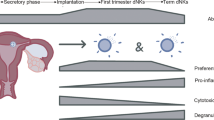
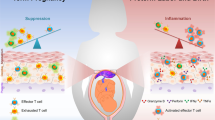
Abstract
Preeclampsia is a hypertensive disorder of major concern in pregnancy than can lead to intrauterine growth restriction, placental abruption and stillbirth. The pathophysiology of preeclampsia is multifactorial, including not only kidney dysfunction but also endothelial dysfunction, as the maternal endothelium becomes exposed to placental factors that are released into the circulation and increase systemic levels of vasoconstrictors, oxidative stress, anti-angiogenic factors and inflammatory mediators. Importantly, inflammation can lead to insufficient placental perfusion and low birthweight in offspring. Various innate and adaptive immune cells and mediators have been implicated in the development of preeclampsia, in which oxidative stress is associated with activation of the maternal inflammatory response. Immune cells such as regulatory T cells, macrophages, natural killer cells, and neutrophils are known to have major causative roles in the pathology of preeclampsia, but the contributions of additional immune cells such as B cells, inflammatory cytokines and anti-angiotensin II type 1 receptor autoantibodies are also now recognized. Immunological interventions, therefore, have therapeutic potential in this disease. Here, we provide an overview of the immune responses that are involved in the pathogenesis of preeclampsia, including the role of innate and adaptive immune cells and mediators.
Key points
-
Endothelial dysfunction, angiogenesis, spiral uterine artery remodelling and inadequate trophoblast invasion are key contributors to the genesis of hypertensive disorders during pregnancy.
-
An altered immune response might have a pivotal role in the development of preeclampsia, eclampsia and haemolysis, elevated liver enzymes and low platelets syndrome.
-
Insufficient or inadequate regulation of the immune system, activation of innate immune cells and imbalanced differentiation of T helper cell subsets create a cytotoxic environment that results in oxidative stress, endothelial dysfunction and intrauterine growth restriction.
-
T helper cells facilitate the activation of B cells that secrete anti-angiotensin II type 1 receptor autoantibodies, which can cause hypertension, cerebral dysfunction, kidney dysfunction and intrauterine growth restriction in response to placental ischaemia.
-
New therapeutics that target the pro-inflammatory response during preeclampsia have potential to attenuate the effects of the systemic factors that promote the development of this hypertensive disorder of pregnancy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Braunthal, S. & Brateanu, A. Hypertension in pregnancy: pathophysiology and treatment. SAGE Open Med. 7, 2050312119843700 (2019).
Wang, W. et al. Epidemiological trends of maternal hypertensive disorders of pregnancy at the global, regional, and national levels: a population‐based study. BMC Pregnancy Childbirth 21, 1–10 (2021).
Aneman, I. et al. Mechanisms of key innate immune cells in early-and late-onset preeclampsia. Front. Immunol. 11, 1864 (2020).
Shen, M. et al. Comparison of risk factors and outcomes of gestational hypertension and pre-eclampsia. PLoS One 12, e0175914 (2017).
Papageorghiou, A. T. et al. Preeclampsia and COVID-19: results from the INTERCOVID prospective longitudinal study. Am. J. Obstet. Gynecol. 225, 289.e1–289.e17 (2021).
Jena, M. K., Sharma, N. R., Petitt, M., Maulik, D. & Nayak, N. R. Pathogenesis of preeclampsia and therapeutic approaches targeting the placenta. Biomolecules 10, 953 (2020).
Deer, E. et al. Vascular endothelial mitochondrial oxidative stress in response to preeclampsia: a role for angiotensin II type 1 autoantibodies. Am. J. Obstet. Gynecol. MFM 3, 100275 (2021).
Magee, L. A. et al. The 2021 International Society for the Study of Hypertension in Pregnancy classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 27, 148–169 (2022).
Redman, C. Early and late onset preeclampsia: two sides of the same coin. Pregnancy Hypertens. 7, 58 (2017).
Martin Jr, J. N. & Morris, R. F. in Sex Differences in Cardiovascular Physiology and Pathophysiology 121–136 (Elsevier, 2019).
Fishel Bartal, M. & Sibai, B. M. Eclampsia in the 21st century. Am. J. Obstet. Gynecol. 226, S1237–S1253 (2020).
Shahzad, N., Irshad, B., Sami, N. & Nadeem, D. Comparison of dexamethasone versus betamethasone for the management of females with HELLP syndrome. Pak. J. Med. Health Sci. 11, 593–597 (2017).
Stojanovska, V. & Zenclussen, A. C. Innate and adaptive immune responses in HELLP syndrome. Front. Immunol. 11, 667 (2020).
Wisner, K. Gestational hypertension and preeclampsia. MCN Am. J. Matern. Child Nurs. 44, 170 (2019).
Burke, S. D. & Karumanchi, S. A. Hypertension 62, 1013–1014 (2013).
Pratt, A. et al. Placenta-derived angiogenic proteins and their contribution to the pathogenesis of preeclampsia. Angiogenesis 18, 115–123 (2015).
Possomato-Vieira, J. S. & Khalil, R. A. Mechanisms of endothelial dysfunction in hypertensive pregnancy and preeclampsia. Adv. Pharmacol. 77, 361–431 (2016).
Geldenhuys, J., Rossouw, T. M., Lombaard, H. A., Ehlers, M. M. & Kock, M. M. Disruption in the regulation of immune responses in the placental subtype of preeclampsia. Front. Immunol. 9, 1659 (2018).
Pijnenborg, R., Vercruysse, L. & Hanssens, M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta 27, 939–958 (2006).
Brosens, I., Robertson, W. B. & Dixon, H. G. The physiological response of the vessels of the placental bed to normal pregnancy. J. Pathol. Bacteriol. 93, 569–579 (1967).
Burton, G. J., Woods, A. W., Jauniaux, E. & Kingdom, J. C. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 30, 473–482 (2009).
Lyall, F., Robson, S. C. & Bulmer, J. N. Spiral artery remodeling and trophoblast invasion in preeclampsia and fetal growth restriction: relationship to clinical outcome. Hypertension 62, 1046–1054 (2013).
Enkhmaa, D. et al. Preeclampsia and vascular function: a window to future cardiovascular disease risk. J. Women’s Health 25, 284–291 (2016).
Cerdeira, A. S., Agrawal, S., Staff, A. C., Redman, C. W. & Vatish, M. Angiogenic factors: potential to change clinical practice in pre-eclampsia? BJOG 125, 1389–1395 (2018).
Bizerea, T. O. et al. The link between selenium, oxidative stress and pregnancy induced hypertensive disorders. Clin. Lab. 64, 1593–1610 (2018).
Sánchez-Aranguren, L. C., Prada, C. E., Riaño-Medina, C. E. & Lopez, M. Endothelial dysfunction and preeclampsia: role of oxidative stress. Front. Physiol. 5, 372 (2014).
McElwain, C. J., Tuboly, E., McCarthy, F. P. & McCarthy, C. M. Mechanisms of endothelial dysfunction in pre-eclampsia and gestational diabetes mellitus: windows into future cardiometabolic health? Front. Endocrinol. 11, 655 (2020).
Deer, E. et al. Vascular endothelial mitochondrial oxidative stress in response to preeclampsia: a role for AT1-AAs. Am. J. Obstet. Gynecol. MFM 3, 100275 (2021).
Mor, G., Aldo, P. & Alvero, A. B. The unique immunological and microbial aspects of pregnancy. Nat. Rev. Immunol. 17, 469–482 (2017).
Gant, N. F., Daley, G. L., Chand, S., Whalley, P. J. & MacDonald, P. C. A study of angiotensin II pressor response throughout primigravid pregnancy. J. Clin. Invest. 52, 2682–2689 (1973).
Madazli, R., Aydin, S., Uludag, S., Vildan, O. & Tolun, N. Maternal plasma levels of cytokines in normal and preeclamptic pregnancies and their relationship with diastolic blood pressure and fibronectin levels. Acta Obstet. Gynecol. Scand. 82, 797–802 (2003).
Szarka, A., Rigó, J., Lázár, L., Bekő, G. & Molvarec, A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol. 11, 1–9 (2010).
Jonsson, Y. et al. Cytokine mapping of sera from women with preeclampsia and normal pregnancies. J. Reprod. Immunol. 70, 83–91 (2006).
Aggarwal, R. et al. Association of pro- and anti-inflammatory cytokines in preeclampsia. J. Clin. Lab. Anal. 33, e22834 (2019).
LaMarca, B. B. D., Cockrell, K., Sullivan, E., Bennett, W. & Granger, J. P. Role of endothelin in mediating tumor necrosis factor-induced hypertension in pregnant rats. Hypertension 46, 82–86 (2005).
Formby, B. Immunologic response in pregnancy: its role in endocrine disorders of pregnancy and influence on the course of maternal autoimmune diseases. Endocrinol. Metab. Clin. North. Am. 24, 187–205 (1995).
Gadonski, G. et al. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of interleukin 6. Hypertension 48, 711–716 (2006).
Prins, J. et al. Altered expression of immune-associated genes in first-trimester human decidua of pregnancies later complicated with hypertension or foetal growth restriction. Placenta 33, 453–455 (2012).
Kharfi, A. et al. Trophoblastic remodeling in normal and preeclamptic pregnancies: implication of cytokines. Clin. Biochem. 36, 323–331 (2003).
Yoshizumi, M., Perrella, M. A., Burnett, J. Jr & Lee, M. E. Tumor necrosis factor downregulates an endothelial nitric oxide synthase mRNA by shortening its half-life. Circulation Res. 73, 205–209 (1993).
Maruotti, N., Cantatore, F. P., Crivellato, E., Vacca, A. & Ribatti, D. Angiogenesis in rheumatoid arthritis. Histol. Histopathol. 21, 557–566 (2006).
LaMarca, B. D., Ryan, M. J., Gilbert, J. S., Murphy, S. R. & Granger, J. P. Inflammatory cytokines in the pathophysiology of hypertension during preeclampsia. Curr. Hypertens. Rep. 9, 480–485 (2007).
LaMarca, B. et al. Autoantibodies to the angiotensin type I receptor in response to placental ischemia and tumor necrosis factor α in pregnant rats. Hypertension 52, 1168–1172 (2008).
LaMarca, B. et al. Hypertension in response to chronic reductions in uterine perfusion in pregnant rats: effect of tumor necrosis factor-α blockade. Hypertension 52, 1161–1167 (2008).
Murphy, S. R., LaMarca, B. B. D., Parrish, M., Cockrell, K. & Granger, J. P. Control of soluble fms-like tyrosine-1 (sFlt-1) production response to placental ischemia/hypoxia: role of tumor necrosis factor-α. Am. J. Physiol. Regul. Integr. Comp. Physiol. 304, R130–R135 (2013).
Cunningham, M. W. et al. Tumor necrosis factor alpha (TNF-α) blockade improves natural killer cell (NK) activation, hypertension, and mitochondrial oxidative stress in a preclinical rat model of preeclampsia. Hypertens. Pregnancy 39, 399–404 (2020).
Dhillion, P. et al. IL-17-mediated oxidative stress is an important stimulator of AT1-AA and hypertension during pregnancy. Am. J. Physiol. Regulatory Integr. Comp. Physiol. 303, R353–R358 (2012).
Boeldt, D. & Bird, I. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J. Endocrinol. 232, R27 (2017).
Krupp, J. et al. The loss of sustained Ca2+ signaling underlies suppressed endothelial nitric oxide production in preeclamptic pregnancies: implications for new therapy. Am. J. Physiol. Heart Circulatory Physiol. 305, H969–H979 (2013).
Maynard, S. E. et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Invest. 111, 649–658 (2003).
Vishnyakova, P., Elchaninov, A., Fatkhudinov, T. & Sukhikh, G. Role of the monocyte-macrophage system in normal pregnancy and preeclampsia. Int. J. Mol. Sci. 20, https://doi.org/10.3390/ijms20153695 (2019).
Faas, M. M. & de Vos, P. Uterine NK cells and macrophages in pregnancy. Placenta 56, 44–52 (2017).
Nguyen, T. G., Ward, C. M. & Morris, J. M. To B or not to B cells-mediate a healthy start to life. Clin. Exp. Immunol. 171, 124–134 (2013).
Lash, G. E. et al. Decidual macrophages: key regulators of vascular remodeling in human pregnancy. J. Leukoc. Biol. 100, 315–325 (2016).
Yao, Y., Xu, X. H. & Jin, L. Macrophage polarization in physiological and pathological pregnancy. Front. Immunol. 10, 792 (2019).
Reyes, L. & Golos, T. G. Hofbauer cells: their role in healthy and complicated pregnancy. Front. Immunol. 9, 2628 (2018).
Reister, F. et al. The distribution of macrophages in spiral arteries of the placental bed in pre-eclampsia differs from that in healthy patients. Placenta 20, 229–233 (1999).
Ma, Y., Ye, Y., Zhang, J., Ruan, C. C. & Gao, P. J. Immune imbalance is associated with the development of preeclampsia. Medicine 98, e15080 (2019).
Tsao, F. Y., Wu, M. Y., Chang, Y. L., Wu, C. T. & Ho, H. N. M1 macrophages decrease in the deciduae from normal pregnancies but not from spontaneous abortions or unexplained recurrent spontaneous abortions. J. Formos. Med. Assoc. 117, 204–211 (2018).
Reister, F. et al. Macrophage-induced apoptosis limits endovascular trophoblast invasion in the uterine wall of preeclamptic women. Lab. Invest. 81, 1143–1152 (2001).
Bert, S., Ward, E. J. & Nadkarni, S. Neutrophils in pregnancy: new insights into innate and adaptive immune regulation. Immunology 164, 665–676 (2021).
Dockree, S., Shine, B., Pavord, S., Impey, L. & Vatish, M. White blood cells in pregnancy: reference intervals for before and after delivery. EBioMedicine 74, 103715 (2021).
Canzoneri, B. J., Lewis, D. F., Groome, L. & Wang, Y. Increased neutrophil numbers account for leukocytosis in women with preeclampsia. Am. J. Perinatol. 26, 729–732 (2009).
Giaglis, S. et al. Neutrophil migration into the placenta: good, bad or deadly? Cell Adhes. Migr. 10, 208–225 (2016).
Gupta, A. K., Hasler, P., Holzgreve, W., Gebhardt, S. & Hahn, S. Induction of neutrophil extracellular DNA lattices by placental microparticles and IL-8 and their presence in preeclampsia. Hum. Immunol. 66, 1146–1154 (2005).
Gupta, A. K., Gebhardt, S., Hillermann, R., Holzgreve, W. & Hahn, S. Analysis of plasma elastase levels in early and late onset preeclampsia. Arch. Gynecol. Obstet. 273, 239–242 (2006).
Bajnok, A., Ivanova, M., Rigó, J. & Toldi, G. The distribution of activation markers and selectins on peripheral T lymphocytes in preeclampsia. Mediators Inflamm. 2017, 8045161 (2017).
Goksu Erol, A. Y., Nazli, M. & Elis Yildiz, S. Significance of platelet endothelial cell adhesion molecule-1 (PECAM-1) and intercellular adhesion molecule-1 (ICAM-1) expressions in preeclamptic placentae. Endocrine 42, 125–131 (2012).
Yang, W. et al. miR-125b enhances IL-8 production in early-onset severe preeclampsia by targeting sphingosine-1-phosphate lyase 1. PLoS One 11, e0166940 (2016).
Wang, Y. et al. Inhibition of pregnancy-associated granulocytic myeloid-derived suppressor cell expansion and arginase-1 production in preeclampsia. J. Reprod. Immunol. 127, 48–54 (2018).
Langers, I., Renoux, V. M., Thiry, M., Delvenne, P. & Jacobs, N. Natural killer cells: role in local tumor growth and metastasis. Biologics 6, 73–82 (2012).
Cornish, E. F., Filipovic, I., Åsenius, F., Williams, D. J. & McDonnell, T. Innate immune responses to acute viral infection during pregnancy. Front. Immunol. 11, 572567 (2020).
Trundley, A. & Moffett, A. Human uterine leukocytes and pregnancy. Tissue Antigens 63, 1–12 (2004).
Nishikawa, K. et al. Accumulation of CD16−CD56+ natural killer cells with high affinity interleukin 2 receptors in human early pregnancy decidua. Int. Immunol. 3, 743–750 (1991).
Kalkunte, S. et al. Evolution of non-cytotoxic uterine natural killer cells. Am. J. Reprod. Immunol. 59, 425–432 (2008).
Hanna, J. et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat. Med. 12, 1065–1074 (2006).
Fukui, A. et al. Uterine and circulating natural killer cells and their roles in women with recurrent pregnancy loss, implantation failure and preeclampsia. J. Reprod. Immunol. 90, 105–110, (2011).
Wallace, A. E., Host, A. J., Whitley, G. S. & Cartwright, J. E. Decidual natural killer cell interactions with trophoblasts are impaired in pregnancies at increased risk of preeclampsia. Am. J. Pathol. 183, 1853–1861 (2013).
Liu, Z., Chen, Y., Yang, Y. & Peng, J.-P. The effect on MHC class II expression and apoptosis in placenta by IFNγ administration. Contraception 65, 177–184 (2002).
Greenwood, J. et al. Ultrastructural studies of implantation sites from mice deficient in uterine natural killer cells. Placenta 21, 693–702 (2000).
Albrecht, E. D. & Pepe, G. J. Regulation of uterine spiral artery remodeling: a review. Reprod. Sci. 27, 1932–1942 (2020).
Sliz, A. et al. Gab3 is required for IL-2- and IL-15-induced NK cell expansion and limits trophoblast invasion during pregnancy. Sci. Immunol. 4, eaav3866 (2019).
Chakraborty, D., Rumi, M. K. & Soares, M. NK cells, hypoxia and trophoblast cell differentiation. Cell Cycle 11, 2427–2430 (2012).
Raghupathy, R. Cytokines as key players in the pathophysiology of preeclampsia. Med. Princ. Pract. 22, 8–19 (2013).
Liu, H. Y. et al. High-dose interferon-γ promotes abortion in mice by suppressing Treg and Th17 polarization. J. Interferon Cytokine Res. 34, 394–403 (2014).
Sun, Q.-H., Peng, J.-P., Xia, H.-F. & Yang, Y. IFN-γ promotes apoptosis of the uterus and placenta in pregnant rat and human cytotrophoblast cells. J. Interferon Cytokine Res. 27, 567–578 (2007).
El Costa, H. et al. Effector functions of human decidual NK cells in healthy early pregnancy are dependent on the specific engagement of natural cytotoxicity receptors. J. Reprod. Immunol. 82, 142–147 (2009).
Koopman, L. A. et al. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J. Exp. Med. 198, 1201–1212 (2003).
Hiby, S. E. et al. Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2. J. Clin. Invest. 120, 4102–4110 (2010).
Peraçoli, J. C., Fortes, M. R., Rudge, M. V., Rezkallah-Iwasso, M. T. & Peraçoli, M. T. Studies of natural killer cells in pregnancy-induced hypertension. Braz. J. Med. Biol. Res. 28, 655–661 (1995).
Borzychowski, A. M., Croy, B. A., Chan, W. L., Redman, C. W. & Sargent, I. L. Changes in systemic type 1 and type 2 immunity in normal pregnancy and pre-eclampsia may be mediated by natural killer cells. Eur. J. Immunol. 35, 3054–3063 (2005).
Zhang, Z. et al. Studies on activity of NK cells in preeclampsia patients. J. Huazhong Univ. Sci. Technol. Med. Sci. 24, 473–475 (2004).
Bachmayer, N., Rafik Hamad, R., Liszka, L., Bremme, K. & Sverremark-Ekström, E. Aberrant uterine natural killer (NK)-cell expression and altered placental and serum levels of the NK-cell promoting cytokine interleukin-12 in pre-eclampsia. Am. J. Reprod. Immunol. 56, 292–301 (2006).
Travis, O. K. et al. Interleukin-17 signaling mediates cytolytic natural killer cell activation in response to placental ischemia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 318, R1036–R1046 (2020).
Travis, O. K. et al. Adoptive transfer of placental ischemia-stimulated natural killer cells causes a preeclampsia-like phenotype in pregnant rats. Am. J. Reprod. Immunol. 85, e13386 (2021).
Richani, K. et al. Normal pregnancy is characterized by systemic activation of the complement system. J. Matern. Fetal Neonatal Med. 17, 239–245 (2005).
Derzsy, Z., Prohászka, Z., Rigó, J., Füst, G. & Molvarec, A. Activation of the complement system in normal pregnancy and preeclampsia. Mol. Immunol. 47, 1500–1506 (2010).
Reis, E. S., Mastellos, D. C., Hajishengallis, G. & Lambris, J. D. New insights into the immune functions of complement. Nat. Rev. Immunol. 19, 503–516 (2019).
Lynch, A. M. et al. Alternative complement pathway activation fragment Bb in early pregnancy as a predictor of preeclampsia. Am. J. Obstet. Gynecol. 198, 385.e1–385.e9 (2008).
Lynch, A. M. et al. Prepregnancy obesity and complement system activation in early pregnancy and the subsequent development of preeclampsia. Am. J. Obstet. Gynecol. 206, 428.e1–428.e8 (2012).
Lillegard, K. E. et al. Complement activation is critical for placental ischemia-induced hypertension in the rat. Mol. Immunol. 56, 91–97 (2013).
Burwick, R. M. et al. Terminal complement activation in preeclampsia. Obstet. Gynecol. 132, 1477–1485 (2018).
Rampersad, R., Barton, A., Sadovsky, Y. & Nelson, D. M. The C5b-9 membrane attack complex of complement activation localizes to villous trophoblast injury in vivo and modulates human trophoblast function in vitro. Placenta 29, 855–861 (2008).
Holers, V. M. et al. Complement C3 activation is required for antiphospholipid antibody-induced fetal loss. J. Exp. Med. 195, 211–220 (2002).
Denny, K. J. et al. Elevated complement factor C5a in maternal and umbilical cord plasma in preeclampsia. J. Reprod. Immunol. 97, 211–216 (2013).
Guseh, S. H. et al. Urinary excretion of C5b-9 is associated with the anti-angiogenic state in severe preeclampsia. Am. J. Reprod. Immunol. 73, 437–444 (2015).
He, Y. et al. Expression of the complement system’s activation factors in plasma of patients with early/late-onset severe pre-eclampsia. Am. J. Reprod. Immunol. 76, 205–211 (2016).
He, Y. D. et al. Dysregulation of complement system during pregnancy in patients with preeclampsia: a prospective study. Mol. Immunol. 122, 69–79 (2020).
Larsen, J. B. et al. Lectin pathway proteins of the complement system in normotensive pregnancy and pre-eclampsia. Am. J. Reprod. Immunol. 81, e13092 (2019).
Wu, W. et al. Polymorphisms in complement genes and risk of preeclampsia in Taiyuan, China. Inflamm. Res. 65, 837–845 (2016).
Lokki, A. I. et al. Analysis of complement. Front. Immunol. 8, 589 (2017).
Salmon, J. E. et al. Mutations in complement regulatory proteins predispose to preeclampsia: a genetic analysis of the PROMISSE cohort. PLoS Med. 8, e1001013 (2011).
Fang, C. J. et al. Membrane cofactor protein mutations in atypical hemolytic uremic syndrome (aHUS), fatal Stx-HUS, C3 glomerulonephritis, and the HELLP syndrome. Blood 111, 624–632 (2008).
Klonoff-Cohen, H. S., Savitz, D. A., Cefalo, R. C. & McCann, M. F. An epidemiologic study of contraception and preeclampsia. JAMA 262, 3143–3147 (1989).
Robertson, S. A., Care, A. S. & Moldenhauer, L. M. Regulatory T cells in embryo implantation and the immune response to pregnancy. J. Clin. Invest. 128, 4224–4235 (2018).
Masoudian, P. et al. Oocyte donation pregnancies and the risk of preeclampsia or gestational hypertension: a systematic review and metaanalysis. Am. J. Obstet. Gynecol. 214, 328–339 (2016).
Laresgoiti‐Servitje, E. A leading role for the immune system in the pathophysiology of preeclampsia. J. Leukoc. Biol. 94, 247–257 (2013).
Darmochwal-Kolarz, D. et al. The predominance of Th17 lymphocytes and decreased number and function of Treg cells in preeclampsia. J. Reprod. Immunol. 93, 75–81 (2012).
Kondelkova, K. et al. Regulatory T cells (TREG) and their roles in immune system with respect to immunopathological disorders. Acta Med. 53, 73–77 (2010).
Hosseini, A., Dolati, S., Hashemi, V., Abdollahpour‐Alitappeh, M. & Yousefi, M. Regulatory T and T helper 17 cells: their roles in preeclampsia. J. Cell. Physiol. 233, 6561–6573 (2018).
Harmon, A. C. et al. The role of inflammation in the pathology of preeclampsia. Clin. Sci. 130, 409–419 (2016).
Jørgensen, N., Persson, G. & Hviid, T. V. F. The tolerogenic function of regulatory T cells in pregnancy and cancer. Front. Immunol. 10, 911 (2019).
Lucca, L. E. & Dominguez-Villar, M. Modulation of regulatory T cell function and stability by co-inhibitory receptors. Nat. Rev. Immunol. 20, 680–693 (2020).
Tilburgs, T. et al. Fetal-maternal HLA-C mismatch is associated with decidual T cell activation and induction of functional T regulatory cells. J. Reprod. Immunol. 82, 148–157 (2009).
Saito, S., Shiozaki, A., Nakashima, A., Sakai, M. & Sasaki, Y. The role of the immune system in preeclampsia. Mol. Asp. Med. 28, 192–209 (2007).
Robertson, S. A. et al. Therapeutic potential of regulatory T cells in preeclampsia — opportunities and challenges. Front. Immunol. 10, 478 (2019).
Santner-Nanan, B. et al. Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J. Immunol. 183, 7023–7030 (2009).
Sasaki, Y. et al. Proportion of peripheral blood and decidual CD4+ CD25bright regulatory T cells in pre-eclampsia. Clin. Exp. Immunol. 149, 139–145 (2007).
Tsuda, S., Nakashima, A., Shima, T. & Saito, S. New paradigm in the role of regulatory T cells during pregnancy. Front. Immunol. 10, 573 (2019).
Novotny, S. R. et al. Activating autoantibodies to the angiotensin II type I receptor play an important role in mediating hypertension in response to adoptive transfer of CD4+ T lymphocytes from placental ischemic rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 302, R1197–R1201 (2012).
Harmon, A. C. et al. Placental CD4+ T cells isolated from preeclamptic women cause preeclampsia-like symptoms in pregnant nude-athymic rats. Pregnancy Hypertens. 15, 7–11 (2019).
Cornelius, D. C. et al. An increased population of regulatory T cells improves the pathophysiology of placental ischemia in a rat model of preeclampsia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 309, R884–R891 (2015).
Care, A. S. et al. Reduction in regulatory T cells in early pregnancy causes uterine artery dysfunction in mice. Hypertension 72, 177–187 (2018).
Deer, E. et al. CD4+ T cells cause renal and placental mitochondrial oxidative stress as mechanisms of hypertension in response to placental ischemia. Am. J. Physiol. Renal Physiol. 320, F47–F54 (2021).
Novotny, S. et al. CD4+ T cells play a critical role in mediating hypertension in response to placental ischemia. J. Hypertens. Open Access 2, 14873 (2013).
Reeve, K. et al. Placental CD4+ T cells from preeclamptic patients cause autoantibodies to the angiotensin II type I receptor and hypertension in a pregnant rat model of preeclampsia. Exploration Med. 3, 99–111 (2022).
Deer, E. et al. Progesterone induced blocking factor reduces hypertension and placental mitochondrial dysfunction in response to sFlt-1 during pregnancy. Cells 10, 2817 (2021).
Singh, R. P. et al. Th17 cells in inflammation and autoimmunity. Autoimmun. Rev. 13, 1174–1181 (2014).
Kamali, A. N. et al. A role for Th1-like Th17 cells in the pathogenesis of inflammatory and autoimmune disorders. Mol. Immunol. 105, 107–115 (2019).
Fu, B. et al. Natural killer cells promote immune tolerance by regulating inflammatory TH17 cells at the human maternal–fetal interface. Proc. Natl Acad. Sci. 110, E231–E240 (2013).
Fu, B., Tian, Z. & Wei, H. TH17 cells in human recurrent pregnancy loss and pre-eclampsia. Cell. Mol. Immunol. 11, 564–570 (2014).
Cornelius, D. C. et al. Reduced uterine perfusion pressure T-helper 17 cells cause pathophysiology associated with preeclampsia during pregnancy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 311, R1192–R1199 (2016).
Nguyen, H. et al. Interleukin-17 causes Rho-kinase-mediated endothelial dysfunction and hypertension. Cardiovascular Res. 97, 696–704 (2013).
Gaffen, S. L. An overview of IL-17 function and signaling. Cytokine 43, 402–407 (2008).
Dinh, Q. N., Drummond, G. R., Sobey, C. G. & Chrissobolis, S. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. Biomed. Res. Int. 2014, 406960 (2014).
Cornelius, D. C. et al. Administration of interleukin-17 soluble receptor C suppresses TH17 cells, oxidative stress, and hypertension in response to placental ischemia during pregnancy. Hypertension 62, 1068–1073 (2013).
Zhou, L. et al. TGF-β-induced Foxp3 inhibits TH17 cell differentiation by antagonizing RORγt function. Nature 453, 236–240 (2008).
Kawahara, T., Ohdan, H., Zhao, G., Yang, Y. G. & Sykes, M. Peritoneal cavity B cells are precursors of splenic IgM natural antibody-producing cells. J. Immunol. 171, 5406–5414 (2003).
Allman, D., Wilmore, J. R. & Gaudette, B. T. The continuing story of T-cell independent antibodies. Immunol. Rev. 288, 128–135 (2019).
Muzzio, D., Zenclussen, A. C. & Jensen, F. The role of B cells in pregnancy: the good and the bad. Am. J. Reprod. Immunol. 69, 408–412 (2013).
Jensen, F. et al. CD19+CD5+ cells as indicators of preeclampsia. Hypertension 59, 861–868 (2012).
Wallukat, G. et al. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J. Clin. Invest. 103, 945–952 (1999).
Torricelli, M. et al. Levels of antibodies against protein C and protein S in pregnancy and in preeclampsia. J. Matern. Fetal Neonatal Med. 22, 993–999 (2009).
Nor Azlin, M. I. et al. Thyroid autoantibodies and associated complications during pregnancy. J. Obstet. Gynaecol. 30, 675–678 (2010).
Hubel, C. A. et al. Agonistic angiotensin II type 1 receptor autoantibodies in postpartum women with a history of preeclampsia. Hypertension 49, 612–617 (2007).
Rieber-Mohn, A. B. et al. Auto-antibodies against the angiotensin II type I receptor in women with uteroplacental acute atherosis and preeclampsia at delivery and several years postpartum. J. Reprod. Immunol. 128, 23–29 (2018).
Zeng, B., Kwak-Kim, J., Liu, Y. & Liao, A. H. Treg cells are negatively correlated with increased memory B cells in pre-eclampsia while maintaining suppressive function on autologous B-cell proliferation. Am. J. Reprod. Immunol. 70, 454–463 (2013).
Cornelius, D. C. et al. Blockade of CD40 ligand for intercellular communication reduces hypertension, placental oxidative stress, and AT1-AA in response to adoptive transfer of CD4+ T lymphocytes from RUPP rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 309, R1243–R1250 (2015).
Robillard, P.-Y., Dekker, G., Scioscia, M. & Saito, S. Progress in the understanding of the pathophysiology of immunologic maladaptation related to early-onset preeclampsia and metabolic syndrome related to late-onset preeclampsia. Am. J. Obstet. Gynecol. 226, S867–S875 (2022).
Robillard, P.-Y. Epidemiological evidence that severe obese women (pre-pregnancy BMI≥ 40 kg/m2) should lose weight during their pregnancy. J. Matern. Fetal Neonatal Med. 35, 6618–6623 (2021).
Dechend, R. et al. AT1 receptor agonistic antibodies from preeclamptic patients cause vascular cells to express tissue factor. Circulation 101, 2382–2387 (2000).
Regal, J. F. et al. Role of IgM and angiotensin II type I receptor autoantibodies in local complement activation in placental ischemia-induced hypertension in the rat. Mol. Immunol. 78, 38–47 (2016).
Murphy, S. R. & Cockrell, K. Regulation of soluble fms-like tyrosine kinase-1 production in response to placental ischemia/hypoxia: role of angiotensin II. Physiol. Rep. 3, https://doi.org/10.14814/phy2.12310 (2015).
Quan, A. Fetopathy associated with exposure to angiotensin converting enzyme inhibitors and angiotensin receptor antagonists. Early Hum. Dev. 82, 23–28 (2006).
Elliott, S. E. et al. Characterization of antibody specificities associated with preeclampsia. Hypertension 63, 1086–1093 (2014).
Wenzel, K. et al. Angiotensin II type 1 receptor antibodies and increased angiotensin II sensitivity in pregnant rats. Hypertension 58, 77–84 (2011).
Irani, R. A. et al. The detrimental role of angiotensin receptor agonistic autoantibodies in intrauterine growth restriction seen in preeclampsia. J. Exp. Med. 206, 2809–2822 (2009).
Xia, Y., Wen, H., Bobst, S., Day, M. C. & Kellems, R. E. Maternal autoantibodies from preeclamptic patients activate angiotensin receptors on human trophoblast cells. J. Soc. Gynecol. Investig. 10, 82–93 (2003).
Thway, T. M. et al. Antibodies from preeclamptic patients stimulate increased intracellular Ca2+ mobilization through angiotensin receptor activation. Circulation 110, 1612–1619 (2004).
Brewer, J. et al. Endothelin-1, oxidative stress, and endogenous angiotensin II: mechanisms of angiotensin II type I receptor autoantibody-enhanced renal and blood pressure response during pregnancy. Hypertension 62, 886–892 (2013).
Cunningham, M. W. et al. AT1-AA (Angiotensin II Type 1 receptor agonistic autoantibody) blockade prevents preeclamptic symptoms in placental ischemic rats. Hypertension 71, 886–893 (2018).
Vaka, V. R. et al. Blockade of endogenous angiotensin II type I receptor agonistic autoantibody activity improves mitochondrial reactive oxygen species and hypertension in a rat model of preeclampsia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 318, R256–R262 (2020).
Duncan, J. W. et al. Angiotensin II type 1 receptor autoantibody blockade improves cerebral blood flow autoregulation and hypertension in a preclinical model of preeclampsia. Hypertens. Pregnancy 39, 451–460 (2020).
Panayi, G. S. B cells: a fundamental role in the pathogenesis of rheumatoid arthritis? Rheumatology 44, ii3–ii7 (2005).
Cianchini, G. et al. Treatment of severe pemphigus with rituximab: report of 12 cases and a review of the literature. Arch. Dermatol. 143, 1033–1038 (2007).
Cianchini, G. et al. Severe persistent pemphigoid gestationis: long-term remission with rituximab. Br. J. Dermatol. 157, 388–389 (2007).
LaMarca, B. et al. Hypertension in response to placental ischemia during pregnancy: role of B lymphocytes. Hypertension 57, 865–871 (2011).
Chakravarty, E. F., Murray, E. R., Kelman, A. & Farmer, P. Pregnancy outcomes after maternal exposure to rituximab. Blood 117, 1499–1506 (2011).
Das, G. et al. Rituximab before and during pregnancy: a systematic review, and a case series in MS and NMOSD. Neurol. Neuroimmunol. Neuroinflamm. 5, e453 (2018).
Smith, J. B. et al. Rituximab, MS, and pregnancy. Neurol. Neuroimmunol. Neuroinflamm. 7, https://doi.org/10.1212/NXI.0000000000000734 (2020).
Friedrichs, B. et al. The effects of rituximab treatment during pregnancy on a neonate. Haematologica 91, 1426–1427 (2006).
Vaught, A. J. et al. Direct evidence of complement activation in HELLP syndrome: a link to atypical hemolytic uremic syndrome. Exp. Hematol. 44, 390–398 (2016).
Burwick, R. M., Fichorova, R. N., Dawood, H. Y., Yamamoto, H. S. & Feinberg, B. B. Urinary excretion of C5b-9 in severe preeclampsia: tipping the balance of complement activation in pregnancy. Hypertension 62, 1040–1045 (2013).
Stefanovic, V. The extended use of eculizumab in pregnancy and complement activation-associated diseases affecting maternal, fetal and neonatal kidneys — the future is now? J. Clin. Med. 8, 407 (2019).
Lokki, A. I., Haapio, M. & Heikkinen-Eloranta, J. Eculizumab treatment for postpartum HELLP syndrome and aHUS — case report. Front. Immunol. 11, 548 (2020).
Smith, D. D. & Costantine, M. M. The role of statins in the prevention of preeclampsia. Am. J. Obstet. Gynecol. 226, S1171–S1181 (2020).
Lefkou, E. et al. Pravastatin improves pregnancy outcomes in obstetric antiphospholipid syndrome refractory to antithrombotic therapy. J. Clin. Investig. 126, 2933–2940 (2016).
Rolnik, D. L. et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N. Engl. J. Med. 377, 613–622 (2017).
Dodd, J. M., Jones, L., Flenady, V., Cincotta, R. & Crowther, C. A. Prenatal administration of progesterone for preventing preterm birth in women considered to be at risk of preterm birth. Cochrane Database Syst. Rev. (2013).
Amaral, L. M. et al. 17-Hydroxyprogesterone caproate improves hypertension and renal endothelin-1 in response to sFlt-1 induced hypertension in pregnant rats. Pregnancy Hypertens. 22, 151–155 (2020).
Singh, J., Ahmed, A. & Girardi, G. Role of complement component C1q in the onset of preeclampsia in mice. Hypertension 58, 716–724 (2011).
Shah, D. M. Role of the renin-angiotensin system in the pathogenesis of preeclampsia. Am. J. Physiol. Renal Physiol. 288, F614–F625 (2005).
Xia, Y., Ramin, S. M. & Kellems, R. E. Potential roles of angiotensin receptor-activating autoantibody in the pathophysiology of preeclampsia. Hypertension 50, 269–275 (2007).
Cunningham, M. W. et al. Agonistic autoantibodies to the angiotensin II type 1 receptor enhance angiotensin II-induced renal vascular sensitivity and reduce renal function during pregnancy. Hypertension 68, 1308–1313 (2016).
Zhang, W. et al. Mechanism of agonistic angiotensin II type I receptor autoantibody-amplified contractile response to Ang II in the isolated rat thoracic aorta. Acta Biochim. Biophys. Sin. 47, 851–856 (2015).
Singh, K. D. et al. Novel allosteric ligands of the angiotensin receptor AT1R as autoantibody blockers. Proc. Natl Acad. Sci. USA 118, https://doi.org/10.1073/pnas.2019126118 (2021).
LaMarca, B. et al. Hypertension in response to autoantibodies to the angiotensin II type I receptor (AT1-AA) in pregnant rats: role of endothelin-1. Hypertension 54, 905–909 (2009).
Parrish, M. R. et al. Hypertension in response to AT1-AA: role of reactive oxygen species in pregnancy-induced hypertension. Am. J. Hypertens. 24, 835–840 (2011).
Zhou, C. C. et al. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat. Med. 14, 855–862 (2008).
Parrish, M. R. et al. The effect of immune factors, tumor necrosis factor-α, and agonistic autoantibodies to the angiotensin II type I receptor on soluble fms-like tyrosine-1 and soluble endoglin production in response to hypertension during pregnancy. Am. J. Hypertens. 23, 911–916 (2010).
Zhou, C. C. et al. Angiotensin II induces soluble fms-like tyrosine kinase-1 release via calcineurin signaling pathway in pregnancy. Circ. Res. 100, 88–95 (2007).
Zhou, C. C. Upregulation of placental soluble fms-like tyrosine kinase 1 by AT1 receptor agonistic autoantibodies in preeclampsia. Hypertens. Pregnancy 25, 38 (2006).
Zhou, C. C. et al. Angiotensin receptor agonistic autoantibody-mediated tumor necrosis factor-α induction contributes to increased soluble endoglin production in preeclampsia. Circulation 121, 436–444 (2010).
Cunningham, M. W. et al. Renal natural killer cell activation and mitochondrial oxidative stress; new mechanisms in AT1-AA mediated hypertensive pregnancy. Pregnancy Hypertens. 15, 72–77 (2019).
Wang, Z. C. et al. Valsartan reduces AT1-AA-induced apoptosis through suppression oxidative stress mediated ER stress in endothelial progenitor cells. Eur. Rev. Med. Pharmacol. Sci. 21, 1159–1168 (2017).
Dechend, R. et al. AT1 receptor agonistic antibodies from preeclamptic patients stimulate NADPH oxidase. Circulation 107, 1632–1639 (2003).
Vaka, V. R. et al. Role of mitochondrial dysfunction and reactive oxygen species in mediating hypertension in the reduced uterine perfusion pressure rat model of preeclampsia. Hypertension 72, 703–711 (2018).
Parrish, M. R. et al. Angiotensin II type 1 autoantibody induced hypertension during pregnancy is associated with renal endothelial dysfunction. Gend. Med. 8, 184–188 (2011).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article and wrote the manuscript. E.D. and B.L. made substantial contributions to discussions of the content, and reviewed or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks S. Saito and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Fetal resorption
-
The disintegration and absorption of one or more fetuses in the uterus after the completion of organogenesis.
- Flow-mediated dilation
-
A vascular function test traditionally performed in the brachial artery, which measures the change in artery diameter in response to reactive hyperaemia.
- Hofbauer cells
-
A diverse population of fetal macrophages that reside within placental tissue (in the chorionic villus); they are present as early as 18 days post-conception and persist throughout pregnancy.
- Spiral uterine artery remodelling
-
An adaptive process in pregnancy that allows placental blood flow volume to increase while blood flow resistance decreases.
- Syncytiotrophoblasts
-
A specialized, continuous layer of epithelial cells that cover the surface of embryonic placental villi and are in direct contact with maternal blood.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Deer, E., Herrock, O., Campbell, N. et al. The role of immune cells and mediators in preeclampsia. Nat Rev Nephrol 19, 257–270 (2023). https://doi.org/10.1038/s41581-022-00670-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-022-00670-0
This article is cited by
-
Characterizing immune variation and diagnostic indicators of preeclampsia by single-cell RNA sequencing and machine learning
Communications Biology (2024)
-
Advances in pathogenesis of preeclampsia
Archives of Gynecology and Obstetrics (2024)
-
Identification of novel first-trimester serum biomarkers for early prediction of preeclampsia
Journal of Translational Medicine (2023)
-
IL-32-driven neutrophil activation in preeclampsia
Cellular & Molecular Immunology (2023)