799.e5 (2019).
Maekawa, H. et al. Mitochondrial damage causes inflammation via cGAS-STING signaling in acute kidney injury. Cell Rep. 29, 1261–1273.e6 (2019).
Janikiewicz, J. et al. Mitochondria-associated membranes in aging and senescence: structure, function, and dynamics. Cell Death Dis. 9, 332 (2018).
Yang, M. et al. DsbA-L ameliorates high glucose induced tubular damage through maintaining MAM integrity. EBioMedicine 43, 607–619 (2019).
Xue, M. et al. PACS-2 attenuates diabetic kidney disease via the enhancement of mitochondria-associated endoplasmic reticulum membrane formation. Cell Death Dis. 12, 1107 (2021).
Canaud, G. & Bonventre, J. V. Cell cycle arrest and the evolution of chronic kidney disease from acute kidney injury. Nephrol. Dial. Transpl. 30, 575–583 (2015).
He, W. et al. Wnt/β-catenin signaling promotes renal interstitial fibrosis. J. Am. Soc. Nephrol. 20, 765–776 (2009).
Liu, B. C., Tang, T. T., Lv, L. L. & Lan, H. Y. Renal tubule injury: a driving force toward chronic kidney disease. Kidney Int. 93, 568–579 (2018).
Steinhubl, S. R. Why have antioxidants failed in clinical trials? Am. J. Cardiol. 101, 14d–19d (2008).
Ardanaz, N. et al. Lack of glutathione peroxidase 1 accelerates cardiac-specific hypertrophy and dysfunction in angiotensin II hypertension. Hypertension 55, 116–123 (2010).
Daenen, K. et al. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 34, 975–991 (2019).
Griendling, K. K. et al. Oxidative stress and hypertension. Circ. Res. 128, 993–1020 (2021).
Te Riet, L., van Esch, J. H., Roks, A. J., van den Meiracker, A. H. & Danser, A. H. Hypertension: renin-angiotensin-aldosterone system alterations. Circ. Res. 116, 960–975 (2015).
Ruggenenti, P. et al. Preventing microalbuminuria in type 2 diabetes. N. Engl. J. Med. 351, 1941–1951 (2004).
Lewis, E. J. et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N. Engl. J. Med. 345, 851–860 (2001).
Brenner, B. M. et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 345, 861–869 (2001).
Nistala, R., Wei, Y., Sowers, J. R. & Whaley-Connell, A. Renin-angiotensin-aldosterone system-mediated redox effects in chronic kidney disease. Transl. Res. 153, 102–113 (2009).
Terami, N. et al. Long-term treatment with the sodium glucose cotransporter 2 inhibitor, dapagliflozin, ameliorates glucose homeostasis and diabetic nephropathy in db/db mice. PLoS ONE 9, e100777 (2014).
Novikov, A. et al. SGLT2 inhibition and renal urate excretion: role of luminal glucose, GLUT9, and URAT1. Am. J. Physiol. Ren. Physiol. 316, F173–F185 (2019).
Wilcox, C. S. Antihypertensive and renal mechanisms of SGLT2 (sodium-glucose linked transporter 2) inhibitors. Hypertension 75, 894–901 (2020).
Gager, G. M. et al. Effects of SGLT2 inhibitors on ion homeostasis and oxidative stress associated mechanisms in heart failure. Biomed. Pharmacother. 143, 112169 (2021).
Carlström, M., Wilcox, C. S. & Arendshorst, W. J. Renal autoregulation in health and disease. Physiol. Rev. 95, 405–511 (2015).
Yaribeygi, H., Butler, A. E., Atkin, S. L., Katsiki, N. & Sahebkar, A. Sodium-glucose cotransporter 2 inhibitors and inflammation in chronic kidney disease: possible molecular pathways. J. Cell Physiol. 234, 223–230 (2018).
Aroor, A. R. et al. Glycemic control by the SGLT2 inhibitor empagliflozin decreases aortic stiffness, renal resistivity index and kidney injury. Cardiovasc. Diabetol. 17, 108 (2018).
Lei, C. et al. Nitric oxide decreases acute kidney injury and stage 3 chronic kidney disease after cardiac surgery. Am. J. Respir. Crit. Care Med. 198, 1279–1287 (2018).
Marrazzo, F. et al. Protocol of a randomised controlled trial in cardiac surgical patients with endothelial dysfunction aimed to prevent postoperative acute kidney injury by administering nitric oxide gas. BMJ Open. 9, e026848 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02836899 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04022161 (2022).
Stasch, J. P., Schlossmann, J. & Hocher, B. Renal effects of soluble guanylate cyclase stimulators and activators: a review of the preclinical evidence. Curr. Opin. Pharmacol. 21, 95–104 (2015).
Hanrahan, J. P. et al. Effects of the soluble guanylate cyclase stimulator praliciguat in diabetic kidney disease: a randomized placebo-controlled clinical trial. Clin. J. Am. Soc. Nephrol. 16, 59–69 (2020).
Breyer, M. D. & Susztak, K. The next generation of therapeutics for chronic kidney disease. Nat. Rev. Drug. Discov. 15, 568–588 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT02156843 (2016).
Jha, J. C. et al. Genetic targeting or pharmacologic inhibition of NADPH oxidase Nox4 provides renoprotection in long-term diabetic nephropathy. J. Am. Soc. Nephrol. 25, 1237–1254 (2014).
Reutens, A. T. et al. A physician-initiated double-blind, randomised, placebo-controlled, phase 2 study evaluating the efficacy and safety of inhibition of NADPH oxidase with the first-in-class Nox-1/4 inhibitor, GKT137831, in adults with type 1 diabetes and persistently elevated urinary albumin excretion: protocol and statistical considerations. Contemp. Clin. Trials 90, 105892 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04534439 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05758896 (2023).
Tesch, G. H., Ma, F. Y. & Nikolic-Paterson, D. J. ASK1: a new therapeutic target for kidney disease. Am. J. Physiol. Ren. Physiol. 311, F373–F381 (2016).
Ma, F. Y., Tesch, G. H. & Nikolic-Paterson, D. J. ASK1/p38 signaling in renal tubular epithelial cells promotes renal fibrosis in the mouse obstructed kidney. Am. J. Physiol. Ren. Physiol. 307, F1263–F1273 (2014).
Terada, Y. et al. Important role of apoptosis signal-regulating kinase 1 in ischemic acute kidney injury. Biochem. Biophys. Res. Commun. 364, 1043–1049 (2007).
Liles, J. T. et al. ASK1 contributes to fibrosis and dysfunction in models of kidney disease. J. Clin. Invest. 128, 4485–4500 (2018).
Chertow, G. M. et al. Effects of selonsertib in patients with diabetic kidney disease. J. Am. Soc. Nephrol. 30, 1980–1990 (2019).
Mills, E. L. et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 556, 113–117 (2018).
Lampropoulou, V. et al. Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metab. 24, 158–166 (2016).
He, R. et al. Itaconate inhibits ferroptosis of macrophage via Nrf2 pathways against sepsis-induced acute lung injury. Cell Death Discov. 8, 43 (2022).
Hong, D. S. et al. A phase I first-in-human trial of bardoxolone methyl in patients with advanced solid tumors and lymphomas. Clin. Cancer Res. 18, 3396–3406 (2012).
Pergola, P. E. et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N. Engl. J. Med. 365, 327–336 (2011).
de Zeeuw, D. et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N. Engl. J. Med. 369, 2492–2503 (2013).
Nangaku, M. et al. Randomized clinical trial on the effect of bardoxolone methyl on GFR in diabetic kidney disease patients (TSUBAKI study). Kidney Int. Rep. 5, 879–890 (2020).
Nangaku, M. et al. Randomized, double-blind, placebo-controlled phase 3 study of bardoxolone methyl in patients with diabetic kidney disease: design and baseline characteristics of AYAME study. Nephrol. Dial. Transpl. 38, 1204–1216 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03019185 (2022).
Warady, B. A. et al. Effects of bardoxolone methyl in Alport syndrome. Clin. J. Am. Soc. Nephrol. 17, 1763–1774 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03918447 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03749447 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04702997 (2022).
Author information
Authors and Affiliations
Contributions
N.K. developed the article outline, and S.K. drafted the first version. All authors contributed to researching data for the article, participated in discussions about the content, and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
N.K. has received lecture fees from Astellas, AstraZeneca, Kyowa-Kirin, Novartis and Otsuka; research funding from AstraZeneca, Daiichi Sankyo, Kyowa-Kirin, Novartis and Otsuka; and has served as an adviser for Kyowa-Kirin and Novartis. The other authors declare no conflicts of interest.
Peer review
Peer review information
Nature Reviews Nephrology thanks Lin Sun, Mattias Carlstrom and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kishi, S., Nagasu, H., Kidokoro, K. et al. Oxidative stress and the role of redox signalling in chronic kidney disease. Nat Rev Nephrol 20, 101–119 (2024). https://doi.org/10.1038/s41581-023-00775-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-023-00775-0
This article is cited by
-
Impact of resistance exercise on patients with chronic kidney disease
BMC Nephrology (2024)
-
Targeting ferroptosis for treating kidney disease
Clinical and Experimental Nephrology (2024)