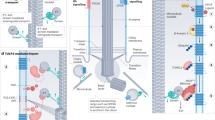
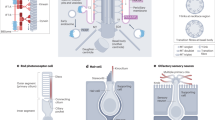
Abstract
Primary cilia act as cell surface antennae, coordinating cellular responses to sensory inputs and signalling molecules that regulate developmental and homeostatic pathways. Cilia are therefore critical to physiological processes, and defects in ciliary components are associated with a large group of inherited pleiotropic disorders — known collectively as ciliopathies — that have a broad spectrum of phenotypes and affect many or most tissues, including the kidney. A central feature of the cilium is its compartmentalized structure, which imparts its unique molecular composition and signalling environment despite its membrane and cytosol being contiguous with those of the cell. Such compartmentalization is achieved via active transport pathways that bring protein cargoes to and from the cilium, as well as gating pathways at the ciliary base that establish diffusion barriers to protein exchange into and out of the organelle. Many ciliopathy-linked proteins, including those involved in kidney development and homeostasis, are components of the compartmentalizing machinery. New insights into the major compartmentalizing pathways at the cilium, namely, ciliary gating, intraflagellar transport, lipidated protein flagellar transport and ciliary extracellular vesicle release pathways, have improved our understanding of the mechanisms that underpin ciliary disease and associated renal disorders.
Key points
-
Cilia at the cell plasma membrane serve motility, sensory and signalling roles that are essential for cell and tissue formation, homeostasis and function. Cilia defects cause ciliopathy disorders that affect many tissue types.
-
The unique and dynamic molecular composition of the ciliary organelle and its biochemically compartmentalized state are established and maintained via interconnected barrier and active transport systems.
-
The ciliary base, which encompasses the transition zone and basal body regions, and associated ‘gating’ complexes (MKS, NPHP, TAF and NUP modules), establish barriers to regulate the movement of cytosolic and membrane proteins into and out of cilia.
-
Membrane and cytosolic proteins move into and out of cilia by binding to cargo adaptor complexes (intraflagellar transport (IFT)-A, IFT-B and the BBSome) present on kinesin-2 and cytoplasmic dynein-driven IFT trains that run bidirectionally along ciliary microtubules.
-
Myristoyl-anchored and palmitoyl-anchored membrane proteins enter cilia via lipidated IFT, which involves shuttling carriers (UNC119B and PDE6D), cargo displacement factors (ARL3) and associated regulators (RP2 and ARL13B).
-
Extracellular vesicles that bud from the ciliary membrane regulate ciliary structure, composition and function by dispatching molecules and membrane from the organelle. Ciliary extracellular vesicles may serve as waste disposal and/or cell–cell communication devices.
-
Better knowledge of the ciliary compartmentalization pathways is essential for understanding mechanisms of ciliary disease such as the renal ciliopathies that include autosomal dominant polycystic kidney disease and nephronophthisis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Anvarian, Z., Mykytyn, K., Mukhopadhyay, S., Pedersen, L. B. & Christensen, S. T. Cellular signalling by primary cilia in development, organ function and disease. Nat. Rev. Nephrol. 15, 199–219 (2019).
Carrisoza-Gaytan, R., Carattino, M. D., Kleyman, T. R. & Satlin, L. M. An unexpected journey: conceptual evolution of mechanoregulated potassium transport in the distal nephron. Am. J. Physiol. Cell Physiol. 310, C243–C259 (2016).
Satir, P., Pedersen, L. B. & Christensen, S. T. The primary cilium at a glance. J. Cell Sci. 123, 499–503 (2010).
Benmerah, A. The ciliary pocket. Curr. Opin. Cell Biol. 25, 78–84 (2013).
Nachury, M. V. The molecular machines that traffic signaling receptors into and out of cilia. Curr. Opin. Cell Biol. 51, 124–131 (2018).
Garcia-Gonzalo, F. R. & Reiter, J. F. Open sesame: how transition fibers and the transition zone control ciliary composition. Cold Spring Harb. Perspect. Biol. 9, a028134 (2017).
Wingfield, J. L., Lechtreck, K.-F. & Lorentzen, E. Trafficking of ciliary membrane proteins by the intraflagellar transport/BBSome machinery. Essays Biochem. 62, 753–763 (2018).
Naharros, I. O. & Nachury, M. V. Shedding of ciliary vesicles at a glance. J. Cell Sci. 135, jcs246553 (2022).
Park, K. & Leroux, M. R. Composition, organization and mechanisms of the transition zone, a gate for the cilium. EMBO Rep. 23, e55420 (2022).
Carter, S. P. & Blacque, O. E. Membrane retrieval, recycling and release pathways that organise and sculpt the ciliary membrane. Curr. Opin. Cell Biol. 59, 133–139 (2019).
Long, H. & Huang, K. Transport of ciliary membrane proteins. Front. Cell Dev. Biol. 7, 381 (2020).
Waters, A. M. & Beales, P. L. Ciliopathies: an expanding disease spectrum. Pediatr. Nephrol. 26, 1039–1056 (2011).
Reiter, J. F. & Leroux, M. R. Genes and molecular pathways underpinning ciliopathies. Nat. Rev. Mol. Cell Biol. 18, 533–547 (2017).
Lambacher, N. J. et al. TMEM107 recruits ciliopathy proteins to subdomains of the ciliary transition zone and causes Joubert syndrome. Nat. Cell Biol. 18, 122–131 (2016).
Schouteden, C., Serwas, D., Palfy, M. & Dammermann, A. The ciliary transition zone functions in cell adhesion but is dispensable for axoneme assembly in C. elegans. J. Cell Biol. 210, 35–44 (2015).
Trépout, S., Tassin, A.-M., Marco, S. & Bastin, P. STEM tomography analysis of the trypanosome transition zone. J. Struct. Biol. 202, 51–60 (2018).
van den Hoek, H. et al. In situ architecture of the ciliary base reveals the stepwise assembly of intraflagellar transport trains. Science 377, 543–548 (2022).
Gilula, N. B. & Satir, P. The ciliary necklace. A ciliary membrane specialization. J. Cell Biol. 53, 494–509 (1972).
Kee, H. L. et al. A size-exclusion permeability barrier and nucleoporins characterize a ciliary pore complex that regulates transport into cilia. Nat. Cell Biol. 14, 431–437 (2012).
Breslow, D. K., Koslover, E. F., Seydel, F., Spakowitz, A. J. & Nachury, M. V. An in vitro assay for entry into cilia reveals unique properties of the soluble diffusion barrier. J. Cell Biol. 203, 129–147 (2013).
Endicott, S. J. & Brueckner, M. NUP98 sets the size-exclusion diffusion limit through the ciliary base. Curr. Biol. 28, 1643–1650 (2018).
Lin, Y.-C. et al. Chemically inducible diffusion trap at cilia reveals molecular sieve-like barrier. Nat. Chem. Biol. 9, 437–443 (2013).
Najafi, M., Maza, N. A. & Calvert, P. D. Steric volume exclusion sets soluble protein concentrations in photoreceptor sensory cilia. Proc. Natl Acad. Sci. USA 109, 203–208 (2012).
Takao, D. et al. An assay for clogging the ciliary pore complex distinguishes mechanisms of cytosolic and membrane protein entry. Curr. Biol. 24, 2288–2294 (2014).
Dishinger, J. F. et al. Ciliary entry of the kinesin-2 motor KIF17 is regulated by importin-beta2 and RanGTP. Nat. Cell Biol. 12, 703–710 (2010).
Hurd, T. W., Fan, S. & Margolis, B. L. Localization of retinitis pigmentosa 2 to cilia is regulated by Importin beta2. J. Cell Sci. 124, 718–726 (2011).
Han, Y. et al. Regulation of Gli ciliary localization and Hedgehog signaling by the PY-NLS/karyopherin-β2 nuclear import system. PLoS Biol. 15, e2002063 (2017).
Funabashi, T. et al. Ciliary entry of KIF17 is dependent on its binding to the IFT-B complex via IFT46-IFT56 as well as on its nuclear localization signal. Mol. Biol. Cell 28, 624–633 (2017).
Del Viso, F. et al. Congenital heart disease genetics uncovers context-dependent organization and function of nucleoporins at cilia. Dev. Cell 38, 478–492 (2016).
Endicott, S. J., Basu, B., Khokha, M. & Brueckner, M. The NIMA-like kinase Nek2 is a key switch balancing cilia biogenesis and resorption in the development of left-right asymmetry. Development 142, 4068–4079 (2015).
Takao, D., Wang, L., Boss, A. & Verhey, K. J. Protein interaction analysis provides a map of the spatial and temporal organization of the ciliary gating zone. Curr. Biol. 27, 2296–2306.e3 (2017).
Blasius, T. L., Takao, D. & Verhey, K. J. NPHP proteins are binding partners of nucleoporins at the base of the primary cilium. PLoS ONE 14, e0222924 (2019).
Garcia-Gonzalo, F. R. et al. A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nat. Genet. 43, 776–784 (2011).
Craige, B. et al. CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. J. Cell Biol. 190, 927–940 (2010).
Chih, B. et al. A ciliopathy complex at the transition zone protects the cilia as a privileged membrane domain. Nat. Cell Biol. 14, 61–72 (2011).
Williams, C. L. et al. MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis. J. Cell Biol. 192, 1023–1041 (2011).
Wang, L. et al. Ciliary transition zone proteins coordinate ciliary protein composition and ectosome shedding. Nat. Commun. 13, 3997 (2022).
Yinsheng, Z. et al. TMEM67 is required for the gating function of the transition zone that controls entry of membrane-associated proteins ARL13B and INPP5E into primary cilia. Biochem. Biophys. Res. Commun. 636, 162–169 (2022).
Cevik, S. et al. Active transport and diffusion barriers restrict Joubert syndrome-associated ARL13B/ARL-13 to an Inv-like ciliary membrane subdomain. PLoS Genet. 9, e1003977 (2013).
Jensen, V. L. et al. Formation of the transition zone by Mks5/Rpgrip1L establishes a ciliary zone of exclusion (CIZE) that compartmentalises ciliary signalling proteins and controls PIP2 ciliary abundance. EMBO J. 34, 2537–2556 (2015).
van Krugten, J., Danné, N. & Peterman, E. J. G. A local interplay between diffusion and intraflagellar transport distributes TRPV-channel OCR-2 along C. elegans chemosensory cilia. Commun. Biol. 5, 720 (2022).
Nechipurenko, I. V. The enigmatic role of lipids in cilia signaling. Front. Cell Dev. Biol. 8, 777 (2020).
Lin, H., Guo, S. & Dutcher, S. K. RPGRIP1L helps to establish the ciliary gate for entry of proteins. J. Cell Sci. 131, jcs220905 (2018).
Shi, X. et al. Super-resolution microscopy reveals that disruption of ciliary transition-zone architecture causes Joubert syndrome. Nat. Cell Biol. 19, 1178–1188 (2017).
Jana, S. C. et al. Differential regulation of transition zone and centriole proteins contributes to ciliary base diversity. Nat. Cell Biol. 20, 928–941 (2018).
Yang, T. T. et al. Superresolution pattern recognition reveals the architectural map of the ciliary transition zone. Sci. Rep. 5, 14096 (2015).
Weng, R. R. et al. Super-resolution imaging reveals TCTN2 depletion-induced IFT88 lumen leakage and ciliary weakening. Biophys. J. 115, 263–275 (2018).
Gogendeau, D. et al. MKS-NPHP module proteins control ciliary shedding at the transition zone. PLoS Biol. 18, e3000640 (2020).
Li, C. et al. MKS5 and CEP290 dependent assembly pathway of the ciliary transition zone. PLoS Biol. 14, e1002416 (2016).
Awata, J. et al. NPHP4 controls ciliary trafficking of membrane proteins and large soluble proteins at the transition zone. J. Cell Sci. 127, 4714–4727 (2014).
Leterrier, C. The axon initial segment: an updated viewpoint. J. Neurosci. 38, 2135–2145 (2018).
Heiman, M. G. & Shaham, S. DEX-1 and DYF-7 establish sensory dendrite length by anchoring dendritic tips during cell migration. Cell 137, 344–355 (2009).
Diener, D. R., Lupetti, P. & Rosenbaum, J. L. Proteomic analysis of isolated ciliary transition zones reveals the presence of ESCRT proteins. Curr. Biol. 25, 379–384 (2015).
Theerthagiri, G., Eisenhardt, N., Schwarz, H. & Antonin, W. The nucleoporin NUP188 controls passage of membrane proteins across the nuclear pore complex. J. Cell Biol. 189, 1129–1142 (2010).
Yang, T. T. et al. Super-resolution architecture of mammalian centriole distal appendages reveals distinct blade and matrix functional components. Nat. Commun. 9, 2023 (2018).
Yan, H. et al. TALPID3 and ANKRD26 selectively orchestrate FBF1 localization and cilia gating. Nat. Commun. 11, 2196 (2020).
Wei, Q. et al. The hydrolethalus syndrome protein HYLS-1 regulates formation of the ciliary gate. Nat. Commun. 7, 12437 (2016).
Hu, Q. et al. A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science 329, 436–439 (2010).
Kim, S. K. et al. Planar cell polarity acts through septins to control collective cell movement and ciliogenesis. Science 329, 1337–1340 (2010).
Ghossoub, R. et al. Septins 2, 7 and 9 and MAP4 colocalize along the axoneme in the primary cilium and control ciliary length. J. Cell Sci. 126, 2583–2594 (2013).
Kanamaru, T., Neuner, A., Kurtulmus, B. & Pereira, G. Balancing the length of the distal tip by septins is key for stability and signalling function of primary cilia. EMBO J. 41, e108843 (2022).
Fliegauf, M., Kahle, A., Häffner, K. & Zieger, B. Distinct localization of septin proteins to ciliary sub-compartments in airway epithelial cells. Biol. Chem. 395, 151–156 (2014).
Palander, O., El-Zeiry, M. & Trimble, W. S. Uncovering the roles of septins in cilia. Front. Cell Dev. Biol. 5, 36 (2017).
Kaplan, O. I. et al. Endocytosis genes facilitate protein and membrane transport in C. elegans sensory cilia. Curr. Biol. 22, 451–460 (2012).
Clement, C. A. et al. TGF-β signaling is associated with endocytosis at the pocket region of the primary cilium. Cell Rep. 3, 1806–1814 (2013).
Cheng, H. et al. Actin filaments form a size-dependent diffusion barrier around centrosomes. EMBO Rep. 24, e54935 (2023).
Kozminski, K. G., Johnson, K. A., Forscher, P. & Rosenbaum, J. L. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc. Natl Acad. Sci. USA 90, 5519–5523 (1993).
van Dam, T. J. P. et al. Evolution of modular intraflagellar transport from a coatomer-like progenitor. Proc. Natl Acad. Sci. USA 110, 6943–6948 (2013).
Kozminski, K. G., Beech, P. L. & Rosenbaum, J. L. The Chlamydomonas kinesin-like protein FLA10 is involved in motility associated with the flagellar membrane. J. Cell Biol. 131, 1517–1527 (1995).
Pazour, G. J., Dickert, B. L. & Witman, G. B. The DHC1b (DHC2) isoform of cytoplasmic dynein is required for flagellar assembly. J. Cell Biol. 144, 473–481 (1999).
Signor, D. et al. Role of a class DHC1b dynein in retrograde transport of IFT motors and IFT raft particles along cilia, but not dendrites, in chemosensory neurons of living Caenorhabditis elegans. J. Cell Biol. 147, 519–530 (1999).
Vuolo, L., Stevenson, N. L., Mukhopadhyay, A. G., Roberts, A. J. & Stephens, D. J. Cytoplasmic dynein-2 at a glance. J. Cell Sci. 133, jcs240614 (2020).
Cole, D. G. et al. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J. Cell Biol. 141, 993–1008 (1998).
Taschner, M. & Lorentzen, E. The intraflagellar transport machinery. Cold Spring Harb. Perspect. Biol. 8, a028092 (2016).
Nachury, M. V. et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 129, 1201–1213 (2007).
Snow, J. J. et al. Two anterograde intraflagellar transport motors cooperate to build sensory cilia on C. elegans neurons. Nat. Cell Biol. 6, 1109–1113 (2004).
Juhl, A. D. et al. Transient accumulation and bidirectional movement of KIF13B in primary cilia. J. Cell Sci. 136, jcs259257 (2023).
Peden, E. M. & Barr, M. M. The KLP-6 kinesin is required for male mating behaviors and polycystin localization in Caenorhabditis elegans. Curr. Biol. 15, 394–404 (2005).
Wachter, S. et al. Binding of IFT22 to the intraflagellar transport complex is essential for flagellum assembly. EMBO J. 38, e101251 (2019).
Bhogaraju, S. et al. Molecular basis of tubulin transport within the cilium by IFT74 and IFT81. Science 341, 1009–1012 (2013).
Taschner, M. et al. Intraflagellar transport proteins 172, 80, 57, 54, 38, and 20 form a stable tubulin‐binding IFT‐B2 complex. EMBO J. 35, 773–790 (2016).
Lacey, S. E., Foster, H. E. & Pigino, G. The molecular structure of IFT-A and IFT-B in anterograde intraflagellar transport trains. Nat. Struct. Mol. Biol. 30, 584–593 (2023).
Petriman, N. A. et al. Biochemically validated structural model of the 15-subunit intraflagellar transport complex IFT-B. EMBO J. 41, e112440 (2022).
McCafferty, C. L. et al. Integrative modeling reveals the molecular architecture of the intraflagellar transport A (IFT-A) complex. eLife 11, e81977 (2022).
Hesketh, S. J., Mukhopadhyay, A. G., Nakamura, D., Toropova, K. & Roberts, A. J. IFT-A structure reveals carriages for membrane protein transport into cilia. Cell 185, 4971–4985 (2022).
Meleppattu, S., Zhou, H., Dai, J., Gui, M. & Brown, A. Mechanism of IFT-A polymerization into trains for ciliary transport. Cell 185, 4986–4998 (2022).
Jiang, M. et al. Human IFT-A complex structures provide molecular insights into ciliary transport. Cell Res. 33, 288–298 (2023).
Ma, Y. et al. Structural insight into the intraflagellar transport complex IFT-A and its assembly in the anterograde IFT train. Nat. Commun. 14, 1506 (2023).
Chou, H.-T. et al. The molecular architecture of native BBSome obtained by an integrated structural approach. Structure 27, 1384–1394 (2019).
Yang, S. et al. Near-atomic structures of the BBSome reveal the basis for BBSome activation and binding to GPCR cargoes. eLife 9, e55954 (2020).
Klink, B. U., Gatsogiannis, C., Hofnagel, O., Wittinghofer, A. & Raunser, S. Structure of the human BBSome core complex. eLife 9, e53910 (2020).
Kobayashi, T., Ishida, Y., Hirano, T., Katoh, Y. & Nakayama, K. Cooperation of the IFT-A complex with the IFT-B complex is required for ciliary retrograde protein trafficking and GPCR import. Mol. Biol. Cell 32, 45–56 (2021).
Nozaki, S., Castro Araya, R. F., Katoh, Y. & Nakayama, K. Requirement of IFT-B-BBSome complex interaction in export of GPR161 from cilia. Biol. Open. 8, bio043786 (2019).
Funabashi, T., Katoh, Y., Okazaki, M., Sugawa, M. & Nakayama, K. Interaction of heterotrimeric kinesin-II with IFT-B-connecting tetramer is crucial for ciliogenesis. J. Cell Biol. 217, 2867–2876 (2018).
Jordan, M. A., Diener, D. R., Stepanek, L. & Pigino, G. The cryo-EM structure of intraflagellar transport trains reveals how dynein is inactivated to ensure unidirectional anterograde movement in cilia. Nat. Cell Biol. 20, 1250–1255 (2018).
Kiesel, P. et al. The molecular structure of mammalian primary cilia revealed by cryo-electron tomography. Nat. Struct. Mol. Biol. 27, 1115–1124 (2020).
Toropova, K. et al. Structure of the dynein-2 complex and its assembly with intraflagellar transport trains. Nat. Struct. Mol. Biol. 26, 823–829 (2019).
Williams, C. L. et al. Direct evidence for BBSome-associated intraflagellar transport reveals distinct properties of native mammalian cilia. Nat. Commun. 5, 5813 (2014).
Lechtreck, K.-F. et al. The Chlamydomonas reinhardtii BBSome is an IFT cargo required for export of specific signaling proteins from flagella. J. Cell Biol. 187, 1117–1132 (2009).
Stepanek, L. & Pigino, G. Microtubule doublets are double-track railways for intraflagellar transport trains. Science 352, 721–724 (2016).
Kuhns, S. & Blacque, O. E. Cilia train spotting. Dev. Cell 37, 395–396 (2016).
Bertiaux, E. et al. Bidirectional intraflagellar transport is restricted to two sets of microtubule doublets in the trypanosome flagellum. J. Cell Biol. 217, 4284–4297 (2018).
Mallet, A. & Bastin, P. Restriction of intraflagellar transport to some microtubule doublets: an opportunity for cilia diversification? Bioessays 44, e2200031 (2022).
Kimura, Y. et al. Environmental responsiveness of tubulin glutamylation in sensory cilia is regulated by the p38 MAPK pathway. Sci. Rep. 8, 8392 (2018).
O’Hagan, R. et al. The tubulin deglutamylase CCPP-1 regulates the function and stability of sensory cilia in C. elegans. Curr. Biol. 21, 1685–1694 (2011).
Wingfield, J. L. et al. IFT trains in different stages of assembly queue at the ciliary base for consecutive release into the cilium. eLife 6, e26609 (2017).
Buisson, J. et al. Intraflagellar transport proteins cycle between the flagellum and its base. J. Cell Sci. 126, 327–338 (2013).
Mijalkovic, J., Prevo, B., Oswald, F., Mangeol, P. & Peterman, E. J. G. Ensemble and single-molecule dynamics of IFT dynein in Caenorhabditis elegans cilia. Nat. Commun. 8, 14591 (2017).
Prevo, B., Mangeol, P., Oswald, F., Scholey, J. M. & Peterman, E. J. G. Functional differentiation of cooperating kinesin-2 motors orchestrates cargo import and transport in C. elegans cilia. Nat. Cell Biol. 17, 1536–1545 (2015).
Yi, P., Li, W.-J., Dong, M.-Q. & Ou, G. Dynein-driven retrograde intraflagellar transport is triphasic in C. elegans sensory cilia. Curr. Biol. 27, 1448–1461 (2017).
Hirano, T., Katoh, Y. & Nakayama, K. Intraflagellar transport-A complex mediates ciliary entry and retrograde trafficking of ciliary G protein-coupled receptors. Mol. Biol. Cell 28, 429–439 (2017).
Blacque, O. E., Cevik, S. & Kaplan, O. I. Intraflagellar transport: from molecular characterisation to mechanism. Front. Biosci. 13, 2633–2652 (2008).
Oswald, F., Prevo, B., Acar, S. & Peterman, E. J. G. Interplay between ciliary ultrastructure and IFT-train dynamics revealed by single-molecule super-resolution imaging. Cell Rep. 25, 224–235 (2018).
De-Castro, A. R. G. et al. WDR60-mediated dynein-2 loading into cilia powers retrograde IFT and transition zone crossing. J. Cell Biol. 221, e202010178 (2022).
Scheidel, N. & Blacque, O. E. Intraflagellar transport complex A genes differentially regulate cilium formation and transition zone gating. Curr. Biol. 28, 3279–3287 (2018).
Jensen, V. L. et al. Role for intraflagellar transport in building a functional transition zone. EMBO Rep. 19, e45862 (2018).
Vuolo, L., Stevenson, N. L., Heesom, K. J. & Stephens, D. J. Dynein-2 intermediate chains play crucial but distinct roles in primary cilia formation and function. eLife 7, e39655 (2018).
Zhang, Z., Danné, N., Meddens, B., Heller, I. & Peterman, E. J. G. Direct imaging of intraflagellar-transport turnarounds reveals that motors detach, diffuse, and reattach to opposite-direction trains. Proc. Natl Acad. Sci. USA 118, e2115089118 (2021).
Jiang, L. et al. Kinesin family 17 (osmotic avoidance abnormal-3) is dispensable for photoreceptor morphology and function. FASEB J. 29, 4866–4880 (2015).
Wingfield, J. L. et al. In vivo imaging shows continued association of several IFT A, B and dynein complexes while IFT trains U-turn at the tip. J. Cell Sci. 134, jcs259010 (2021).
Chien, A. et al. Dynamics of the IFT machinery at the ciliary tip. eLife 6, 28606 (2017).
Mijalkovic, J., van Krugten, J., Oswald, F., Acar, S. & Peterman, E. J. G. Single-molecule turnarounds of intraflagellar transport at the C. elegans ciliary tip. Cell Rep. 25, 1701–1707 (2018).
Engel, B. D. et al. The role of retrograde intraflagellar transport in flagellar assembly, maintenance, and function. J. Cell Biol. 199, 151–167 (2012).
Nakamura, K. et al. Anterograde trafficking of ciliary MAP kinase-like ICK/CILK1 by the intraflagellar transport machinery is required for intraciliary retrograde protein trafficking. J. Biol. Chem. 295, 13363–13376 (2020).
Chaya, T., Omori, Y., Kuwahara, R. & Furukawa, T. ICK is essential for cell type-specific ciliogenesis and the regulation of ciliary transport. EMBO J. 33, 1227–1242 (2014).
Satoda, Y. et al. BROMI/TBC1D32 together with CCRK/CDK20 and FAM149B1/JBTS36 contributes to intraflagellar transport turnaround involving ICK/CILK1. Mol. Biol. Cell 33, ar79 (2022).
Jin, H. et al. The conserved Bardet–Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell 141, 1208–1219 (2010).
Nievergelt, A. P. et al. Conversion of anterograde into retrograde trains is an intrinsic property of intraflagellar transport. Curr. Biol. 32, 4071–4078 (2022).
Lechtreck, K. F. et al. ARMC2/PF27 is an obligate cargo adapter for intraflagellar transport of radial spokes. eLife 11, e74993 (2022).
Craft, J. M., Harris, J. A., Hyman, S., Kner, P. & Lechtreck, K. F. Tubulin transport by IFT is upregulated during ciliary growth by a cilium-autonomous mechanism. J. Cell Biol. 208, 223–237 (2015).
Hao, L. et al. Intraflagellar transport delivers tubulin isotypes to sensory cilium middle and distal segments. Nat. Cell Biol. 13, 790–798 (2011).
Wren, K. N. et al. A differential cargo-loading model of ciliary length regulation by IFT. Curr. Biol. 23, 2463–2471 (2013).
Dai, J., Barbieri, F., Mitchell, D. R. & Lechtreck, K. F. In vivo analysis of outer arm dynein transport reveals cargo-specific intraflagellar transport properties. Mol. Biol. Cell 29, 2553–2565 (2018).
Lechtreck, K. F., Mengoni, I., Okivie, B. & Hilderhoff, K. B. In vivo analyses of radial spoke transport, assembly, repair and maintenance. Cytoskeleton 75, 352–362 (2018).
Ye, F., Nager, A. R. & Nachury, M. V. BBSome trains remove activated GPCRs from cilia by enabling passage through the transition zone. J. Cell Biol. 217, 1847–1868 (2018).
Lechtreck, K. F. et al. Chlamydomonas ARMC2/PF27 is an obligate cargo adapter for intraflagellar transport of radial spokes. eLife 11, e74993 (2022).
Ye, F. et al. Single molecule imaging reveals a major role for diffusion in the exploration of ciliary space by signaling receptors. eLife 2, e00654 (2013).
Milenkovic, L. et al. Single-molecule imaging of Hedgehog pathway protein Smoothened in primary cilia reveals binding events regulated by Patched1. Proc. Natl Acad. Sci. USA 112, 8320–8325 (2015).
Weiss, L. E., Milenkovic, L., Yoon, J., Stearns, T. & Moerner, W. E. Motional dynamics of single patched1 molecules in cilia are controlled by Hedgehog and cholesterol. Proc. Natl Acad. Sci. USA 116, 5550–5557 (2019).
Mukhopadhyay, S. et al. TULP3 bridges the IFT-A complex and membrane phosphoinositides to promote trafficking of G protein-coupled receptors into primary cilia. Genes. Dev. 24, 2180–2193 (2010).
Badgandi, H. B., Hwang, S.-H., Shimada, I. S., Loriot, E. & Mukhopadhyay, S. Tubby family proteins are adapters for ciliary trafficking of integral membrane proteins. J. Cell Biol. 216, 743–760 (2017).
Nager, A. R. et al. An actin network dispatches ciliary GPCRs into extracellular vesicles to modulate signaling. Cell 168, 252–263 (2017).
Mukhopadhyay, S. et al. The ciliary G-protein-coupled receptor Gpr161 negatively regulates the Sonic hedgehog pathway via cAMP signaling. Cell 152, 210–223 (2013).
Eguether, T. et al. IFT27 links the BBSome to IFT for maintenance of the ciliary signaling compartment. Dev. Cell 31, 279–290 (2014).
Liu, Y.-X. et al. Bardet–Biedl syndrome 3 protein promotes ciliary exit of the signaling protein phospholipase D via the BBSome. eLife 10, e59119 (2021).
Lechtreck, K. F. et al. Cycling of the signaling protein phospholipase D through cilia requires the BBSome only for the export phase. J. Cell Biol. 201, 249–261 (2013).
Hou, Y. & Witman, G. B. The N-terminus of IFT46 mediates intraflagellar transport of outer arm dynein and its cargo-adaptor ODA16. Mol. Biol. Cell 28, 2420–2433 (2017).
Klink, B. U. et al. A recombinant BBSome core complex and how it interacts with ciliary cargo. eLife 6, e27434 (2017).
Jiang, X. et al. DYF-5/MAK-dependent phosphorylation promotes ciliary tubulin unloading. Proc. Natl Acad. Sci. USA 119, e2207134119 (2022).
Craft Van De Weghe, J., Harris, J. A., Kubo, T., Witman, G. B. & Lechtreck, K. F. Diffusion rather than intraflagellar transport likely provides most of the tubulin required for axonemal assembly in Chlamydomonas. J. Cell Sci. 133, jcs249805 (2020).
Belzile, O., Hernandez-Lara, C. I., Wang, Q. & Snell, W. J. Regulated membrane protein entry into flagella is facilitated by cytoplasmic microtubules and does not require IFT. Curr. Biol. 23, 1460–1465 (2013).
Hao, K., Chen, Y., Yan, X. & Zhu, X. Cilia locally synthesize proteins to sustain their ultrastructure and functions. Nat. Commun. 12, 6971 (2021).
Quidwai, T. et al. A WDR35-dependent coat protein complex transports ciliary membrane cargo vesicles to cilia. eLife 10, e69786 (2021).
Wu, D. et al. Ciliogenesis requires sphingolipid-dependent membrane and axoneme interaction. Proc. Natl Acad. Sci. USA 119, e2201096119 (2022).
Jékely, G. & Arendt, D. Evolution of intraflagellar transport from coated vesicles and autogenous origin of the eukaryotic cilium. Bioessays 28, 191–198 (2006).
Jensen, V. L. & Leroux, M. R. Gates for soluble and membrane proteins, and two trafficking systems (IFT and LIFT), establish a dynamic ciliary signaling compartment. Curr. Opin. Cell Biol. 47, 83–91 (2017).
Gotthardt, K. et al. A G-protein activation cascade from Arl13B to Arl3 and implications for ciliary targeting of lipidated proteins. eLife 4, e11859 (2015).
Ismail, S. A. et al. Structural basis for Arl3-specific release of myristoylated ciliary cargo from UNC119. EMBO J. 31, 4085–4094 (2012).
Jaiswal, M. et al. Novel biochemical and structural insights into the interaction of myristoylated cargo with Unc119 protein and their release by Arl2/3. J. Biol. Chem. 291, 20766–20778 (2016).
Wright, K. J. et al. An ARL3-UNC119-RP2 GTPase cycle targets myristoylated NPHP3 to the primary cilium. Genes Dev. 25, 2347–2360 (2011).
Humbert, M. C. et al. ARL13B, PDE6D, and CEP164 form a functional network for INPP5E ciliary targeting. Proc. Natl Acad. Sci. USA 109, 19691–19696 (2012).
Farazi, T. A., Waksman, G. & Gordon, J. I. The biology and enzymology of protein N-myristoylation. J. Biol. Chem. 276, 39501–39504 (2001).
Wang, M. & Casey, P. J. Protein prenylation: unique fats make their mark on biology. Nat. Rev. Mol. Cell Biol. 17, 110–122 (2016).
Zhang, Q. et al. GTP-binding of ARL-3 is activated by ARL-13 as a GEF and stabilized by UNC-119. Sci. Rep. 6, 24534 (2016).
Thomas, S. et al. A homozygous PDE6D mutation in Joubert syndrome impairs targeting of farnesylated INPP5E protein to the primary cilium. Hum. Mutat. 35, 137–146 (2014).
Fansa, E. K., Kösling, S. K., Zent, E., Wittinghofer, A. & Ismail, S. PDE6δ-mediated sorting of INPP5E into the cilium is determined by cargo-carrier affinity. Nat. Commun. 7, 11366 (2016).
Akella, J. S. et al. Ciliary Rab28 and the BBSome negatively regulate extracellular vesicle shedding. eLife 9, e50580 (2020).
Carter, S. P. et al. Genetic deletion of zebrafish Rab28 causes defective outer segment shedding, but not retinal degeneration. Front. Cell Dev. Biol. 8, 136 (2020).
Ying, G. et al. The small GTPase RAB28 is required for phagocytosis of cone outer segments by the murine retinal pigmented epithelium. J. Biol. Chem. 293, 17546–17558 (2018).
Dutta, N. & Seo, S. RPGR, a prenylated retinal ciliopathy protein, is targeted to cilia in a prenylation- and PDE6D-dependent manner. Biol. Open 5, 1283–1289 (2016).
Zhang, H. et al. Deletion of PrBP/delta impedes transport of GRK1 and PDE6 catalytic subunits to photoreceptor outer segments. Proc. Natl Acad. Sci. USA 104, 8857–8862 (2007).
Faber, S. et al. PDE6D mediates trafficking of prenylated proteins NIM1K and UBL3 to primary cilia. Cells 12, 312 (2023).
Zhang, H. et al. UNC119 is required for G protein trafficking in sensory neurons. Nat. Neurosci. 14, 874–880 (2011).
Pandey, M., Huang, Y., Lim, T. K., Lin, Q. & He, C. Y. Flagellar targeting of an arginine kinase requires a conserved lipidated protein intraflagellar transport (LIFT) pathway in Trypanosoma brucei. J. Biol. Chem. 295, 11326–11336 (2020).
Stephen, L. A. & Ismail, S. Shuttling and sorting lipid-modified cargo into the cilia. Biochem. Soc. Trans. 44, 1273–1280 (2016).
Hanzal-Bayer, M. The complex of Arl2-GTP and PDEdelta: from structure to function. EMBO J. 21, 2095–2106 (2002).
Chandra, A. et al. The GDI-like solubilizing factor PDEδ sustains the spatial organization and signalling of Ras family proteins. Nat. Cell Biol. 14, 148–158 (2012).
Yelland, T., Garcia, E., Samarakoon, Y. & Ismail, S. The structural and biochemical characterization of UNC119B cargo binding and release mechanisms. Biochemistry 60, 1952–1963 (2021).
Grayson, C. Localization in the human retina of the X-linked retinitis pigmentosa protein RP2, its homologue cofactor C and the RP2 interacting protein Arl3. Hum. Mol. Genet. 11, 3065–3074 (2002).
Lokaj, M. et al. The interaction of CCDC104/BARTL1 with Arl3 and implications for ciliary function. Structure 23, 2122–2132 (2015).
Cevik, S. et al. Joubert syndrome Arl13b functions at ciliary membranes and stabilizes protein transport in Caenorhabditis elegans. J. Cell Biol. 188, 953–969 (2010).
Kühnel, K., Veltel, S., Schlichting, I. & Wittinghofer, A. Crystal structure of the human retinitis pigmentosa 2 protein and its interaction with Arl3. Structure 14, 367–378 (2006).
Evans, R. J. et al. The retinitis pigmentosa protein RP2 links pericentriolar vesicle transport between the Golgi and the primary cilium. Hum. Mol. Genet. 19, 1358–1367 (2010).
ElMaghloob, Y. et al. ARL3 activation requires the co-GEF BART and effector-mediated turnover. eLife 10, e64624 (2021).
Zhang, R.-K. et al. RABL2 promotes the outward transition zone passage of signaling proteins in cilia via ARL3. Proc. Natl Acad. Sci. USA 120, e2302603120 (2023).
Nozaki, S. et al. Regulation of ciliary retrograde protein trafficking by the Joubert syndrome proteins ARL13B and INPP5E. J. Cell Sci. 130, 563–576 (2017).
Jensen, V. L. et al. Whole-organism developmental expression profiling identifies RAB-28 as a novel ciliary GTPase associated with the BBSome and intraflagellar transport. PLoS Genet. 12, e1006469 (2016).
Kösling, S. K., Fansa, E. K., Maffini, S. & Wittinghofer, A. Mechanism and dynamics of INPP5E transport into and inside the ciliary compartment. Biol. Chem. 399, 277–292 (2018).
Wang, J. & Barr, M. M. Cell–cell communication via ciliary extracellular vesicles: clues from model systems. Essays Biochem. 62, 205–213 (2018).
Phua, S. C. et al. Dynamic remodeling of membrane composition drives cell cycle through primary cilia excision. Cell 168, 264–279 (2017).
Wang, J. et al. C. elegans ciliated sensory neurons release extracellular vesicles that function in animal communication. Curr. Biol. 24, 519–525 (2014).
Dentler, W. A role for the membrane in regulating Chlamydomonas flagellar length. PLoS ONE 8, e53366 (2013).
Wood, C. R., Huang, K., Diener, D. R. & Rosenbaum, J. L. The cilium secretes bioactive ectosomes. Curr. Biol. 23, 906–911 (2013).
Hoang-Minh, L. B., Dutra-Clarke, M., Breunig, J. J. & Sarkisian, M. R. Glioma cell proliferation is enhanced in the presence of tumor-derived cilia vesicles. Cilia 7, 6 (2018).
Long, H. et al. Comparative analysis of ciliary membranes and ectosomes. Curr. Biol. 26, 3327–3335 (2016).
Wang, J. et al. Sensory cilia act as a specialized venue for regulated extracellular vesicle biogenesis and signaling. Curr. Biol. 31, 3943–3951 (2021).
Bergman, K., Goodenough, U. W., Goodenough, D. A., Jawitz, J. & Martin, H. Gametic differentiation in Chlamydomonas reinhardtii. II. Flagellar membranes and the agglutination reaction. J. Cell Biol. 67, 606–622 (1975).
Wang, G. et al. Rab7 regulates primary cilia disassembly through cilia excision. J. Cell Biol. 218, 4030–4041 (2019).
Cocucci, E. & Meldolesi, J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol. 25, 364–372 (2015).
Volz, A.-K. et al. Bardet–Biedl syndrome proteins modulate the release of bioactive extracellular vesicles. Nat. Commun. 12, 5671 (2021).
Razzauti, A. & Laurent, P. Ectocytosis prevents accumulation of ciliary cargo in sensory neurons. eLife 10, e67670 (2021).
Clupper, M. et al. Kinesin-2 motors differentially impact biogenesis of extracellular vesicle subpopulations shed from sensory cilia. iScience 25, 105262 (2022).
Maguire, J. E. et al. Myristoylated CIL-7 regulates ciliary extracellular vesicle biogenesis. Mol. Biol. Cell 26, 2823–2832 (2015).
Nikonorova, I. A. et al. Isolation, profiling, and tracking of extracellular vesicle cargo in Caenorhabditis elegans. Curr. Biol. 32, 1924–1936 (2022).
Mohieldin, A. M. et al. Ciliary extracellular vesicles are distinct from the cytosolic extracellular vesicles. J. Extracell. Vesicles 10, e12086 (2021).
Salinas, R. Y. et al. Photoreceptor discs form through peripherin-dependent suppression of ciliary ectosome release. J. Cell Biol. 216, 1489–1499 (2017).
Meldolesi, J. Exosomes and ectosomes in intercellular communication. Curr. Biol. 28, R435–R444 (2018).
Stilling, S., Kalliakoudas, T., Benninghoven-Frey, H., Inoue, T. & Falkenburger, B. H. PIP2 determines length and stability of primary cilia by balancing membrane turnovers. Commun. Biol. 5, 1–15 (2022).
Silva, M. et al. Cell-specific α-tubulin isotype regulates ciliary microtubule ultrastructure, intraflagellar transport, and extracellular vesicle biology. Curr. Biol. 27, 968–980 (2017).
Akella, J. S. & Barr, M. M. The tubulin code specializes neuronal cilia for extracellular vesicle release. Dev. Neurobiol. 81, 231–252 (2020).
Patel, V., Chowdhury, R. & Igarashi, P. Advances in the pathogenesis and treatment of polycystic kidney disease. Curr. Opin. Nephrol. Hyper. 18, 99–106 (2009).
Rozycki, M. et al. The fate of the primary cilium during myofibroblast transition. Mol. Biol. Cell 25, 643–657 (2014).
Pazour, G. J. et al. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J. Cell Biol. 151, 709–718 (2000).
Barr, M. M. & Sternberg, P. W. A polycystic kidney-disease gene homologue required for male mating behaviour in C. elegans. Nature 401, 386–389 (1999).
Pazour, G. J., San Agustin, J. T., Follit, J. A., Rosenbaum, J. L. & Witman, G. B. Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr. Biol. 12, R378–80 (2002).
Yoder, B. K., Hou, X. & Guay-Woodford, L. M. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J. Am. Soc. Nephrol. 13, 2508–2516 (2002).
Bergmann, C. et al. Polycystic kidney disease. Nat. Rev. Dis. Prim. 4, 50 (2018).
Ward, C. J. et al. Cellular and subcellular localization of the ARPKD protein; fibrocystin is expressed on primary cilia. Hum. Mol. Genet. 12, 2703–2710 (2003).
Lu, H. et al. Mutations in DZIP1L, which encodes a ciliary-transition-zone protein, cause autosomal recessive polycystic kidney disease. Nat. Genet. 49, 1025–1034 (2017).
Su, Q. et al. Structure of the human PKD1–PKD2 complex. Science 361, eaat9519 (2018).
Maser, R. L., Calvet, J. P. & Parnell, S. C. The GPCR properties of polycystin-1 — a new paradigm. Front. Mol. Biosci. 9, 1035507 (2022).
Liu, X. et al. Polycystin-2 is an essential ion channel subunit in the primary cilium of the renal collecting duct epithelium. eLife 7, e33183 (2018).
Wang, Z. et al. The ion channel function of polycystin-1 in the polycystin-1/polycystin-2 complex. EMBO Rep. 20, e48336 (2019).
Fedeles, S. V. et al. A genetic interaction network of five genes for human polycystic kidney and liver diseases defines polycystin-1 as the central determinant of cyst formation. Nat. Genet. 43, 639–647 (2011).
Walker, R. V. et al. Ciliary exclusion of polycystin-2 promotes kidney cystogenesis in an autosomal dominant polycystic kidney disease model. Nat. Commun. 10, 4072 (2019).
Wang, W. et al. Ttc21b deficiency attenuates autosomal dominant polycystic kidney disease in a kidney tubular- and maturation-dependent manner. Kidney Int. 102, 577–591 (2022).
Gerakopoulos, V., Ngo, P. & Tsiokas, L. Loss of polycystins suppresses deciliation via the activation of the centrosomal integrity pathway. Life Sci. Alliance 3, e202000750 (2020).
Hildebrandt, F. et al. A novel gene encoding an SH3 domain protein is mutated in nephronophthisis type 1. Nat. Genet. 17, 149–153 (1997).
Fliegauf, M. et al. Nephrocystin specifically localizes to the transition zone of renal and respiratory cilia and photoreceptor connecting cilia. J. Am. Soc. Nephrol. 17, 2424–2433 (2006).
Halbritter, J. et al. Identification of 99 novel mutations in a worldwide cohort of 1,056 patients with a nephronophthisis-related ciliopathy. Hum. Genet. 132, 865–884 (2013).
Luo, F. & Tao, Y.-H. Nephronophthisis: a review of genotype–phenotype correlation. Nephrology 23, 904–911 (2018).
Nürnberger, J., Bacallao, R. L. & Phillips, C. L. Inversin forms a complex with catenins and N-cadherin in polarized epithelial cells. Mol. Biol. Cell 13, 3096–3106 (2002).
Donaldson, J. C. et al. Crk-associated substrate p130Cas interacts with nephrocystin and both proteins localize to cell–cell contacts of polarized epithelial cells. Exper. Cell Res. 256, 168–178 (2000).
Choi, H. J. C. et al. NEK8 links the ATR-regulated replication stress response and S phase CDK activity to renal ciliopathies. Mol. Cell 51, 423–439 (2013).
Putoux, A., Attie-Bitach, T., Martinovic, J. & Gubler, M.-C. Phenotypic variability of Bardet–Biedl syndrome: focusing on the kidney. Pediatr. Nephrol. 27, 7–15 (2012).
Forsythe, E. et al. Risk factors for severe renal disease in Bardet–Biedl syndrome. J. Am. Soc. Nephrol. 28, 963–970 (2017).
Zaghloul, N. A. & Katsanis, N. Mechanistic insights into Bardet–Biedl syndrome, a model ciliopathy. J. Clin. Invest. 119, 428–437 (2009).
Davis, E. E. et al. TTC21B contributes both causal and modifying alleles across the ciliopathy spectrum. Nat. Genet. 43, 189–196 (2011).
Halbritter, J. et al. Defects in the IFT-B component IFT172 cause Jeune and Mainzer–Saldino syndromes in humans. Am. J. Hum. Genet. 93, 915–925 (2013).
Senum, S. R. et al. Monoallelic IFT140 pathogenic variants are an important cause of the autosomal dominant polycystic kidney-spectrum phenotype. Am. J. Hum. Genet. 109, 136–156 (2022).
Walczak-Sztulpa, J. et al. Identical variants cause variable skeletal ciliopathy phenotypes — challenges for the accurate diagnosis. Front. Genet. 13, 931822 (2022).
McConnachie, D. J., Stow, J. L. & Mallett, A. J. Ciliopathies and the kidney: a review. Am. J. Kidney Dis. 77, 410–419 (2021).
Barbelanne, M., Hossain, D., Chan, D. P., Peränen, J. & Tsang, W. Y. Nephrocystin proteins NPHP5 and Cep290 regulate BBSome integrity, ciliary trafficking and cargo delivery. Hum. Mol. Genet. 24, 2185–2200 (2015).
Devane, J. et al. Progressive liver, kidney, and heart degeneration in children and adults affected by TULP3 mutations. Am. J. Hum. Genet. 109, 928–943 (2022).
Ma, M., Tian, X., Igarashi, P., Pazour, G. J. & Somlo, S. Loss of cilia suppresses cyst growth in genetic models of autosomal dominant polycystic kidney disease. Nat. Genet. 45, 1004–1012 (2013).
Shao, L. et al. Genetic reduction of cilium length by targeting intraflagellar transport 88 protein impedes kidney and liver cyst formation in mouse models of autosomal polycystic kidney disease. Kidney Int. 98, 1225–1241 (2020).
Hwang, S.-H. et al. Tulp3 regulates renal cystogenesis by trafficking of cystoproteins to cilia. Curr. Biol. 29, 790–802 (2019).
Walker, R. V. et al. Cilia-localized counterregulatory signals as drivers of renal cystogenesis. Front. Mol. Biosci. 9, 936070 (2022).
Legué, E. & Liem, K. F. Jr. Tulp3 is a ciliary trafficking gene that regulates polycystic kidney disease. Curr. Biol. 29, 803–812 (2019).
Alkanderi, S. et al. ARL3 mutations cause Joubert syndrome by disrupting ciliary protein composition. Am. J. Hum. Genet. 103, 612–620 (2018).
Hakim, S. et al. INPP5E suppresses polycystic kidney disease via inhibition of PI3K/Akt-dependent mTORC1 signaling. Hum. Mol. Genet. 25, 2295–2313 (2016).
Nakata, K., Shiba, D., Kobayashi, D. & Yokoyama, T. Targeting of Nphp3 to the primary cilia is controlled by an N-terminal myristoylation site and coiled–coil domains. Cytoskeleton 69, 221–234 (2012).
Lea, W. A. et al. Analysis of the polycystin complex (PCC) in human urinary exosome-like vesicles (ELVs). Sci. Rep. 10, 1500 (2020).
Hogan, M. C. et al. Identification of biomarkers for PKD1 using urinary exosomes. J. Am. Soc. Nephrol. 26, 1661–1670 (2015).
Ding, H., Li, L. X., Harris, P. C., Yang, J. & Li, X. Extracellular vesicles and exosomes generated from cystic renal epithelial cells promote cyst growth in autosomal dominant polycystic kidney disease. Nat. Commun. 12, 4548 (2021).
Mohieldin, A. M., Alachkar, A., Yates, J. & Nauli, S. M. Novel biomarkers of ciliary extracellular vesicles interact with ciliopathy and Alzheimer’s associated proteins. Commun. Integr. Biol. 14, 264–269 (2021).
Hua, K. & Ferland, R. J. Primary cilia proteins: ciliary and extraciliary sites and functions. Cell. Mol. Life Sci. 75, 1521–1540 (2018).
Hwang, S.-H., Somatilaka, B. N., White, K. & Mukhopadhyay, S. Ciliary and extraciliary Gpr161 pools repress Hedgehog signaling in a tissue-specific manner. eLife 10, e67121 (2021).
Gigante, E. D., Taylor, M. R., Ivanova, A. A., Kahn, R. A. & Caspary, T. ARL13B regulates Sonic hedgehog signaling from outside primary cilia. eLife 9, e50434 (2020).
Silva, L. M. et al. Analysis of primary cilia in renal tissue and cells. Methods Cell Biol. 153, 205–229 (2019).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article. D.P.N., D.J.M.P. and O.E.B. contributed substantially to discussion of the content. All authors wrote the article, and A.L.M., D.J.M.P. and O.E.B. reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Nehprology thanks Saikat Mukhopadhyay and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moran, A.L., Louzao-Martinez, L., Norris, D.P. et al. Transport and barrier mechanisms that regulate ciliary compartmentalization and ciliopathies. Nat Rev Nephrol 20, 83–100 (2024). https://doi.org/10.1038/s41581-023-00773-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-023-00773-2