Abstract
Sepsis-associated acute kidney injury (SA-AKI) is common in critically ill patients and is strongly associated with adverse outcomes, including an increased risk of chronic kidney disease, cardiovascular events and death. The pathophysiology of SA-AKI remains elusive, although microcirculatory dysfunction, cellular metabolic reprogramming and dysregulated inflammatory responses have been implicated in preclinical studies. SA-AKI is best defined as the occurrence of AKI within 7 days of sepsis onset (diagnosed according to Kidney Disease Improving Global Outcome criteria and Sepsis 3 criteria, respectively). Improving outcomes in SA-AKI is challenging, as patients can present with either clinical or subclinical AKI. Early identification of patients at risk of AKI, or at risk of progressing to severe and/or persistent AKI, is crucial to the timely initiation of adequate supportive measures, including limiting further insults to the kidney. Accordingly, the discovery of biomarkers associated with AKI that can aid in early diagnosis is an area of intensive investigation. Additionally, high-quality evidence on best-practice care of patients with AKI, sepsis and SA-AKI has continued to accrue. Although specific therapeutic options are limited, several clinical trials have evaluated the use of care bundles and extracorporeal techniques as potential therapeutic approaches. Here we provide graded recommendations for managing SA-AKI and highlight priorities for future research.
Similar content being viewed by others
Introduction
Sepsis is characterized by a dysregulated host response to infection that leads to life-threatening organ dysfunction, commonly including acute kidney injury (AKI)1. Sepsis accounts for 45–70% of all cases of AKI among critically ill patients2,3. Sepsis-associated AKI (SA-AKI) portends a worse prognosis than either syndrome in isolation3,4 and is associated with longer intensive care unit (ICU) and hospital stays, higher mortality, increased rate of long-term disability and reduced quality of life in adult and paediatric populations5,6,7,8,9. AKI associated with sepsis can present with different phenotypes and prognoses10,11. Many aspects of SA-AKI remain poorly described, especially in the paediatric population, including its clinical definition, epidemiology, pathophysiology, impact of resuscitative and fluid strategies, role of biomarkers in risk stratification, diagnosis, and treatment guidance, and the effect of extracorporeal and novel therapies on patient outcomes. The 28th Acute Disease and Quality Initiative (ADQI) was aimed at identifying these knowledge gaps in both the adult and the paediatric populations, propose definitions, develop a common framework for further research in this important area, and provide recommendations for clinical practice.
Methods
The Conference Chairs of the 28th ADQI consensus committee (L.G.F., A.Z., M.K.N. and C.R.) convened a diverse panel of adult and paediatric clinicians and researchers representing relevant disciplines — critical-care medicine, anaesthesiology, nephrology and pharmacology — from Europe, North and South America, and Australia, to discuss SA-AKI. The conference was held over 2.5 days in Vicenza, Italy, on 17–19 June 2022. This consensus meeting followed the established ADQI process and used a modified Delphi method to achieve consensus, as previously described12,13. Briefly, the ADQI approach uses methods that involve a combination of both expert panel and evidence appraisal, and this approach was chosen to achieve the best of both options. Each ADQI conference is divided into three phases: pre-conference, conference, and post-conference. In the pre-conference phase, the groups that are assigned to specific topics identify a list of key questions, conduct a systematic literature search, and generate a bibliography of key studies. Studies are identified via Medline search and bibliographies of review articles; searches are generally limited to articles written in English. The conference itself is divided into breakout sessions, where workgroups address the issues in their assigned topic area, and plenary sessions, where their findings are presented, debated and refined. This approach has led to important practice guidelines with wide acceptance and adoption into clinical practice. If further research is needed, the ADQI group proposes research questions that should be addressed in the future to facilitate advances in the field. Conference participants were divided into five working groups to discuss the epidemiology and definition of SA-AKI; the pathophysiology of SA-AKI and novel underlying mechanisms; the use of fluids and resuscitative strategies to treat SA-AKI; the use of biomarkers for aiding diagnosis and guiding therapy, and in the design of clinical trials; and the use of extracorporeal treatments and novel therapies. Members of the five workgroups reviewed the literature systematically and, where possible, developed a consensus that was backed by evidence, and proposed a research agenda to address important unanswered questions. In addition, the members were asked to note the level of evidence for all consensus statements using the Grades of Recommendation Assessment, Development and Evaluation system14. In several cycles of presentations, feedback and adjustments, all of the individual workgroups presented their output to conference participants. The final output was then assessed and aggregated in a session attended by all attendees, who formally voted and approved the consensus recommendations.
Definition and epidemiology of SA-AKI
Definition of SA-AKI and sepsis-induced AKI
Currently, no universally accepted definition of SA-AKI exists15. To support clinical guidelines, quality improvement initiatives, and future research, we propose that the presence of both sepsis (as currently defined in adults by the Sepsis-3 criteria) and AKI (as presently defined by the Kidney Disease: Improving Global Outcomes (KDIGO) criteria) should define SA-AKI1,16 (Box 1). SA-AKI is a heterogeneous syndrome that occurs as the consequence of either direct mechanisms related to infection or the host response to infection, or indirect mechanisms driven by unwanted sequelae of sepsis or sepsis therapies17. As such, the term SA-AKI operationally unifies the presence of AKI (according to clinical, biochemical and functional criteria) in the context of sepsis as a specific disease phenotype that is characterized by a specific trajectory and outcome18,19.
Sepsis-induced AKI (SI-AKI) can be considered to be a subphenotype of SA-AKI, in which sepsis-induced mechanisms drive kidney damage directly. Thus, by definition, SI-AKI excludes injury that primarily develops as the indirect consequence of sepsis or sepsis therapies (for example, AKI caused by antimicrobial agent-induced nephrotoxicity or abdominal compartment syndrome)20,21. Importantly, mechanisms that underlie cellular and organ injury in ischaemic AKI or nephrotoxic AKI, such as microcirculation failure, inflammation and mitochondrial injury, might also contribute to SI-AKI. The limited availability of clinical tools such as biomarkers that can aid early identification complicate the distinction between SI-AKI and other causes of SA-AKI. Of note, although the development of AKI is associated with an increased risk of infection, this definition intentionally excludes sepsis following an AKI event, as the aetiology is likely different from that of SA-AKI.
To capture the temporal relationship between the two conditions, SA-AKI should be considered when AKI occurs within 7 days of sepsis diagnosis, and can be further differentiated into early (AKI occurs up to 48 h after sepsis diagnosis) or late SA-AKI (AKI occurs between 48 h and 7 days of sepsis diagnosis), to align with current AKI criteria (Box 1). The rationale for the proposed 7-day window in the definition of SA-AKI is based on the observation that, in most cases of sepsis, AKI occurs within a few days of sepsis onset and consensus was that AKI occurring after this timeframe was probably not directly related to the initial septic insult. The rationale for establishing a separation between early and late presentation is based on the observation that the development of AKI late in the course of sepsis is associated with worse clinical outcomes and increased mortality compared with early AKI development22. Distinguishing early versus late SA-AKI might improve phenotyping for targeted assessments and management, as patients with sepsis that is untreated or early in the course of treatment are more likely to have SI-AKI, whereas in patients who have received sepsis-related interventions, other factors might have also contributed to AKI development.
Epidemiology of SA-AKI
Sepsis and AKI are common in the setting of critical illness, with 25–75% of all AKI being associated with sepsis or septic shock globally23,24,25,26,27. The epidemiology of SA-AKI is highly variable owing to the lack of a standardized definition for SA-AKI, the loose implementation of standardized nomenclature for sepsis and AKI, the diversity of clinical settings and patient populations, and the inconsistent reporting of relevant outcomes (Supplementary Table 1). A 2020 systematic review of observational studies in SA-AKI illustrates these challenges in describing SA-AKI epidemiology15. Of the 47 studies identified, four definitions of sepsis and three definitions of AKI were used. Several studies did not report sepsis criteria, and only a few included the urine output criteria to define AKI or reported the timing of AKI relative to the onset of sepsis. Moreover, the patient populations were considerably heterogeneous, with varying incidence of sepsis, severe sepsis and/or septic shock, as well as differences in the clinical settings, which included the emergency department, medical, surgical and general ICUs, and medical wards (Box 1). The study also identified several risk factors for SA-AKI15 which included the presence of septic shock, the use of vasopressors and mechanical ventilation, Gram-negative bacteraemia, use of renin–angiotensin–aldosterone system inhibitors, presence of chronic liver disease and chronic kidney disease (CKD), pre-existent hypertension and diabetes, and smoking. The reported incidence of SA-AKI ranged from 14–87% and the association with mortality (including ICU mortality, hospital mortality, 28-day and 90-day mortality) was also highly variable, ranging from 11 to 77%.
Research questions
-
1.
What is the epidemiology of SA-AKI based on the proposed definition?
-
2.
What is the epidemiology and the clinically relevant time frame for early versus late SA-AKI?
-
3.
What are the aetiology, incidence and severity, risk factors, and renal and non-renal outcomes of both SA-AKI and SI-AKI?
-
4.
How can the proposed definition of SA-AKI be operationalized in electronic health records?
Pathophysiology of SA-AKI and novel mechanisms
Mechanisms underlying the development of SA-AKI
Depending on the interaction between genotype and exposures, SA-AKI can lead to a variety of clinical phenotypes (that is, observable disease characteristics) and sub-phenotypes. Moreover, multiple pathophysiological mechanisms of injury (that is, the disease endotype) might underlie the same disease phenotype18,19 (Box 2). This heterogeneity complicates the assessment of therapeutic efficacy in clinical trials of sepsis interventions, as different therapies might only be beneficial in the treatment of specific disease endotypes. Importantly, multiple pathophysiological mechanisms might simultaneously lead to AKI in an individual patient with sepsis. Therefore, the ability to identify the specific SA-AKI endotypes will be crucial to the development of effective therapies. Multiple mechanisms can contribute to injury in SA-AKI (Box 2), including systemic and renal inflammation, complement activation, RAAS dysregulation, mitochondrial dysfunction, metabolic reprogramming, microcirculatory dysfunction and macrocirculatory abnormalities (Fig. 1). Several additional processes might indirectly contribute to SA-AKI, such as exposure to nephrotoxic drugs, hyperchloraemia and abdominal compartment syndrome. Of note, some of these mechanisms might have temporal association with the onset and treatment of sepsis. The ability to recognize and link endotypes, subphenotypes and phenotypes therefore represents a major future research focus10,28,29. The biological and clinical characterization of endotypes and of the interactions between endotypes and sepsis-related treatments will be the key to refining the definitions of SI-AKI and SA-AKI set forth in this manuscript.
The release of pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharide, and of damage-associated molecular patterns (DAMPs) from injured cells and tissues can lead to the dysregulated activation of the immune system that characterizes sepsis. Background susceptibility to tissue and organ injury varies across individuals, according to non-modifiable factors such as comorbidities, current lifestyle choices (for example, smoking), genetic variants (for example, single nucleotide polymorphisms), premorbid comorbidities and medication use (for example, the use of renin–angiotensin–aldosterone system (RAAS) inhibitors for blood pressure control), and modifiable factors such as the use of vasopressors, mechanical ventilation or the presence of bacteraemia. The pathways induced in response to sepsis (or sepsis therapies) are modulated by background susceptibility and determine the endotype-defining pathophysiological mechanisms that underlie acute kidney injury (AKI) in patients with sepsis. The combination of different disease mechanisms can therefore result in a variety of disease endotypes. Sepsis-associated AKI (SA-AKI) includes cases of sepsis-induced AKI, whereby the response to sepsis causes kidney injury directly, as well as cases in which other sepsis-associated factors (such as therapeutic interventions) indirectly contribute to AKI. For example, very high doses of norepinephrine can decrease microvascular blood flow and exacerbate the microvascular dysfunction induced by sepsis. Several tissue tolerance mechanisms, such as the activation of haem-oxygenase 1 or mitochondrial autophagy (that is, mitophagy), can protect cells and tissues from injury caused by PAMPs and DAMPs. Importantly, the disease phenotypes observed in the clinic (for example, sub-phenotypes 1–3) reflect a complex interplay between background susceptibility, disease endotypes and tolerance capacity. Consequently, phenotypes cannot be directly traced to a specific disease mechanism or endotype, and therefore clinical subphenotyping of patients with sepsis might not be sufficient to identify relevant therapeutic targets. The figure is a simplified representation of these complex interactions but also illustrates a roadmap for investigating mechanism-specific biomarkers that can identify whether specific endotypes and tolerance mechanisms are operational, thereby enabling the development and assessment of mechanism-specific therapies. BM, biomarker; AKD, acute kidney disease; CKD, chronic kidney disease; GFR, glomerular filtration rate; KDIGO, Kidney Disease Improving Global Outcomes.
Determinants of susceptibility and recovery trajectory
Several modifiable and non-modifiable factors affect susceptibility to AKI and disease severity in patients with sepsis. As discussed earlier, a 2020 meta-analysis identified ten clinical risk factors with prognostic value15. Although useful for risk stratification, such clinical factors only partly explain an individual’s susceptibility to developing SA-AKI and do not consider susceptibility within the conceptual framework of different SA-AKI endotypes.
Genetic and epigenetic variability, as well as the interplay between resistance and tolerance mechanisms during sepsis, have been recognized as potential key factors underlying individual susceptibility (Box 2). In patients with sepsis, single nucleotide polymorphisms (SNPs) in genes involved in both inflammatory (TNF, IL6 and IL10)30,31,32,33 and vascular (VEGF)34 pathways have been implicated in the development of AKI35,36. However, study results have been inconsistent and three independent systematic reviews did not find a clear link between specific genetic variants and AKI risk in patients with sepsis37,38,39. Epigenetic control of gene expression is mediated by enzymatic DNA methylation or histone modification without changes in the genetic code. This type of control has been implicated in the induction of cross-tolerance in immune cells and kidney tubular epithelial cells, whereby innate and adaptive immune responses to a subsequent insult are attenuated40,41,42,43. However, exposure to sublethal ischaemic or toxic AKI can also be followed by a local hyper-inflammatory response in animals subsequently challenged with lipopolysaccharide or lipoteichoic acid44,45. This ‘biological memory’ and the capacity to reprogram future responses is probably induced by epigenetic mechanisms, whereby histone-modifying enzymes enhance the expression rate of inflammatory genes45,46,47. The influence of a previous insult on the response to a second insult are likely dependent on both the extent of the initial insult and the timing in relation to the initial event. Resistance and tolerance capacity (not to be confused with cross-tolerance described above) might explain an individual’s susceptibility to SA-AKI. Resistance capacity refers to the ability of the immune system to control or eliminate the microbial burden, whereas tolerance capacity has a critical role in sepsis because it reflects the ability of a cell, tissue, or organ to attenuate its susceptibility to injury during infection48,49. Tolerance mechanisms protect the host from the potential harm associated with resistance mechanisms. Several protective tolerance mechanisms against AKI have been identified including in preclinical models of malaria50,51, viral and bacterial sepsis52,53,54, ischaemia–reperfusion injury, and nephrotoxicity50,51,52,53,54. Tolerance mechanisms seem to be specific to the type of insult or infection and are thus not entirely generalizable. For instance, starvation protects from tissue injury and death in rodents with bacterial sepsis but worsens outcomes in viral sepsis55.
Similar to individual susceptibility to AKI, the trajectory of post-AKI recovery — determined by adaptive or maladaptive repair processes — is influenced not only by genetic variation, but also injury severity, recurrent insults, and the presence of underlying CKD. Within the nephron, adaptive repair involves the proliferation and re-differentiation of tubular epithelial cells, as well as the repair and regeneration of endothelial cells. By contrast, maladaptive repair manifests as tubular atrophy and dilation56, expansion of interstitial fibroblasts and myofibroblasts57, endothelial-to-mesenchymal transition and a reduction in peritubular capillary density58,59. Together, these maladaptive processes culminate in interstitial fibrosis, tissue hypoxia, increased oxidative stress and accelerated senescence56. Progressive fibrosis is followed by loss of functional renal reserve, glomerular hypertension and the development of CKD60.
SA-AKI mechanisms and novel therapeutic targets
As mentioned above, many clinical studies have attempted to investigate the benefit of therapeutic interventions in unselected patient populations, which might have reduced therapeutic efficacy signals and led to negative results. The framework proposed in this consensus statement advocates for a strategic shift in randomized controlled trial (RCT) design, whereby the deployment of any therapeutic strategy is targeted to subgroups of patients defined according to disease likelihood endotypes or therapy-responsive subphenotypes to enhance the possibility of discovering effective therapies. In addition, endotyping and subphenotyping will provide a platform to better understand the interaction between pathogenic mechanisms induced by sepsis directly or by sepsis-related factors (for example, nephrotoxins or complications such as abdominal compartment syndrome). Moreover, this granular approach will help to define the relationship between pathogenic mechanisms and time, and the therapeutic potential of different interventions in early and late SA-AKI or SI-AKI (Box 2).
Several interventions that modulate pathogenic processes involved in SA-AKI have been tested. For example, anti-inflammatory agents were not found to be beneficial but post hoc analyses demonstrated that dexamethasone was associated with a reduced need for kidney replacement therapy (KRT) in patients with sepsis61. A phase II trial showed long-term kidney benefit and lower mortality in patients who received the anti-inflammatory recombinant alkaline phosphatase (NCT04411472)62. With regard to haemodynamics and oxygen delivery, studies using angiotensin 2 (ref. 63) (ASK-IT trial, NCT00711789) and levosimendan64,65 suggest that these agents might protect the kidneys. Of note, although mitochondrial dysfunction is a feature of SA-AKI, no compounds targeting this impairment are in the clinical development phase thus far. Interventions related to cellular repair and fibrosis, including mesenchymal stem cell therapy66, protein-7 agonist67 and mimetics of hepatocyte growth factor68, have been studied but not yet found to decrease the incidence or severity of AKI.
Research questions
-
1.
How can we validate mechanisms recognized in preclinical models in the clinical setting?
-
2.
How can we identify distinct endotypes of SA-AKI?
-
3.
How can we leverage molecular diagnostic technologies to identify novel therapeutic targets?
-
4.
How can we match distinct endotypes of SI-AKI to targeted therapies?
-
5.
How can we optimize the delivery of novel therapies to maximize efficacy within the kidney while minimizing remote toxicity?
-
6.
What is the role of damage and systemic markers of sepsis in defining the mechanism and time course of SA-AKI and its endotypes?
Fluid and resuscitation therapy
Goals of fluid management in SA-AKI
Restoring intravascular volume through redistribution of fluid is a therapeutic target in sepsis to sustain adequate perfusion and tissue oxygen delivery. Together with source control and treatment with antimicrobials, the administration of fluids and vasopressors are key management strategies in SA-AKI. The main goal of fluid administration is to increase preload and cardiac output to maintain adequate oxygen delivery to vital organs. The haemodynamic targets for SA-AKI should be consistent with those outlined in the Surviving Sepsis Campaign Guidelines 2021 and our previous report on haemodynamics for monitoring fluid therapy from the 12th ADQI Consensus Conference69,70 (Box 3). The utility of central venous pressure (CVP) as a haemodynamic indicator in SA-AKI is unclear and, although a high CVP might reflect congestion in the capacitance vessels71,72, CVP correlates only moderately with overall volume status, given that CVP is also influenced by right ventricular function73. Assessment of fluid status and response to fluid administration (that is, fluid responsiveness) should be undertaken to prevent under- or over-hydration. Urine output should be closely monitored but should not be used to guide fluid therapy in patients with SA-AKI. Measurement of intra-abdominal pressure can be useful in patients at risk of AKI. Daily and cumulative fluid balance should inform fluid management in patients with SA-AKI, as many studies have shown that fluid overload in critically ill patients is associated with excess mortality74,75. Assessment of fluid responsiveness should include clinical perfusion markers, and advanced haemodynamic monitoring, invasive or non-invasive, where available, should be considered76. Of note, the rate and duration of intravascular volume expansion following fluid administration are crucial given the role of the endothelial glycocalyx layer in vascular permeability where injury to this layer might lead to increased rates of fluid loss from the intravascular into the extravascular space and further fluid administration could cause fluid overload77,78,79.
Role of fluid protocols for the treatment of SA-AKI
Given the need to manage fluid volume, composition and distribution concurrently with AKI and sepsis of varying severity, the potential for toxicity from fluid therapy is substantial. The fluid management goals can be protocolized, utilizing the type, rate and duration of fluid delivery to target the interdependent relationship between sepsis and AKI (Box 3). Early and late SA-AKI might require different treatment protocols. Whereas haemodynamic stabilization is a priority in early SA-AKI, targeting fluid overload might be more relevant in late SA-AKI. The ongoing CLOVERS trial and ARISE FLUIDS observational study are expected to provide additional evidence in this field80,81. The use of fluid-restrictive protocols is feasible in SA-AKI, but a beneficial effect has not yet been demonstrated. The REVERSE-AKI trial, suggested that restricted daily fluid intake, aiming for a negative daily fluid balance with unrestricted use of diuretics was associated with reduced use of KRT compared with usual care. However, only ~50% of participants with AKI also had sepsis82. The recently published CLASSIC trial found that intravenous fluid restriction after initial fluid resuscitation was not superior to liberal fluid management with regard to kidney outcomes in adult patients with septic shock in the ICU83. As described previously at the 12th ADQI conference, the four phases of intravenous fluid therapy — resuscitation, optimization, stabilization and de-escalation — form an appropriate conceptual framework for tailoring fluid therapy to the individual patient context70. Although (balanced) crystalloids are often used, the recent BaSICS and PLUS trials and meta-analysis found no clinical benefit of balanced solutions over the use of 0.9% saline solutions84,85,86,87. The SMART trial reported that the use of balanced crystalloids significantly decreased major adverse kidney events at day 90 (MAKE90) and a composite end point (death, KRT, or persistent kidney dysfunction) compared saline; however, only ~15% of the patient population had sepsis at baseline88. Similarly, the SALT-ED trial noted a significant decrease in MAKE90 with the use of balanced crystalloids versus saline, but the study cohort comprised a heterogenous population of patients receiving treatment in the emergency department89.
Colloids of high molecular weight theoretically cause selective expansion of the intravascular space but this effect is impaired when vascular permeability is altered and the endothelial glycocalyx is damaged in inflammation. Supplemental albumin administration, as a preferred colloid over synthetic colloids, might be considered if substantial fluid replacement is required; however, to date, no data support its routine use for volume resuscitation in sepsis and data to inform a suggested cut-off value for crystalloid infusion above which albumin should be considered as part of resuscitation fluid are limited69,85,90,91. According to the subgroup analyses of patients with severe sepsis or septic shock in the SAFE and ALBIOS trials, the administration of albumin, either as a primary resuscitation fluid or as a supplement to crystalloid resuscitation, might be associated with a lower mortality trend85,90. However, these were post hoc analyses and must be interpreted with caution. We await the results of the ongoing ALBumin Italian Outcome Septic Shock-BALANCED trial (ALBIOSS-BALANCED) to provide evidence as to whether albumin, as a primary or supplemental resuscitation fluid, improves outcomes in patients with septic shock92. Of note, the use of hydroxyethyl starch (HES) has been associated with increased mortality risk and other adverse outcomes compared with crystalloids, including the need for KRT in patients with severe sepsis; accordingly, the FDA has mandated changes to safety labelling in HES products and its use was suspended in the European Union93. Hence, we recommend against the use of HES for fluid resuscitation in patients with SA-AKI. Similarly, compared with crystalloids or albumin, use of gelatin was associated with an increased risk of anaphylaxis, mortality, AKI and bleeding in a 2016 meta-analysis of 30 RCTs, 8 non-randomized studies and 22 animal studies94. Dextrans have also been associated with anaphylaxis, coagulation disorders, osmotic nephrosis and AKI in observational studies95,96. The BICAR-ICU study found that treatment with intravenous 4.2% sodium bicarbonate for severe metabolic acidaemia (pH <7.20) and moderate-to-severe AKI in the ICU reduced the primary composite outcome (death from any cause by day 28 and the presence of at least one organ failure at day 7) and 28-day mortality97. However, only ~60% of the trial population in each study arm had sepsis at the time of randomization, so the results cannot be fully extrapolated to patients with SA-AKI, particularly as these findings are specific to patients with severe acidaemia in the presence of AKI.
Combination of adjunctive therapies with fluid management
Adjunctive therapies should be used to optimize haemodynamic status and enhance fluid management, and should be adjusted based on the clinical condition of the patient (Box 3). Vasoactive agents are also key to haemodynamic optimization, and their use should not be limited by the presence or absence of central venous access. If clinically required, peripheral use of vasoactive agents should be initiated with careful monitoring for extravasation. Norepinephrine remains the first-line vasopressor for sepsis and SA-AKI69,98. The early use of vasopressors might have a volume-sparing effect. Conversely, diuretics have an essential role in the treatment of volume overload, potentially reducing mortality99. In the FFAKI, REVERSE-AKI and RADAR-2 RCTs, forced fluid removal prevented and treated fluid overload effectively in critically ill patients82,100,101. However, depending on the severity of AKI, significant increases in diuretic therapy dosage might be required to achieve a sufficient effect100,102. Moreover, specific phenotypes of SA-AKI might benefit from specific vasopressors. A secondary analysis of the VASST trial29 showed improved survival compared with norepinephrine in patients with a subphenotype of SA-AKI that was characterized by a low severity of disease and low levels of angiotensin 1, angiotensin 2 and IL-8. A posthoc analysis of the ATHOS-3 trial found that patients with vasodilatory shock and AKI requiring KRT had significantly greater 28-day survival with a higher mean arterial pressure response and a higher rate of KRT discontinuation when treated with intravenous angiotensin II compared with placebo103. In the case of impaired cardiac function, inotropes should be considered to optimize oxygen delivery.
Research questions
-
1.
What is the utility of haemodynamic monitoring in SA-AKI?
-
2.
What is the role of the assessment of renal perfusion using ultrasound, measurement of intra-abdominal pressure and assessment of mean perfusion pressure in managing SA-AKI?
-
3.
What is the effect of 0.9% saline versus balanced crystalloids on outcomes for patients with SA-AKI, particularly with regard to hyperchloraemia and hyper- and hyponatraemia in patients with SA-AKI?
-
4.
Is there an indication for using albumin for fluid resuscitation in patients with SA-AKI?
-
5.
What is the clinical utility of markers of glycocalyx damage during fluid therapy and their correlation with markers of kidney dysfunction?
-
6.
Does the choice of vasopressor agent affect the course of SA-AKI?
-
7.
What is the role of diuretics in treating fluid overload in SA-AKI?
Biomarkers for diagnosis and guiding treatment
Measures for SA-AKI prediction and diagnosis
The ADQI 23 Consensus Conference statement104 proposed combining damage and functional biomarkers to increase the sensitivity of AKI definitions. For example, stage 1S (‘subclinical AKI’), could be defined by biomarker-positive evidence of kidney injury that does not meet the KDIGO criteria (that is, AKI stage 1 defined by creatinine and urine output criteria are not achieved). Data from the Protocolized Care for Early Septic Shock (ProCESS) cohort reported in 2022, demonstrate that, for a given stage of KDIGO-defined AKI, higher biomarker values (stages 1B, 2B and 3B) were associated with a higher risk of 30-day mortality than stages 1A, 2A and 3A105. However, 30-day survival did not differ between biomarker-positive (stage 1S) and biomarker-negative cases in the absence of KDIGO AKI criteria.
Emerging data demonstrate that plasma proenkephalin (penKid) identifies patients with sepsis who are at an increased risk of developing KDIGO-defined functional AKI and MAKE; patients with stage 1S AKI (defined by plasma penKid increases) had higher 28-day mortality than those with KDIGO-defined functional AKI106,107. Similarly, plasma cystatin C has been proposed as an alternative to serum creatinine as a functional marker of glomerular filtration rate changes to identify AKI in patients with critical illnesses, including those with SA-AKI108. Whether these functional markers, which have shorter half-lives than serum creatinine, provide a swifter diagnosis of decreasing kidney function, can assist in appropriate drug dosing, and/or if other biological and analytical features improve the diagnosis and prognostication of AKI in sepsis, remains unclear109,110. Functional biomarkers (for example, cystatin C and penKid) and damage or stress biomarkers (for example, neutrophil gelatinase-associated lipocalin (NGAL) and (TIMP2) × (IGFBP7)) predict SA-AKI with high accuracy104,106,111,112,113,114,115. Additionally, several non-biochemical tools can forecast SA-AKI, including logistic or artificial intelligence-driven prediction models based on available clinical information116,117,118,119. The clinical information used for these models includes clinical and physiological data, volume assessment and other laboratory information. These forecasting models and biomarkers could be used in combination to assign patients with SA-AKI to specific phenotypes and subphenotypes117,120,121 (Box 4). Several investigations have demonstrated the clinical potential for using biological, genetic and machine-learning multidimensional models for assessing AKI risk and improving outcomes29,118,119. Further research is needed to distinguish SA-AKI from other AKI aetiologies using validated measures, including biomarkers, and to characterize and differentiate SA-AKI endotypes or sub-phenotypes (Fig. 2).
At the top are the characteristics of an individual’s acute kidney injury (AKI) journey — severity, duration and trajectory. Each episode of AKI will have unique biological characteristics. In addition to pre-sepsis comorbidities, several tests will be performed, including standard laboratory measures, advanced biomarker assessment and genetic, proteomic and metabolic tests. When combining these tests with clinical and environmental factors, distinct sepsis-associated AKI (SA-AKI) phenotypes will be characterized, each with its distinct course and response to treatment plans. In the near future, biomarkers and machine-learning algorithms might be used to characterize patients by phenotype and endotype more rapidly to optimize their care or expedite enrolment into clinical trials. Adapted from Maslove et al.193, Springer Nature Limited.
Measures for SA-AKI course and outcome prediction
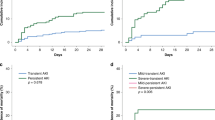
The duration of AKI has gained relevance as an additional dimension in AKI phenotyping122, given that a pattern of persistent, non-resolving AKI is associated with poorer short-term20 and long-term outcomes123,124,125 than transient AKI, regardless of disease severity. Early patient risk identification could aid the development of personalized in-hospital126 and outpatient127 care strategies to reduce AKI progression, the development of AKI-related complications and the risk of sequelae of SA-AKI, as well as enabling predictive enrichment in randomized trials of potential therapeutic targets. Various markers, including clinical risk scores, functional, stress- and injury-related biomarkers, and imaging tests, have been described in patients admitted to the ICU (Tables 1 and 2). Notably, many markers were tested in heterogeneous cohorts of critically ill patients; thus, their generalizability to patients with sepsis might require further investigation. Scoring systems (for example, the renal angina index) and imaging tests, such as the renal resistive index, can be considered complementary to direct AKI biomarker testing to optimize their use for the prediction of persistent AKI and other outcomes128,129 (Box 4).
Various sepsis-associated biomarkers have been evaluated to assess the prognosis of SA-AKI (Supplementary Table 2). However, the timing of SA-AKI diagnosis relative to the timing of biomarker assessment is often highly variable, thus complicating the differentiation of early versus late SA-AKI and indeed, most sepsis-associated biomarker monitoring is anchored at the time of admission to the ICU. Of note, prognosis determination could be influenced by the introduction of a confounding variable after SA-AKI diagnosis but before the outcome measure. Many studies evaluating the association between biomarkers and AKI considered single or multiple biomarkers (Supplementary Table 3), but few directly compared or measured the additive impact of combining sepsis and kidney biomarkers to determine prognosis. Of note, AKI biomarkers predicting acute kidney disease (that is, 7 to 90 days post-insult) in patients with sepsis are less well-defined130.
Research questions
-
1.
Do kidney injury biomarkers add prognostic discrimination in patients with SA-AKI, and can they identify a high-risk 1S subgroup in the absence of KDIGO-defined functional AKI (subclinical AKI)?
-
2.
What is the impact of individual biomarkers on SA-AKI clinical trajectories, including severity, duration, recovery and non-kidney-related outcomes?
-
3.
What are the best methods for integrating clinical information, identification of phenotypes and single or serial use of validated measures to predict clinical course and the likelihood of response to interventions?
-
4.
What is the role of the measurement of sepsis and kidney markers for targeted intervention in different subphenotypes and endotypes of SA-AKI?
Extracorporeal therapies for SA-AKI
Extracorporeal blood purification in SA-AKI
Extracorporeal blood purification (EBP) can be performed using various techniques (Supplementary Fig. 1); the most common techniques involve systems that are mainly employed for KRT with the aim of re-establishing homeostasis (Table 3). These techniques affect the molecular and electrolyte composition of blood directly, which might enable the correction, replacement and maintenance of homeostasis in multi-organ dysfunction through the control of acid–base, electrolyte and fluid balances. EBP techniques might also facilitate the control of immune dysregulation in sepsis by removing endotoxins, cytokines, pathogens and inflammatory factors131,132,133,134,135. The selection of a specific EBP modality or combination of selected modalities should be based on the patient’s needs (Table 3). Global practice is very heterogeneous owing to the lack of consensus guidelines and high-grade evidence, and the limited availability and approval of specific devices and therapies.
Accepted indications for commencing KRT to support kidney function during SA-AKI are consistent with those in place for other causes of AKI16. Although early KRT initiation for SA-AKI has been used for fluid and solute control and to prevent multi-organ dysfunction, no clear benefit has been demonstrated for earlier initiation136. Of the latest RCTs focused on the timing of initiation of KRT in critically ill AKI patients137,138,139, the IDEAL-ICU study140 is the only one that focused on SA-AKI and demonstrated that earlier initiation of KRT had no significant survival benefit compared with ‘standard’ initiation, although a significant number of patients in the ‘delayed’ group were not treated with KRT, owing to spontaneous kidney recovery. Of note, a further study on the initiation of KRT in SA-AKI is currently underway141. Initiation of KRT in both septic and non-septic conditions should be based on clinical assessment and goals of EBP for kidney support, not just on creatinine levels and oliguria69. In patients with SA-AKI for whom KRT is indicated and with explicit clinical (for example, shock) and/or biological (for example, the detection of damage-associated molecular patterns and pathogen-associated molecular patterns) criteria are recognized, EBP for immunomodulatory support might be considered in combination with KRT, either concurrently (for example, hybrid treatments) or following KRT (Box 5). EBP for immunomodulatory support can be considered in patients with sepsis as a stand-alone treatment if kidney support is not required142. Despite the biological rationale for using EBP approaches in SA-AKI, namely their potential to limit the pathophysiology of organ damage, mitigate homeostatic derangements and prevent multi-organ dysfunction in sepsis, the lack of robust data precludes definitive recommendations with regard to its use in patients with sepsis or SA-AKI, including its timing in the clinical course of the disease.
Delivery and monitoring of extracorporeal blood purification therapies
For haemoadsorption therapies, anticoagulation is recommended, and the indications for venous access are similar to those of KRT143. Haemoadsorption cartridges can be combined with the KRT circuit with variable blood flow rates144,145,146. Initiation of haemoadsorption to remove endotoxins has been based on the result of the endotoxin activity assay, which compares the activation of neutrophils caused by endotoxin to the theoretical maximum response when exogenous endotoxin is added to the blood sample147,148,149,150,151. Polymyxin B haemoadsorption has been used in sepsis with variable results. When Polymyxin B haemoadsorption was applied for 2-h sessions for 2 consecutive days, it was found to be safe but without a survival benefit. However, a potential survival benefit was observed in patients with an endotoxin activity assay of 0.6–0.9 EAA units, indicative of a high but measurable endotoxin burden151,152,153. These effects are being investigated in an ongoing trial (the TIGRIS trial, ClinicalTrials.gov Identifier: NCT03901807).
New synthetic polymeric resins enable highly biocompatible haemoadsorption designed for the non-specific adsorption of damage-associated molecular patterns and other mediators. The rationale for their use is based on the peak concentration hypothesis, which postulates that haemoadsorption might enable removal of the solutes with the highest concentration in blood (either pro- or anti-inflammatory mediators), helping to restore immunohomeostasis by mitigating the uncontrolled response of the innate and/or the adaptive immunity of the patient154,155. Additional research on clinical benefits is warranted. Notably, the unselective nature of such EBP interventions might result in unrecognized losses of electrolytes, nutrients and drugs. As significant losses of amino acids and several micronutrients, such as vitamins B1 and C, copper and selenium can occur, careful monitoring in prolonged KRT should be considered156. Importantly, discrepancies exist in the observed and predicted removal of antimicrobials with haemoadsorption in critically ill patients157,158,159,160,161,162,163. In patients undergoing continuous KRT, antimicrobial clearance depends on the effluent fluid rate and therapeutic drug monitoring should therefore be considered where available157. The identification of subphenotypes of patients and the delivery of EBP should be assessed and supported by a multidisciplinary team of trained personnel to improve patient selection and safety164. Optimal EBP delivery demands timely communication between stakeholders, iterative adjustment of therapy and quality assurance systems165,166. Patient selection, timing, duration and appropriate primary clinical endpoints are crucial elements for well-conducted clinical studies in this area. Moreover, given the phenotypic variability of SA-AKI, one extracorporeal therapy might be effective in a specific phenotype, while having no effect, or even causing harm, in others. Investigators should refrain from choosing mortality as the primary end point because of the well-known variation in mortality across centres, sepsis and AKI phenotypes167. RCTs examining the effects of EBP, in which patient heterogeneity is reduced through specific inclusion criteria with clinically relevant endpoints, including haemodynamic and organ improvement, as well as ICU stay rather than only mortality, should be performed.
Research questions
-
1.
How do the EBP therapies affect the pathophysiology of SA-AKI?
-
2.
In which subgroup of patients, and when in the clinical course of the disease, might EBP therapies be beneficial?
-
3.
Are EBP therapies safe, efficacious and cost-effective?
-
4.
What meaningful target molecules can guide EBP therapy, and can their kinetics be employed to assess response to treatment?
-
5.
What is the effect of EBP therapies on other organ systems during sepsis?
SA-AKI: the paediatric perspective
This Consensus statement has thus far been based on a systematic review of the literature as it pertains to adult medicine, especially with the use of Sepsis-3 as the definition of sepsis in adult patients. Although there is undoubtedly much overlap in the pathophysiology of SA-AKI throughout the lifespan, neonates and children do merit particular attention. SA-AKI is a common cause of AKI in critically ill children168 but the definition of SA-AKI in paediatrics is currently limited by the reliance on adult sepsis criteria. The 26th ADQI recently published consensus recommendations for the advancement in paediatric AKI with particular attention to the role of development as a biological variable that modulates the development of and recovery from AKI169. AKI prediction in paediatric patients continues to progress. The renal angina index has been modified for paediatric patients with sepsis and shown to reliably predict SA-AKI, particularly when platelet count is incorporated within the scoring system170. Another study demonstrated the use of prognostic biomarkers for diagnostic and predictive enrichment in paediatric SA-AKI171. However, notable differences remain between current recommendations of management of SA-AKI for adult and paediatric patients, particularly with regard to fluid management and the type of fluid, with a preference for the use of balanced crystalloids in children. The aforementioned fluid recommendations in this manuscript apply to adults specifically172. Of note, a 2021 study reported the inclusion of paediatric populations in the study of EBP therapies to treat SA-AKI via a selective cytopheretic device173. Future work should align paediatric-specific sepsis definitions with SA-AKI management and research agendas (Box 6). A Society of Critical Care Medicine (SCCM) task force is currently working to streamline a definition of paediatric sepsis, but, for now, a precise definition of paediatric SA-AKI remains unavailable.
Research questions
-
1.
How should SA-AKI be defined in the paediatric population?
-
2.
Are there differences in the pathophysiology of SA-AKI across the lifespan?
-
3.
How can the proposed research agenda incorporate the concept of development as a biological variable in the diagnosis and management of SA-AKI?
Conclusions
The presence of AKI in patients with sepsis is common and SA-AKI is best defined by both consensus sepsis criteria and AKI criteria, with early SA-AKI occurring within 48 h of diagnosis of sepsis and late SA-AKI occurring between 48 h and 7 days of diagnosis of sepsis. Multiple mechanisms can contribute to the development of SA-AKI and their relative contributions might define distinct SA-AKI endotypes. These endotypes might be identified through the use of biomarkers, including functional, stress and tissue damage-related biomarkers, as well as clinical information. Prognostic information should help to determine treatment, which should follow currently accepted guidelines, but the use of specific therapies might be influenced by the endotype. For example, the SA-AKI endotype might affect the choice of vasopressor or dictate whether EBP techniques might be used for immunomodulatory support in patients who meet explicit criteria.
References
Singer, M. et al. The Third International Consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315, 801–810 (2016).
Uchino, S. et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294, 813–818 (2005).
Hoste, E. A. et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 41, 1411–1423 (2015).
Peerapornratana, S., Manrique-Caballero, C. L., Gomez, H. & Kellum, J. A. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 96, 1083–1099 (2019).
Poston, J. T. & Koyner, J. L. Sepsis associated acute kidney injury. BMJ 364, k4891 (2019).
Schuler, A. et al. The impact of acute organ dysfunction on long-term survival in Sepsis. Crit. Care Med. 46, 843–849 (2018).
Stanski, N. L. et al. Severe acute kidney injury is independently associated with mortality in children with septic shock. Intensive Care Med. 46, 1050–1051 (2020).
Zarbock, A., Gomez, H. & Kellum, J. A. Sepsis-induced acute kidney injury revisited: pathophysiology, prevention and future therapies. Curr. Opin. Crit. Care 20, 588–595 (2014).
Kaddourah, A., Basu, R. K., Bagshaw, S. M., Goldstein, S. L. & Investigators, A. Epidemiology of acute kidney injury in critically ill children and young adults. N. Engl. J. Med. 376, 11–20 (2017).
Wiersema, R. et al. Two subphenotypes of septic acute kidney injury are associated with different 90-day mortality and renal recovery. Crit. Care 24, 150 (2020).
Basu, R. K. et al. Clinical phenotypes of acute kidney injury are associated with unique outcomes in critically ill septic children. Pediatr. Res. 90, 1031–1038 (2021).
Kellum, J. A., Bellomo, R. & Ronco, C. Acute Dialysis Quality Initiative (ADQI): methodology. Int. J. Artif. Organs 31, 90–93 (2008).
Nadim, M. K. et al. Cardiac and vascular surgery-associated acute kidney injury: the 20th International Consensus Conference of the ADQI (Acute Disease Quality Initiative) Group. J. Am. Heart Assoc. https://doi.org/10.1161/JAHA.118.008834 (2018).
Alonso-Coello, P. et al. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: introduction. BMJ 353, i2016 (2016).
Liu, J., Xie, H., Ye, Z., Li, F. & Wang, L. Rates, predictors, and mortality of sepsis-associated acute kidney injury: a systematic review and meta-analysis. BMC Nephrol. 21, 318 (2020).
Kellum, J. A. & Lameire, N., KDIGO AKI Guideline Work Group Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit. Care 17, 204 (2013).
Mehta, R. L. et al. Sepsis as a cause and consequence of acute kidney injury: program to improve care in acute renal disease. Intensive Care Med. 37, 241–248 (2011).
Lotvall, J. et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J. Allergy Clin. Immunol. 127, 355–360 (2011).
Seymour, C. W. et al. Precision medicine for all? Challenges and opportunities for a precision medicine approach to critical illness. Crit. Care 21, 257 (2017).
Bhatraju, P. K. et al. Acute kidney injury subphenotypes based on creatinine trajectory identifies patients at increased risk of death. Crit. Care 20, 372 (2016).
Kellum, J. A., Sileanu, F. E., Bihorac, A., Hoste, E. A. & Chawla, L. S. Recovery after acute kidney injury. Am. J. Respir. Crit. Care Med. 195, 784–791 (2017).
Lima, R. S. et al. Comparison between early and delayed acute kidney injury secondary to infectious disease in the intensive care unit. Int. Urol. Nephrol. 40, 731–739 (2008).
Bagshaw, S. M., George, C., Bellomo, R. & Committee, A. D. M. Early acute kidney injury and sepsis: a multicentre evaluation. Crit. Care 12, R47 (2008).
Bagshaw, S. M. et al. Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes. Clin. J. Am. Soc. Nephrol. 2, 431–439 (2007).
Vincent, J. L. et al. Sepsis in European intensive care units: results of the SOAP study. Crit. Care Med. 34, 344–353 (2006).
Cruz, D. N. et al. North east Italian prospective hospital renal outcome survey on acute kidney injury (NEiPHROS-AKI): targeting the problem with the RIFLE criteria. Clin. J. Am. Soc. Nephrol. 2, 418–425 (2007).
Kolhe, N. V., Stevens, P. E., Crowe, A. V., Lipkin, G. W. & Harrison, D. A. Case mix, outcome and activity for patients with severe acute kidney injury during the first 24 hours after admission to an adult, general critical care unit: application of predictive models from a secondary analysis of the ICNARC Case Mix Programme database. Crit. Care 12 (Suppl 1), S2 (2008).
Xu, K. et al. Unique transcriptional programs identify subtypes of AKI. J. Am. Soc. Nephrol. 28, 1729–1740 (2017).
Bhatraju, P. K. et al. Identification of acute kidney injury subphenotypes with differing molecular signatures and responses to vasopressin therapy. Am. J. Respir. Crit. Care Med. 199, 863–872 (2019).
Treszl, A. et al. Interleukin genetic variants and the risk of renal failure in infants with infection. Pediatr. Nephrol. 17, 713–717 (2002).
Gordon, A. C. et al. TNF and TNFR polymorphisms in severe sepsis and septic shock: a prospective multicentre study. Genes Immun. 5, 631–640 (2004).
Jaber, B. L. et al. Cytokine gene promoter polymorphisms and mortality in acute renal failure. Cytokine 25, 212–219 (2004).
Wattanathum, A., Manocha, S., Groshaus, H., Russell, J. A. & Walley, K. R. Interleukin-10 haplotype associated with increased mortality in critically ill patients with sepsis from pneumonia but not in patients with extrapulmonary sepsis. Chest 128, 1690–1698 (2005).
Cardinal-Fernandez, P. et al. Genetic predisposition to acute kidney injury induced by severe sepsis. J. Crit. Care 28, 365–370 (2013).
Frank, A. J. et al. BCL2 genetic variants are associated with acute kidney injury in septic shock*. Crit. Care Med. 40, 2116–2123 (2012).
Vilander, L. M., Kaunisto, M. A., Vaara, S. T. & Pettila, V., group, F. s. Genetic variants in SERPINA4 and SERPINA5, but not BCL2 and SIK3 are associated with acute kidney injury in critically ill patients with septic shock. Crit. Care 21, 47 (2017).
Lu, J. C. et al. Searching for genes that matter in acute kidney injury: a systematic review. Clin. J. Am. Soc. Nephrol. 4, 1020–1031 (2009).
Vilander, L. M., Kaunisto, M. A. & Pettila, V. Genetic predisposition to acute kidney injury — a systematic review. BMC Nephrol. 16, 197 (2015).
Cardinal-Fernandez, P. et al. [Genetic determinants of acute renal damage risk and prognosis: a systematic review]. Med. Intensiv. 36, 626–633 (2012).
Allis, C. D. & Jenuwein, T. The molecular hallmarks of epigenetic control. Nat. Rev. Genet. 17, 487–500 (2016).
Lehner, M. D., Morath, S., Michelsen, K. S., Schumann, R. R. & Hartung, T. Induction of cross-tolerance by lipopolysaccharide and highly purified lipoteichoic acid via different Toll-like receptors independent of paracrine mediators. J. Immunol. 166, 5161–5167 (2001).
Hato, T. et al. The macrophage mediates the renoprotective effects of endotoxin preconditioning. J. Am. Soc. Nephrol. 26, 1347–1362 (2015).
He, K. et al. Lipopolysaccharide-induced cross-tolerance against renal ischemia-reperfusion injury is mediated by hypoxia-inducible factor-2α-regulated nitric oxide production. Kidney Int. 85, 276–288 (2014).
Zager, R. A. ‘Biologic memory’ in response to acute kidney injury: cytoresistance, Toll-like receptor hyper-responsiveness and the onset of progressive renal disease. Nephrol. Dial. Transplant. 28, 1985–1993 (2013).
Zager, R. A., Johnson, A. C., Lund, S. & Hanson, S. Acute renal failure: determinants and characteristics of the injury-induced hyperinflammatory response. Am. J. Physiol. Renal Physiol. 291, F546–F556 (2006).
Naito, M., Bomsztyk, K. & Zager, R. A. Endotoxin mediates recruitment of RNA polymerase II to target genes in acute renal failure. J. Am. Soc. Nephrol. 19, 1321–1330 (2008).
Naito, M., Zager, R. A. & Bomsztyk, K. BRG1 increases transcription of proinflammatory genes in renal ischemia. J. Am. Soc. Nephrol. 20, 1787–1796 (2009).
Medzhitov, R., Schneider, D. S. & Soares, M. P. Disease tolerance as a defense strategy. Science 335, 936–941 (2012).
Ayres, J. S. & Schneider, D. S. Tolerance of infections. Annu. Rev. Immunol. 30, 271–294 (2012).
Ferreira, A. et al. Sickle hemoglobin confers tolerance to Plasmodium infection. Cell 145, 398–409 (2011).
Ramos, S. et al. Renal control of disease tolerance to malaria. Proc. Natl Acad. Sci. USA 116, 5681–5686 (2019).
Larsen, R. et al. A central role for free heme in the pathogenesis of severe sepsis. Sci. Transl. Med. 2, 51ra71 (2010).
Jin, K. et al. Activation of AMP-activated protein kinase during sepsis/inflammation improves survival by preserving cellular metabolic fitness. FASEB J. 34, 7036–7057 (2020).
Toro, J., Manrique-Caballero, C. L. & Gomez, H. Metabolic reprogramming and host tolerance: a novel concept to understand sepsis-associated AKI. J. Clin. Med. https://doi.org/10.3390/jcm10184184 (2021).
Wang, A. et al. Opposing effects of fasting metabolism on tissue tolerance in bacterial and viral inflammation. Cell 166, 1512–1525 e1512 (2016).
Basile, D. P., Leonard, E. C., Tonade, D., Friedrich, J. L. & Goenka, S. Distinct effects on long-term function of injured and contralateral kidneys following unilateral renal ischemia-reperfusion. Am. J. Physiol. Renal Physiol. 302, F625–F635 (2012).
Grgic, I. et al. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int. 82, 172–183 (2012).
Basile, D. P., Donohoe, D., Roethe, K. & Osborn, J. L. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am. J. Physiol. Renal Physiol. 281, F887–F899 (2001).
Pagtalunan, M. E., Olson, J. L., Tilney, N. L. & Meyer, T. W. Late consequences of acute ischemic injury to a solitary kidney. J. Am. Soc. Nephrol. 10, 366–373 (1999).
Sharma, A., Mucino, M. J. & Ronco, C. Renal functional reserve and renal recovery after acute kidney injury. Nephron Clin. Pract. 127, 94–100 (2014).
Jacob, K. A. et al. Intraoperative high-dose dexamethasone and severe AKI after cardiac surgery. J. Am. Soc. Nephrol. 26, 2947–2951 (2015).
Pickkers, P. et al. Effect of human recombinant alkaline phosphatase on 7-day creatinine clearance in patients with sepsis-associated acute kidney injury: a randomized clinical trial. JAMA 320, 1998–2009 (2018).
Tumlin, J. A. et al. Outcomes in patients with vasodilatory shock and renal replacement therapy treated with intravenous angiotensin II. Crit. Care Med. 46, 949–957 (2018).
Zhou, C. et al. Levosimendan for prevention of acute kidney injury after cardiac surgery: a meta-analysis of randomized controlled trials. Am. J. Kidney Dis. 67, 408–416 (2016).
Tholen, M., Ricksten, S. E. & Lannemyr, L. Effects of levosimendan on renal blood flow and glomerular filtration in patients with acute kidney injury after cardiac surgery: a double blind, randomized placebo-controlled study. Crit. Care 25, 207 (2021).
Swaminathan, M. et al. Allogeneic mesenchymal stem cells for treatment of AKI after cardiac surgery. J. Am. Soc. Nephrol. 29, 260–267 (2018).
Himmelfarb, J. et al. Perioperative THR-184 and AKI after cardiac surgery. J. Am. Soc. Nephrol. 29, 670–679 (2018).
Bromberg, J. S. et al. Renal function improvement following ANG-3777 treatment in patients at high risk for delayed graft function after kidney transplantation. Transplantation 105, 443–450 (2021).
Evans, L. et al. Surviving Sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit. Care Med. 49, e1063–e1143 (2021).
Kellum, J. A., Mythen, M. G. & Shaw, A. D. The 12th Consensus Conference of the Acute Dialysis Quality Initiative (ADQI XII). Br. J. Anaesth. 113, 729–731 (2014).
Chen, K. P. et al. Peripheral edema, central venous pressure, and risk of AKI in critical illness. Clin. J. Am. Soc. Nephrol. 11, 602–608 (2016).
Legrand, M. et al. Association between systemic hemodynamics and septic acute kidney injury in critically ill patients: a retrospective observational study. Crit. Care 17, R278 (2013).
Eskesen, T. G., Wetterslev, M. & Perner, A. Systematic review including re-analyses of 1148 individual data sets of central venous pressure as a predictor of fluid responsiveness. Intensive Care Med. 42, 324–332 (2016).
Payen, D. et al. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit. Care 12, R74 (2008).
Garzotto, F. et al. The dose response multicentre investigation on fluid assessment (DoReMIFA) in critically ill patients. Crit. Care 20, 196 (2016).
Douglas, I. S. et al. Fluid response evaluation in sepsis hypotension and shock: a randomized clinical trial. Chest 158, 1431–1445 (2020).
Woodcock, T. E. & Woodcock, T. M. Revised Starling equation and the glycocalyx model of transvascular fluid exchange: an improved paradigm for prescribing intravenous fluid therapy. Br. J. Anaesth. 108, 384–394 (2012).
Milford, E. M. & Reade, M. C. Resuscitation fluid choices to preserve the endothelial glycocalyx. Crit. Care 23, 77 (2019).
Byrne, L. et al. Unintended consequences: fluid resuscitation worsens shock in an ovine model of endotoxemia. Am. J. Respir. Crit. Care Med. 198, 1043–1054 (2018).
Self, W. H. et al. Liberal versus restrictive intravenous fluid therapy for early septic shock: rationale for a randomized trial. Ann. Emerg. Med. 72, 457–466 (2018).
Keijzers, G. et al. The Australasian resuscitation in sepsis evaluation: FLUid or vasopressors in emergency department Sepsis, a multicentre observational study (ARISE FLUIDS observational study): rationale, methods and analysis plan. Emerg. Med. Australas. 31, 90–96 (2019).
Vaara, S. T. et al. Restrictive fluid management versus usual care in acute kidney injury (REVERSE-AKI): a pilot randomized controlled feasibility trial. Intensive Care Med. 47, 665–673 (2021).
Meyhoff, T. S. et al. Restriction of intravenous fluid in ICU patients with septic shock. N. Engl. J. Med. 386, 2459–2470 (2022).
Zampieri, F. G. et al. Effect of intravenous fluid treatment with a balanced solution vs 0.9% saline solution on mortality in critically ill patients: the BaSICS randomized clinical trial. JAMA https://doi.org/10.1001/jama.2021.11684 (2021).
Finfer, S. et al. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N. Engl. J. Med. 350, 2247–2256 (2004).
Beran, A. et al. Balanced crystalloids versus normal saline in adults with sepsis: a comprehensive systematic review and meta-analysis. J. Clin. Med. https://doi.org/10.3390/jcm11071971 (2022).
Hammond, D. A. et al. Balanced crystalloids versus saline in critically ill adults: a systematic review and meta-analysis. Ann. Pharmacother. 54, 5–13 (2020).
Semler, M. W. et al. Balanced crystalloids versus saline in critically ill adults. N. Engl. J. Med. 378, 829–839 (2018).
Self, W. H. et al. Balanced crystalloids versus saline in noncritically ill adults. N. Engl. J. Med. 378, 819–828 (2018).
Caironi, P. et al. Albumin replacement in patients with severe sepsis or septic shock. N. Engl. J. Med. 370, 1412–1421 (2014).
Lewis, S. R. et al. Colloids versus crystalloids for fluid resuscitation in critically ill people. Cochrane Database Syst. Rev. 8, CD000567 (2018).
Sakr, Y. et al. Randomized controlled multicentre study of albumin replacement therapy in septic shock (ARISS): protocol for a randomized controlled trial. Trials 21, 1002 (2020).
Perner, A. et al. Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. N. Engl. J. Med. 367, 124–134 (2012).
Moeller, C. et al. How safe is gelatin? A systematic review and meta-analysis of gelatin-containing plasma expanders vs crystalloids and albumin. J. Crit. Care. 35, 75–83 (2016).
Ragaller, M. J., Theilen, H. & Koch, T. Volume replacement in critically ill patients with acute renal failure. J. Am. Soc. Nephrol. 12 (Suppl 17), S33–S39 (2001).
Dickenmann, M., Oettl, T. & Mihatsch, M. J. Osmotic nephrosis: acute kidney injury with accumulation of proximal tubular lysosomes due to administration of exogenous solutes. Am. J. Kidney Dis. 51, 491–503 (2008).
Jaber, S. et al. Sodium bicarbonate therapy for patients with severe metabolic acidaemia in the intensive care unit (BICAR-ICU): a multicentre, open-label, randomised controlled, phase 3 trial. Lancet 392, 31–40 (2018).
Russell, J. A. et al. Vasopressin versus norepinephrine infusion in patients with septic shock. N. Engl. J. Med. 358, 877–887 (2008).
Wichmann, S. et al. Loop diuretics in adult intensive care patients with fluid overload: a systematic review of randomised clinical trials with meta-analysis and trial sequential analysis. Ann. Intensive Care 12, 52 (2022).
Berthelsen, R. E. et al. Forced fluid removal in intensive care patients with acute kidney injury: the randomised FFAKI feasibility trial. Acta Anaesthesiol. Scand. 62, 936–944 (2018).
Silversides, J. A. et al. Feasibility of conservative fluid administration and deresuscitation compared with usual care in critical illness: the role of active deresuscitation after resuscitation-2 (RADAR-2) randomised clinical trial. Intensive Care Med. 48, 190–200 (2022).
Silbert, B. I. et al. Determinants of urinary output response to IV furosemide in acute kidney injury: a pharmacokinetic/pharmacodynamic study. Crit. Care Med. 44, e923–e929 (2016).
Khanna, A., Ostermann, M. & Bellomo, R. Angiotensin II for the treatment of vasodilatory shock. N. Engl. J. Med. 377, 2604 (2017).
Ostermann, M. et al. Recommendations on acute kidney injury biomarkers from the acute disease quality initiative consensus conference: a consensus statement. JAMA Netw. Open 3, e2019209 (2020).
Molinari, L. et al. Utility of biomarkers for sepsis-associated acute kidney injury staging. JAMA Netw. Open 5, e2212709 (2022).
Depret, F. et al. Incidence and outcome of subclinical acute kidney injury using penKid in critically ill patients. Am. J. Respir. Crit. Care Med. 202, 822–829 (2020).
Hollinger, A. et al. Proenkephalin A 119-159 (Penkid) is an early biomarker of septic acute kidney injury: the kidney in sepsis and septic shock (Kid-SSS) study. Kidney Int. Rep. 3, 1424–1433 (2018).
Martensson, J., Martling, C. R., Oldner, A. & Bell, M. Impact of sepsis on levels of plasma cystatin C in AKI and non-AKI patients. Nephrol. Dial. Transplant. 27, 576–581 (2012).
Frazee, E. et al. Cystatin C-guided vancomycin dosing in critically ill patients: a quality improvement project. Am. J. Kidney Dis. 69, 658–666 (2017).
Peters, B. J. et al. Impact of serum cystatin C-based glomerular filtration rate estimates on drug dose selection in hospitalized patients. Pharmacotherapy 38, 1068–1073 (2018).
Kashani, K. et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit. Care 17, R25 (2013).
Bagshaw, S. M. et al. Plasma and urine neutrophil gelatinase-associated lipocalin in septic versus non-septic acute kidney injury in critical illness. Intensive Care Med. 36, 452–461 (2010).
de Geus, H. R., Betjes, M. G., Schaick, R. & Groeneveld, J. A. Plasma NGAL similarly predicts acute kidney injury in sepsis and nonsepsis. Biomark. Med. 7, 415–421 (2013).
Basu, R. K. et al. Incorporation of biomarkers with the renal angina index for prediction of severe AKI in critically ill children. Clin. J. Am. Soc. Nephrol. 9, 654–662 (2014).
Honore, P. M. et al. Urinary tissue inhibitor of metalloproteinase-2 and insulin-like growth factor-binding protein 7 for risk stratification of acute kidney injury in patients with sepsis. Crit. Care Med. 44, 1851–1860 (2016).
R, D. S. & D, C. S. Recent advances in bedside device-based early detection of sepsis. J. Intensive Care Med. 37, 849–856 (2022).
Sinha, P., Churpek, M. M. & Calfee, C. S. Machine learning classifier models can identify acute respiratory distress syndrome phenotypes using readily available clinical data. Am. J. Respir. Crit. Care Med. 202, 996–1004 (2020).
Bhatraju, P. K. et al. Genetic variation implicates plasma angiopoietin-2 in the development of acute kidney injury sub-phenotypes. BMC Nephrol. 21, 284 (2020).
Knox, D. B., Lanspa, M. J., Kuttler, K. G., Brewer, S. C. & Brown, S. M. Phenotypic clusters within sepsis-associated multiple organ dysfunction syndrome. Intensive Care Med. 41, 814–822 (2015).
Chaudhary, K. et al. Utilization of deep learning for subphenotype identification in sepsis-associated acute kidney injury. Clin. J. Am. Soc. Nephrol. 15, 1557–1565 (2020).
Kwong, Y. D. et al. Using best subset regression to identify clinical characteristics and biomarkers associated with sepsis-associated acute kidney injury. Am. J. Physiol. Renal Physiol. 319, F979–F987 (2020).
Chawla, L. S. et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 workgroup. Nat. Rev. Nephrol. 13, 241–257 (2017).
Bhatraju, P. K. et al. Association between early recovery of kidney function after acute kidney injury and long-term clinical outcomes. JAMA Netw. Open 3, e202682 (2020).
Siew, E. D. et al. Timing of recovery from moderate to severe AKI and the risk for future loss of kidney function. Am. J. Kidney Dis. 75, 204–213 (2020).
Uhel, F. et al. Mortality and host response aberrations associated with transient and persistent acute kidney injury in critically ill patients with sepsis: a prospective cohort study. Intensive Care Med. 46, 1576–1589 (2020).
Macedo, E. et al. Quality of care after AKI development in the hospital: consensus from the 22nd Acute Disease Quality Initiative (ADQI) conference. Eur. J. Intern. Med. 80, 45–53 (2020).
Karsanji, D. J. et al. Disparity between nephrologists’ opinions and contemporary practices for community follow-up after AKI hospitalization. Clin. J. Am. Soc. Nephrol. 12, 1753–1761 (2017).
Menon, S. et al. Urinary biomarker incorporation into the renal angina index early in intensive care unit admission optimizes acute kidney injury prediction in critically ill children: a prospective cohort study. Nephrol. Dial. Transplant. 31, 586–594 (2016).
Darmon, M., Truche, A. S., Abdel-Nabey, M., Schnell, D. & Souweine, B. Early recognition of persistent acute kidney injury. Semin. Nephrol. 39, 431–441 (2019).
Peerapornratana, S. et al. Sepsis-associated acute kidney disease. Kidney Int. Rep. 5, 839–850 (2020).
Cutuli, S. L., Carelli, S., Grieco, D. L. & De Pascale, G. Immune modulation in critically ill septic patients. Medicina https://doi.org/10.3390/medicina57060552 (2021).
Jarczak, D., Kluge, S. & Nierhaus, A. Sepsis-pathophysiology and therapeutic concepts. Front. Med. 8, 628302 (2021).
Rimmele, T. & Kellum, J. A. Clinical review: blood purification for sepsis. Crit. Care 15, 205 (2011).
Girardot, T., Schneider, A. & Rimmele, T. Blood purification techniques for sepsis and septic AKI. Semin. Nephrol. 39, 505–514 (2019).
Husain-Syed, F. et al. Extracorporeal organ support (ECOS) in critical illness and acute kidney injury: from native to artificial organ crosstalk. Intensive Care Med. 44, 1447–1459 (2018).
Romagnoli, S., Ricci, Z. & Ronco, C. CRRT for sepsis-induced acute kidney injury. Curr. Opin. Crit. Care 24, 483–492 (2018).
Zarbock, A. et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: the ELAIN randomized clinical trial. JAMA 315, 2190–2199 (2016).
Gaudry, S. et al. Initiation strategies for renal-replacement therapy in the intensive care unit. N. Engl. J. Med. 375, 122–133 (2016).
Investigators, S.-A. et al. Timing of initiation of renal-replacement therapy in acute kidney injury. N. Engl. J. Med. 383, 240–251 (2020).
Barbar, S. D. et al. Timing of renal-replacement therapy in patients with acute kidney injury and sepsis. N. Engl. J. Med. 379, 1431–1442 (2018).
Chen, W. Y. et al. The timing of continuous renal replacement therapy initiation in sepsis-associated acute kidney injury in the intensive care unit: the CRTSAKI Study (Continuous RRT Timing in Sepsis-associated AKI in ICU): study protocol for a multicentre, randomised controlled trial. BMJ Open 11, e040718 (2021).
Martin-Loeches, I. et al. Surviving sepsis campaign: research opportunities for infection and blood purification therapies. Crit. Care Explor. 3, e0511 (2021).
Benichou, N. et al. Vascular access for renal replacement therapy among 459 critically ill patients: a pragmatic analysis of the randomized AKIKI trial. Ann. Intensive Care 11, 56 (2021).
Ronco, C. & Reis, T. Continuous renal replacement therapy and extended indications. Semin. Dial. 34, 550–560 (2021).
Stockmann, H. et al. CytoSorb rescue for COVID-19 patients with vasoplegic shock and multiple organ failure: a prospective, open-label, randomized controlled pilot study. Crit. Care Med. 50, 964–976 (2022).
Diab, M. et al. Cytokine hemoadsorption during cardiac surgery versus standard surgical care for infective endocarditis (REMOVE): results from a multicenter randomized controlled trial. Circulation 145, 959–968 (2022).
Ikeda, T., Ikeda, K., Suda, S. & Ueno, T. Usefulness of the endotoxin activity assay as a biomarker to assess the severity of endotoxemia in critically ill patients. Innate Immun. 20, 881–887 (2014).
Kellum, J. A., Foster, D. & Walker, P. M. Endotoxemic shock: a molecular phenotype in sepsis. Nephron https://doi.org/10.1159/000525548 (2022).
Biagioni, E. et al. Endotoxin activity levels as a prediction tool for risk of deterioration in patients with sepsis not admitted to the intensive care unit: a pilot observational study. J. Crit. Care 28, 612–617 (2013).
Romaschin, A. D., Klein, D. J. & Marshall, J. C. Bench-to-bedside review: clinical experience with the endotoxin activity assay. Crit. Care 16, 248 (2012).
Lee, W. Y., Kim, H. J. & Kim, E. Y. Impact of polymyxin B hemoperfusion therapy on high endotoxin activity level patients after successful infection source control: a prospective cohort study. Sci. Rep. 11, 24132 (2021).
Klein, D. J. et al. Polymyxin B hemoperfusion in endotoxemic septic shock patients without extreme endotoxemia: a post hoc analysis of the EUPHRATES trial. Intensive Care Med. 44, 2205–2212 (2018).
Payen, D. M. et al. Early use of polymyxin B hemoperfusion in patients with septic shock due to peritonitis: a multicenter randomized control trial. Intensive Care Med. 41, 975–984 (2015).
Ronco, C. et al. Extracorporeal therapies in non-renal disease: treatment of sepsis and the peak concentration hypothesis. Blood Purif. 22, 164–174 (2004).
Ronco, C. & Bellomo, R. Hemoperfusion: technical aspects and state of the art. Crit. Care 26, 135 (2022).
Berger, M. M. et al. Nutrients and micronutrients at risk during renal replacement therapy: a scoping review. Curr. Opin. Crit. Care 27, 367–377 (2021).
Shaw, A. R. & Mueller, B. A. Antibiotic dosing in continuous renal replacement therapy. Adv. Chronic Kidney Dis. 24, 219–227 (2017).
de Geus, H. R. H., Smeets, T., Hoek, R. A. S., Endeman, H. & Hunfeld, N. The Seraph-100 microbind affinity blood filter does not affect vancomycin, tacrolimus, and mycophenolic acid plasma concentrations. Blood Purif. 50, 971–975 (2021).
Schmidt, J. J., Eden, G., Seffer, M. T., Winkler, M. & Kielstein, J. T. In vitro elimination of anti-infective drugs by the Seraph® 100 Microbind® affinity blood filter. Clin. Kidney J. 13, 421–424 (2020).
Godi, I. et al. Vancomycin adsorption during in vitro model of hemoperfusion with HA380 cartridge. Nephron 145, 157–163 (2021).
Shimokawa, K. et al. Adsorption of various antimicrobial agents to endotoxin removal polymyxin-B immobilized fiber (Toraymyxin®). Colloids Surf. B Biointerfaces 90, 58–61 (2012).
Liebchen, U. et al. No clinically relevant removal of meropenem by cytokine adsorber CytoSorb® in critically ill patients with sepsis or septic shock. Intensive Care Med. 47, 1332–1333 (2021).
Konig, C. et al. In vitro removal of anti-infective agents by a novel cytokine adsorbent system. Int. J. Artif. Organs 42, 57–64 (2019).
Rhee, H. et al. The role of the specialized team in the operation of continuous renal replacement therapy: a single-center experience. BMC Nephrol. 18, 332 (2017).
Joannes-Boyau, O., Velly, L. & Ichai, C. Optimizing continuous renal replacement therapy in the ICU: a team strategy. Curr. Opin. Crit. Care 24, 476–482 (2018).
Neyra, J. A. & Goldstein, S. L. Optimizing renal replacement therapy deliverables through multidisciplinary work in the intensive care unit. Clin. Nephrol. 90, 1–5 (2018).
de Grooth, H. J., Parienti, J. J. & Oudemans-van Straaten, H. M. Should we rely on trials with disease- rather than patient-oriented endpoints? Intensive Care Med. 44, 464–466 (2018).
Weiss, S. L. et al. Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am. J. Respir. Crit. Care Med. 191, 1147–1157 (2015).
Goldstein, S. L. et al. Consensus-based recommendations on priority activities to address acute kidney injury in children: a modified Delphi consensus statement. JAMA Netw. Open 5, e2229442 (2022).
Stanski, N. L. et al. Recalibration of the renal angina index for pediatric septic shock. Kidney Int. Rep. 6, 1858–1867 (2021).
Stanski, N. L. et al. PERSEVERE biomarkers predict severe acute kidney injury and renal recovery in pediatric septic shock. Am. J. Respir. Crit. Care Med. 201, 848–855 (2020).
Weiss, S. L. et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Intensive Care Med. 46, 10–67 (2020).
Goldstein, S. L. et al. Use of the selective cytopheretic device in critically ill children. Kidney Int. Rep. 6, 775–784 (2021).
Ismail, O. Z. et al. Kidney injury molecule-1 protects against Gα12 activation and tissue damage in renal ischemia-reperfusion injury. Am. J. Pathol. 185, 1207–1215 (2015).
Stevens, P. E., Levin A.; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 3, 1–150 (2013).
Bagshaw, S. M. et al. Urinary biomarkers in septic acute kidney injury. Intensive Care Med. 33, 1285–1296 (2007).
Kamijo-Ikemori, A. et al. [Urinary L-type fatty acid binding protein (L-FABP) as a new urinary biomarker promulgated by the Ministry of Health, Labour and Welfare in Japan]. Rinsho. Byori. 61, 635–640 (2013).
de Geus, H. R., Bakker, J., Lesaffre, E. M. & le Noble, J. L. Neutrophil gelatinase-associated lipocalin at ICU admission predicts for acute kidney injury in adult patients. Am. J. Respir. Crit. Care Med. 183, 907–914 (2011).
Palsson, R. et al. Assessment of interobserver reliability of nephrologist examination of urine sediment. JAMA Netw. Open 3, e2013959 (2020).
Bagshaw, S. M. et al. A prospective evaluation of urine microscopy in septic and non-septic acute kidney injury. Nephrol. Dial. Transplant. 27, 582–588 (2012).
Toh, L., Bitker, L., Eastwood, G. M. & Bellomo, R. The incidence, characteristics, outcomes and associations of small short-term point-of-care creatinine increases in critically ill patients. J. Crit. Care 52, 227–232 (2019).
Rewa, O. G. et al. The furosemide stress test for prediction of worsening acute kidney injury in critically ill patients: a multicenter, prospective, observational study. J. Crit. Care 52, 109–114 (2019).
Husain-Syed, F. et al. Congestive nephropathy: a neglected entity? Proposal for diagnostic criteria and future perspectives. ESC Heart Fail. 8, 183–203 (2021).
Abdelhafez, M. et al. Diagnostic performance of fractional excretion of sodium for the differential diagnosis of acute kidney injury: a systematic review and meta-analysis. Clin. J. Am. Soc. Nephrol. 17, 785–797 (2022).
Basu, R. K., Kaddourah, A., Goldstein, S. L. & Investigators, A. S. Assessment of a renal angina index for prediction of severe acute kidney injury in critically ill children: a multicentre, multinational, prospective observational study. Lancet Child Adolesc. Health 2, 112–120 (2018).
Tverring, J. et al. Heparin-binding protein (HBP) improves prediction of sepsis-related acute kidney injury. Ann. Intensive Care 7, 105 (2017).
Ragan, D., Horvath-Szalai, Z., Szirmay, B. & Muhl, D. Novel damage biomarkers of sepsis-related acute kidney injury. EJIFCC 33, 11–22 (2022).
Hu, Q. et al. Association between admission serum procalcitonin and the occurrence of acute kidney injury in patients with septic shock: a retrospective cohort study. Sci. Prog. 104, 368504211043768 (2021).
Shiao, C. C., Chueh, Y. F., Yang, L. & Nsarf Using procalcitonin to predict acute kidney injury in septic patients: caveat emptor? J. Formos. Med. Assoc. 118, 542–544 (2019).
de Werra, I. et al. Cytokines, nitrite/nitrate, soluble tumor necrosis factor receptors, and procalcitonin concentrations: comparisons in patients with septic shock, cardiogenic shock, and bacterial pneumonia. Crit. Care Med. 25, 607–613 (1997).
Iglesias, J., Marik, P. E., Levine, J. S. & Norasept, I. I. S. I. Elevated serum levels of the type I and type II receptors for tumor necrosis factor-α as predictive factors for ARF in patients with septic shock. Am. J. Kidney Dis. 41, 62–75 (2003).
Su, L. X. et al. Diagnostic value of urine sTREM-1 for sepsis and relevant acute kidney injuries: a prospective study. Crit. Care 15, R250 (2011).
Maslove, D. M. et al. Redefining critical illness. Nat. Med. 28, 1141–1148 (2022).
Acknowledgements
The authors wish to acknowledge Ms. Gioia Vencato and Annamaria Saccardo, for their help in organizing this ADQI meeting. This conference was kindly supported by unrestricted educational grants from AM Pharma, Baxter, Biomerieux, Cytosorbants, Exthera Medical, Jafron, Ortho Clinical Diagnostics, Paion, Spectral and Sphingotec GmbH.
Author information
Authors and Affiliations
Contributions
All authors made equal contributions to the discussion of the content and researching data for the article. Members of each group contributed equally to writing their sections. L.G.F., A.Z. and M.K.N. combined the workgroup drafts and edited for style and length. All authors reviewed the final manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
A.Z. has received consulting fees from Astute-bioMérieux, Baxter, Bayer, Novartis, Guard Therapeutics, AM Pharma, Paion, Fresenius, research funding from Astute-bioMérieux, Fresenius, Baxter, and speakers fees from Astute-bioMérieux, Fresenius, Baxter; P.P. has received speaker’s honoraria/travel/consultancy reimbursements as a member of an advisory board or steering committee from Baxter, EBI, AM-Pharma, Sphingotec, Adrenomed, 4Teen4, and Paion; H.G. serves as scientific adviser for Novartis and Trilinear bioventures, and has received research grants from bioMérieux, Baxter and TES Pharma; M.J. has received honoraria and research support from Baxter Healthcare Corp, AM-Pharma, CLS Behring, Fresenius and Novartis; K.K. has received research grants from Philips Research North America and Google, speaker’s honorarium from Nikkiso Critical Care Medical Supplies (Shanghai) Co., Ltd, funding from National Institute of Diabetes and Digestive and Kidney Diseases grant (R01DK131586) and consulting fees from Baxter Inc. to Mayo Clinic; J.L.K. has received consulting fees from Astute-bioMérieux, Sphingotec, Pfizer, Baxter, Mallinckrodt, Novartis, Guard Therapeutics, research funding from Astute-bioMérieux Medical, Bioporto, NxStage, Fresenius, Satellite Healthcare, and speakers fees from NxStage medical; M.M. received lecture fees from bioMérieux, Fresenius Medical Care and Baxter; T. Reis has received funding for lectures, been consultant or advisory board member for AstraZeneca, B. Braun, Baxter, bioMérieux, Boehringer Ingelheim, Contatti Medical (CytoSorbents), Eurofarma, Fresenius Medical Care, Jafron, Lifepharma and Nova Biomedical; T. Rimmelé serves as a scientific adviser for Jafron and Exthera, has received funding for lectures from B. Braun, Baxter, bioMérieux, Exthera, Fresenius Medical Care, Estor and Jafron, and has received research grants from Baxter and Fresenius Medical Care; S.M.B. is supported by a Canada Research Chair in Critical Care Outcomes and Systems Evaluation; R.B. has received grant money, speaker’s fees and advisory board fees from Baxter Acute Care, Jafron Biomedical, CSL Behring, AM Pharma and Paion; V.C. has received lecture fees from Baxter, Estor-Toray and Aferetica-Cytosorbents; S.L.K.-G. is an elected member of the Executive Committee (or Council) of the Society of Critical Care Medicine (SCCM) (the views presented are those of the author and do not represent the views of SCCM); R.L.M. has consulting/advisory relationships with Baxter, AM Pharma, bioMérieux, Intercept, Mallinckrodt, GE Healthcare, Medtronic, CHF Solutions, Sphingotec, Abiomed, Nova Biomed, Sanofi, Renasym, Alexion, Fresenius, Abbott and Renibus; P.T.M. serves as a scientific adviser for AM-Pharma, Novartis, and Renibus Therapeutics; M.O. has received speaker honoraria from Fresenius Medical, Baxter and bioMérieux, and research funding from Fresenius Medical, Baxter and bioMérieux; J.P. has received research support from Jafron Biomedical Co Ltd and bioMérieux SA, and consultancy or lecture fees from Baxter Inc, Nikkiso Europe GmbH, Mission Therapeutics Ltd and Paion UK Ltd; A.S. has received speaker and/or consulting honoraria from Fresenius Medical Care, CytoSorbents SA, Jafron, Medtronics and B. Braun Avitum, and has received grants from the Leenaards foundation and B Braun Melsungen; A.T. has received consulting fees from Baxter and royalties from 0.5% citrate patent from Baxter; G.V. received lecture fees from Baxter; C.R. has been on the advisory boards or speaker’s bureau for Asahi, Aferetica, Baxter, bioMérieux, Cytosorbents, B. Braun, GE, Medica, Medtronic, Jafron and AstraZeneca; L.G.F. has received research support and lecture fees from Ortho Clinical Diagnostics, Baxter, Exthera and bioMérieux, and consulting fees from La Jolla Pharmaceuticals and Paion; the remaining authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks M. Bestle, K. Doi, D. Ponce and R. Raina for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ADQI: www.adqi.org
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zarbock, A., Nadim, M.K., Pickkers, P. et al. Sepsis-associated acute kidney injury: consensus report of the 28th Acute Disease Quality Initiative workgroup. Nat Rev Nephrol 19, 401–417 (2023). https://doi.org/10.1038/s41581-023-00683-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-023-00683-3
This article is cited by
-
Sepsis-associated acute kidney injury: recent advances in enrichment strategies, sub-phenotyping and clinical trials
Critical Care (2024)
-
Association between triglyceride glucose and acute kidney injury in patients with acute myocardial infarction: a propensity score‑matched analysis
BMC Cardiovascular Disorders (2024)
-
Identifying hub genes of sepsis-associated and hepatic encephalopathies based on bioinformatic analysis—focus on the two common encephalopathies of septic cirrhotic patients in ICU
BMC Medical Genomics (2024)
-
The influence of gender on the epidemiology of and outcome from sepsis associated acute kidney injury in ICU: a retrospective propensity-matched cohort study
European Journal of Medical Research (2024)
-
The pathophysiology of sepsis and precision-medicine-based immunotherapy
Nature Immunology (2024)