Abstract
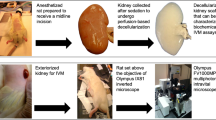
Kidney fibrosis, characterized by excessive deposition of extracellular matrix (ECM) that leads to tissue scarring, is the final common outcome of a wide variety of chronic kidney diseases. Rather than being distributed uniformly across the kidney parenchyma, renal fibrotic lesions initiate at certain focal sites in which the fibrogenic niche is formed in a spatially confined fashion. This niche provides a unique tissue microenvironment that is orchestrated by a specialized ECM network consisting of de novo-induced matricellular proteins. Other structural elements of the fibrogenic niche include kidney resident and infiltrated inflammatory cells, extracellular vesicles, soluble factors and metabolites. ECM proteins in the fibrogenic niche recruit soluble factors including WNTs and transforming growth factor-β from the extracellular milieu, creating a distinctive profibrotic microenvironment. Studies using decellularized ECM scaffolds from fibrotic kidneys show that the fibrogenic niche autonomously promotes fibroblast proliferation, tubular injury, macrophage activation and endothelial cell depletion, pathological features that recapitulate key events in the pathogenesis of chronic kidney disease. The concept of the fibrogenic niche represents a paradigm shift in understanding of the mechanism of kidney fibrosis that could lead to the development of non-invasive biomarkers and novel therapies not only for chronic kidney disease, but also for fibrotic diseases of other organs.
Key points
-
Kidney fibrosis initiates at certain focal sites in which the fibrogenic niche is formed; this niche provides a specialized microenvironment that triggers fibroblast activation and induces fibrotic lesions.
-
The structural elements of the fibrogenic niche include kidney resident and infiltrated inflammatory cells, extracellular matrix (ECM) network, extracellular vesicles, soluble factors and metabolites.
-
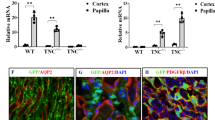
The fibrogenic niche is orchestrated by a specialized ECM network that consists of structurally unrelated, de novo-induced matricellular proteins such as tenascin C, connective tissue growth factor, fibrillin 1 and periostin.
-
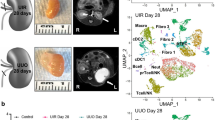
Decellularized ECM scaffolds from fibrotic kidneys spontaneously promote fibroblast proliferation, tubular epithelial-to-mesenchymal transition, macrophage activation and endothelial cell apoptosis, and therefore recapitulate major events in the pathogenesis of chronic kidney disease.
-
Components of the fibrogenic niche such as tenascin C recruit various soluble factors from the extracellular milieu, including WNTs, hedgehog and transforming growth factor-β, resulting in a distinctive microenvironment with high levels of profibrotic factors.
-
The development of therapies that target and disrupt the formation of the fibrogenic niche could be a novel and effective strategy for the treatment of fibrotic chronic kidney disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Webster, A. C., Nagler, E. V., Morton, R. L. & Masson, P. Chronic kidney disease. Lancet 389, 1238–1252 (2017).
GBD Chronic Kidney Disease Collaboration Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 395, 709–733 (2020).
Chen, T. K., Knicely, D. H. & Grams, M. E. Chronic kidney disease diagnosis and management: a review. JAMA 322, 1294–1304 (2019).
Ruiz-Ortega, M., Rayego-Mateos, S., Lamas, S., Ortiz, A. & Rodrigues-Diez, R. R. Targeting the progression of chronic kidney disease. Nat. Rev. Nephrol. 16, 269–288 (2020).
Breyer, M. D. & Susztak, K. The next generation of therapeutics for chronic kidney disease. Nat. Rev. Drug Discov. 15, 568–588 (2016).
Fu, H. et al. Tenascin-C is a major component of the fibrogenic niche in kidney fibrosis. J. Am. Soc. Nephrol. 28, 785–801 (2017).
Tan, R. J.; Bastacky, S. I.; Liu, Y. in Molecular Pathology 2nd edn (eds Coleman W. B. & Tsongalis G. J.) 531–553 (Elsevier, 2018).
Brody, S. L. et al. Chemokine receptor 2-targeted molecular imaging in pulmonary fibrosis. A clinical trial. Am. J. Resp. Crit. Care 203, 78–89 (2021).
Gupta, V., Gupta, I., Park, J., Bram, Y. & Schwartz, R. E. Hedgehog signaling demarcates a niche of fibrogenic peribiliary mesenchymal cells. Gastroenterology 159, 624–638 (2020).
Ramachandran, P. et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature 575, 512–518 (2019).
Herrera, J., Henke, C. A. & Bitterman, P. B. Extracellular matrix as a driver of progressive fibrosis. J. Clin. Invest. 128, 45–53 (2018).
Lovisa, S., Zeisberg, M. & Kalluri, R. Partial epithelial-to-mesenchymal transition and other new mechanisms of kidney fibrosis. Trends Endocrinol. Metab. 27, 681–695 (2016).
Zhou, D. & Liu, Y. Renal fibrosis in 2015: understanding the mechanisms of kidney fibrosis. Nat. Rev. Nephrol. 12, 68–70 (2016).
Liu, Y. Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol. 7, 684–696 (2011).
Humphreys, B. D. Mechanisms of renal fibrosis. Annu. Rev. Physiol. 80, 309–326 (2018).
Schunk, S. J., Floege, J., Fliser, D. & Speer, T. WNT-beta-catenin signalling — a versatile player in kidney injury and repair. Nat. Rev. Nephrol. 17, 172–184 (2021).
Moeller, M. J. et al. New aspects of kidney fibrosis-from mechanisms of injury to modulation of disease. Front. Med. 8, 814497 (2021).
Ding, H. et al. MicroRNA-10 negatively regulates inflammation in diabetic kidney via targeting activation of the NLRP3 inflammasome. Mol. Ther. 29, 2308–2320 (2021).
Lane, S. W., Williams, D. A. & Watt, F. M. Modulating the stem cell niche for tissue regeneration. Nat. Biotechnol. 32, 795–803 (2014).
Potapov, I., Garcia-Prat, L., Ravichandran, S., Munoz-Canoves, P. & Del Sol, A. Computational modelling of stem cell-niche interactions facilitates discovery of strategies to enhance tissue regeneration and counteract ageing. FEBS J. 289, 1486–1491 (2021).
Liu, Y. New insights into epithelial-mesenchymal transition in kidney fibrosis. J. Am. Soc. Nephrol. 21, 212–222 (2010).
Miao, J. et al. Wnt/beta-catenin/RAS signaling mediates age-related renal fibrosis and is associated with mitochondrial dysfunction. Aging Cell 18, e13004 (2019).
Canaud, G. et al. Cyclin G1 and TASCC regulate kidney epithelial cell G2-M arrest and fibrotic maladaptive repair. Sci. Transl Med. 11, eaav4754 (2019).
Ferenbach, D. A. & Bonventre, J. V. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat. Rev. Nephrol. 11, 264–276 (2015).
Sheng, L. & Zhuang, S. New insights into the role and mechanism of partial epithelial-mesenchymal transition in kidney fibrosis. Front. Physiol. 11, 569322 (2020).
Chen, S. et al. β-Catenin-controlled tubular cell-derived exosomes play a key role in fibroblast activation via the OPN-CD44 axis. J. Extracell. Vesicles 11, e12203 (2022).
Xu, J., Zhou, L. & Liu, Y. Cellular senescence in kidney fibrosis: pathologic significance and therapeutic strategies. Front. Pharmacol. 11, 601325 (2020).
Baar, M. P. et al. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell 169, 132–147.e16 (2017).
Tan, H., Xu, J. & Liu, Y. Ageing, cellular senescence and chronic kidney disease: experimental evidence. Curr. Opin. Nephrol. Hypertens. 31, 235–243 (2022).
Hurwitz, S. N. & Meckes, D. G. Jr Extracellular vesicle integrins distinguish unique cancers. Proteomes 7, 14 (2019).
Kang, M., Jordan, V., Blenkiron, C. & Chamley, L. W. Biodistribution of extracellular vesicles following administration into animals: a systematic review. J. Extracell. Vesicles 10, e12085 (2021).
Brigstock, D. R. Extracellular vesicles in organ fibrosis: mechanisms, therapies, and diagnostics. Cells 10, 1596 (2021).
Karpman, D., Stahl, A. L. & Arvidsson, I. Extracellular vesicles in renal disease. Nat. Rev. Nephrol. 13, 545–562 (2017).
Kok, H. M., Falke, L. L., Goldschmeding, R. & Nguyen, T. Q. Targeting CTGF, EGF and PDGF pathways to prevent progression of kidney disease. Nat. Rev. Nephrol. 10, 700–711 (2014).
Meng, X. M., Nikolic-Paterson, D. J. & Lan, H. Y. TGF-beta: the master regulator of fibrosis. Nat. Rev. Nephrol. 12, 325–338 (2016).
Kooman, J. P., Kotanko, P., Schols, A. M., Shiels, P. G. & Stenvinkel, P. Chronic kidney disease and premature ageing. Nat. Rev. Nephrol. 10, 732–742 (2014).
Zhu, H. et al. Tenascin-C promotes acute kidney injury to chronic kidney disease progression by impairing tubular integrity via αvβ6 integrin signaling. Kidney Int. 97, 1017–1031 (2020).
Theocharis, A. D., Manou, D. & Karamanos, N. K. The extracellular matrix as a multitasking player in disease. FEBS J. 286, 2830–2869 (2019).
Vasanthan, K. S., Srinivasan, V. & Pandita, D. Extracellular matrix extraction techniques and applications in biomedical engineering. Regen. Med. 16, 775–802 (2021).
Sobreiro-Almeida, R., Quinteira, R. & Neves, N. M. Renal regeneration: the role of extracellular matrix and current ECM-based tissue engineered strategies. Adv. Healthc. Mater. 10, e2100160 (2021).
Safdari, M., Bibak, B., Soltani, H. & Hashemi, J. Recent advancements in decellularized matrix technology for bone tissue engineering. Differentiation 121, 25–34 (2021).
Li, L. et al. Fibrillin-1-enriched microenvironment drives endothelial injury and vascular rarefaction in chronic kidney disease. Sci. Adv. 7, eabc7170 (2021).
Feng, D., Ngov, C., Henley, N., Boufaied, N. & Gerarduzzi, C. Characterization of matricellular protein expression signatures in mechanistically diverse mouse models of kidney injury. Sci. Rep. 9, 16736 (2019).
Yin, Q. & Liu, H. Connective tissue growth factor and renal fibrosis. Adv. Exp. Med. Biol. 1165, 365–380 (2019).
Mael-Ainin, M., Abed, A., Conway, S. J., Dussaule, J. C. & Chatziantoniou, C. Inhibition of periostin expression protects against the development of renal inflammation and fibrosis. J. Am. Soc. Nephrol. 25, 1724–1736 (2014).
Murphy-Ullrich, J. E. Thrombospondin 1 and its diverse roles as a regulator of extracellular matrix in fibrotic disease. J. Histochem. Cytochem. 67, 683–699 (2019).
Wong, S. L. & Sukkar, M. B. The SPARC protein: an overview of its role in lung cancer and pulmonary fibrosis and its potential role in chronic airways disease. Br. J. Pharmacol. 174, 3–14 (2017).
Gerarduzzi, C. et al. Silencing SMOC2 ameliorates kidney fibrosis by inhibiting fibroblast to myofibroblast transformation. JCI Insight 2, e90299 (2017).
Gerarduzzi, C., Hartmann, U., Leask, A. & Drobetsky, E. The matrix revolution: matricellular proteins and restructuring of the cancer microenvironment. Cancer Res. 80, 2705–2717 (2020).
Wallace, D. P. Periostin in the kidney. Adv. Exp. Med. Biol. 1132, 99–112 (2019).
Theocharis, A. D., Skandalis, S. S., Gialeli, C. & Karamanos, N. K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 97, 4–27 (2016).
Tucic, M., Stamenkovic, V. & Andjus, P. The extracellular matrix glycoprotein tenascin C and adult neurogenesis. Front. Cell Dev. Biol. 9, 674199 (2021).
Midwood, K. S., Chiquet, M., Tucker, R. P. & Orend, G. Tenascin-C at a glance. J. Cell Sci. 129, 4321–4327 (2016).
Rayego-Mateos, S. et al. Interplay between extracellular matrix components and cellular and molecular mechanisms in kidney fibrosis. Clin. Sci. 135, 1999–2029 (2021).
Toda, N., Mukoyama, M., Yanagita, M. & Yokoi, H. CTGF in kidney fibrosis and glomerulonephritis. Inflamm. Regen. 38, 14 (2018).
Chen, Z. et al. Connective tissue growth factor: from molecular understandings to drug discovery. Front. Cell Dev. Biol. 8, 593269 (2020).
Zaykov, V. & Chaqour, B. The CCN2/CTGF interactome: an approach to understanding the versatility of CCN2/CTGF molecular activities. J. Cell Commun. Signal. 15, 567–580 (2021).
Robertson, I., Jensen, S. & Handford, P. TB domain proteins: evolutionary insights into the multifaceted roles of fibrillins and LTBPs. Biochem. J. 433, 263–276 (2011).
Adamo, C. S., Zuk, A. V. & Sengle, G. The fibrillin microfibril/elastic fibre network: a critical extracellular supramolecular scaffold to balance skin homoeostasis. Exp. Dermatol. 30, 25–37 (2021).
Prakoura, N. & Chatziantoniou, C. Periostin in kidney diseases. Cell Mol. Life Sci. 74, 4315–4320 (2017).
An, J. N. et al. Periostin induces kidney fibrosis after acute kidney injury via the p38 MAPK pathway. Am. J. Physiol. Renal Physiol. 316, F426–F437 (2019).
Prakoura, N. et al. NFκB-induced periostin activates integrin-beta3 signaling to promote renal injury in GN. J. Am. Soc. Nephrol. 28, 1475–1490 (2017).
Bian, X. et al. Knockdown of periostin attenuates 5/6 nephrectomy-induced intrarenal renin-angiotensin system activation, fibrosis, and inflammation in rats. J. Cell Physiol. 234, 22857–22873 (2019).
Um, J. E. et al. Periostin-binding DNA aptamer treatment attenuates renal fibrosis under diabetic conditions. Sci. Rep. 7, 8490 (2017).
Julovi, S. M. et al. Blocking thrombospondin-1 signaling via CD47 mitigates renal interstitial fibrosis. Lab. Invest. 100, 1184–1196 (2020).
Maimaitiyiming, H., Clemons, K., Zhou, Q., Norman, H. & Wang, S. Thrombospondin1 deficiency attenuates obesity-associated microvascular complications in ApoE−/− mice. PLoS ONE 10, e0121403 (2015).
Bige, N. et al. Thrombospondin-1 plays a profibrotic and pro-inflammatory role during ureteric obstruction. Kidney Int. 81, 1226–1238 (2012).
Toba, H., Ikemoto, M. J., Kobara, M. & Nakata, T. Secreted protein acidic and rich in cysteine (SPARC) and a disintegrin and metalloproteinase with thrombospondin type 1 motif (ADAMTS1) increments by the renin-angiotensin system induce renal fibrosis in deoxycorticosterone acetate-salt hypertensive rats. Eur. J. Pharmacol. 914, 174681 (2022).
Schmidt, I. M. et al. Cadherin-11, Sparc-related modular calcium binding protein-2, and Pigment epithelium-derived factor are promising non-invasive biomarkers of kidney fibrosis. Kidney Int. 100, 672–683 (2021).
Sorushanova, A. et al. The collagen suprafamily: from biosynthesis to advanced biomaterial development. Adv. Mater. 31, e1801651 (2019).
Karsdal, M. A. et al. The good and the bad collagens of fibrosis — their role in signaling and organ function. Adv. Drug Deliv. Rev. 121, 43–56 (2017).
Buchtler, S. et al. Cellular origin and functional relevance of collagen I production in the kidney. J. Am. Soc. Nephrol. 29, 1859–1873 (2018).
Zollinger, A. J. & Smith, M. L. Fibronectin, the extracellular glue. Matrix Biol. 60–61, 27–37 (2017).
Klingberg, F. et al. The fibronectin ED-A domain enhances recruitment of latent TGF-beta-binding protein-1 to the fibroblast matrix. J. Cell Sci. 131, jcs201293 (2018).
Sun, Q. et al. Elastin imaging enables noninvasive staging and treatment monitoring of kidney fibrosis. Sci. Transl Med. 11, eaat4865 (2019).
Hu, C. et al. Insights into the mechanisms involved in the expression and regulation of extracellular matrix proteins in diabetic nephropathy. Curr. Med. Chem. 22, 2858–2870 (2015).
Afratis, N. A. et al. Syndecans — key regulators of cell signaling and biological functions. FEBS J. 284, 27–41 (2017).
Barbouri, D. et al. Syndecans as modulators and potential pharmacological targets in cancer progression. Front. Oncol. 4, 4 (2014).
Beauvais, D. M. & Rapraeger, A. C. Syndecan-1 couples the insulin-like growth factor-1 receptor to inside-out integrin activation. J. Cell Sci. 123, 3796–3807 (2010).
Cui, J., Jin, S., Jin, C. & Jin, Z. Syndecan-1 regulates extracellular matrix expression in keloid fibroblasts via TGF-beta1/Smad and MAPK signaling pathways. Life Sci. 254, 117326 (2020).
Scarpellini, A. et al. Syndecan-4 knockout leads to reduced extracellular transglutaminase-2 and protects against tubulointerstitial fibrosis. J. Am. Soc. Nephrol. 25, 1013–1027 (2014).
Schulz, M., Diehl, V., Trebicka, J., Wygrecka, M. & Schaefer, L. Biglycan: a regulator of hepatorenal inflammation and autophagy. Matrix Biol. 100-101, 150–161 (2021).
Nastase, M. V. et al. Biglycan, a novel trigger of Th1 and Th17 cell recruitment into the kidney. Matrix Biol. 68–69, 293–317 (2018).
Moreth, K. et al. Biglycan-triggered TLR-2- and TLR-4-signaling exacerbates the pathophysiology of ischemic acute kidney injury. Matrix Biol. 35, 143–151 (2014).
Klingberg, F. et al. Prestress in the extracellular matrix sensitizes latent TGF-beta1 for activation. J. Cell Biol. 207, 283–297 (2014).
Kaul, A. et al. Hyaluronan, a double-edged sword in kidney diseases. Pediatr. Nephrol. 37, 735–744 (2022).
Albeiroti, S., Soroosh, A. & de la Motte, C. A. Hyaluronan’s role in fibrosis: a pathogenic factor or a passive player? Biomed. Res. Int. 2015, 790203 (2015).
Rudnicki, M. et al. Increased renal versican expression is associated with progression of chronic kidney disease. PLoS ONE 7, e44891 (2012).
Wozniak, J., Floege, J., Ostendorf, T. & Ludwig, A. Key metalloproteinase-mediated pathways in the kidney. Nat. Rev. Nephrol. 17, 513–527 (2021).
Liu, Z., Tan, R. J. & Liu, Y. The many faces of matrix metalloproteinase-7 in kidney diseases. Biomolecules 10, 960 (2020).
Tan, R. J. & Liu, Y. Matrix metalloproteinases in kidney homeostasis and diseases. Am. J. Physiol. Renal Physiol. 302, F1351–F1361 (2012).
Zuo, Y. et al. Identification of matrix metalloproteinase-10 as a key mediator of podocyte injury and proteinuria. Kidney Int. 100, 837–849 (2021).
Hu, C. et al. Matrix metalloproteinase-10 protects against acute kidney injury by augmenting epidermal growth factor receptor signaling. Cell Death Dis. 12, 70 (2021).
Fu, H. et al. Matrix metalloproteinase-7 protects against acute kidney injury by priming renal tubules for survival and regeneration. Kidney Int. 95, 1167–1180 (2019).
Tan, R. J. et al. Tubular injury triggers podocyte dysfunction by beta-catenin-driven release of MMP-7. JCI Insight 4, e122399 (2019).
Sun, X. & Liu, Y. Matrix metalloproteinase-10 in kidney injury repair and disease. Int. J. Mol. Sci. 23, 2131 (2022).
Prat-Duran, J., Pinilla, E., Norregaard, R., Simonsen, U. & Buus, N. H. Transglutaminase 2 as a novel target in chronic kidney disease — methods, mechanisms and pharmacological inhibition. Pharmacol. Ther. 222, 107787 (2021).
Nguyen, L. T. et al. Lysyl oxidase inhibitors attenuate cyclosporin A-induced nephropathy in mouse. Sci. Rep. 11, 12437 (2021).
Zhou, D. et al. Early activation of fibroblasts is required for kidney repair and regeneration after injury. FASEB J. 33, 12576–12587 (2019).
Chen, S. et al. Tenascin-C protects against acute kidney injury by recruiting Wnt ligands. Kidney Int. 95, 62–74 (2019).
Yuan, Q., Tan, R. J. & Liu, Y. Myofibroblast in kidney fibrosis: origin, activation, and regulation. Adv. Exp. Med. Biol. 1165, 253–283 (2019).
Xiao, L. et al. Sustained activation of Wnt/β-catenin signaling drives AKI to CKD progression. J. Am. Soc. Nephrol. 27, 1727–1740 (2016).
Zhou, D. et al. Sonic hedgehog is a novel tubule-derived growth factor for interstitial fibroblasts after kidney injury. J. Am. Soc. Nephrol. 25, 2187–2200 (2014).
Cooper, J. G. et al. Fibronectin EDA forms the chronic fibrotic scar after contusive spinal cord injury. Neurobiol. Dis. 116, 60–68 (2018).
Klingberg, F., Hinz, B. & White, E. S. The myofibroblast matrix: implications for tissue repair and fibrosis. J. Pathol. 229, 298–309 (2013).
Zeisberg, M. & Kalluri, R. The role of epithelial-to-mesenchymal transition in renal fibrosis. J. Mol. Med. 82, 175–181 (2004).
Shafieian, M., Chen, S. & Wu, S. Integrin-linked kinase mediates CTGF-induced epithelial to mesenchymal transition in alveolar type II epithelial cells. Pediatr. Res. 77, 520–527 (2015).
Midwood, K. et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat. Med. 15, 774–780 (2009).
Sanchez-Lopez, E. et al. CTGF promotes inflammatory cell infiltration of the renal interstitium by activating NF-κB. J. Am. Soc. Nephrol. 20, 1513–1526 (2009).
Li, Y., Qi, X., Tong, X. & Wang, S. Thrombospondin 1 activates the macrophage Toll-like receptor 4 pathway. Cell Mol. Immunol. 10, 506–512 (2013).
Socha, M. J., Manhiani, M., Said, N., Imig, J. D. & Motamed, K. Secreted protein acidic and rich in cysteine deficiency ameliorates renal inflammation and fibrosis in angiotensin hypertension. Am. J. Pathol. 171, 1104–1112 (2007).
Toba, H. et al. Secreted protein acidic and rich in cysteine facilitates age-related cardiac inflammation and macrophage M1 polarization. Am. J. Physiol. Cell Physiol. 308, C972–C982 (2015).
Poluzzi, C. et al. Biglycan evokes autophagy in macrophages via a novel CD44/Toll-like receptor 4 signaling axis in ischemia/reperfusion injury. Kidney Int. 95, 540–562 (2019).
Zeng-Brouwers, J., Pandey, S., Trebicka, J., Wygrecka, M. & Schaefer, L. Communications via the small leucine-rich proteoglycans: molecular specificity in inflammation and autoimmune diseases. J. Histochem. Cytochem. 68, 887–906 (2020).
Tang, P. C. et al. Smad3 promotes cancer-associated fibroblasts generation via macrophage-myofibroblast transition. Adv. Sci. 9, e2101235 (2022).
Tang, P. M., Nikolic-Paterson, D. J. & Lan, H. Y. Macrophages: versatile players in renal inflammation and fibrosis. Nat. Rev. Nephrol. 15, 144–158 (2019).
Doi, K., Noiri, E. & Fujita, T. Role of vascular endothelial growth factor in kidney disease. Curr. Vasc. Pharmacol. 8, 122–128 (2010).
Belotti, D., Capelli, C., Resovi, A., Introna, M. & Taraboletti, G. Thrombospondin-1 promotes mesenchymal stromal cell functions via TGFβ and in cooperation with PDGF. Matrix Biol. 55, 106–116 (2016).
Sun, D. et al. Thrombospondin-1 short hairpin RNA suppresses tubulointerstitial fibrosis in the kidney of ureteral obstruction by ameliorating peritubular capillary injury. Kidney Blood Press. Res. 35, 35–47 (2012).
Srivastava, S. P., Hedayat, A. F., Kanasaki, K. & Goodwin, J. E. microRNA crosstalk influences epithelial-to-mesenchymal, endothelial-to-mesenchymal, and macrophage-to-mesenchymal transitions in the kidney. Front. Pharmacol. 10, 904 (2019).
Xu-Dubois, Y. C. et al. Markers of endothelial-to-mesenchymal transition: evidence for antibody-endothelium interaction during antibody-mediated rejection in kidney recipients. J. Am. Soc. Nephrol. 27, 324–332 (2016).
Tan, R. J., Zhou, D. & Liu, Y. Signaling crosstalk between tubular epithelial cells and interstitial fibroblasts after kidney injury. Kidney Dis. 2, 136–144 (2016).
Liu, B. C., Tang, T. T., Lv, L. L. & Lan, H. Y. Renal tubule injury: a driving force toward chronic kidney disease. Kidney Int. 93, 568–579 (2018).
Zhou, D. et al. Tubule-derived Wnts are required for fibroblast activation and kidney fibrosis. J. Am. Soc. Nephrol. 28, 2322–2336 (2017).
Liu, X. et al. Tubule-derived exosomes play a central role in fibroblast activation and kidney fibrosis. Kidney Int. 97, 1181–1195 (2020).
Achterberg, V. F. et al. The nano-scale mechanical properties of the extracellular matrix regulate dermal fibroblast function. J. Invest. Dermatol. 134, 1862–1872 (2014).
Pakshir, P. & Hinz, B. The big five in fibrosis: macrophages, myofibroblasts, matrix, mechanics, and miscommunication. Matrix Biol. 68–69, 81–93 (2018).
Lu, P., Weaver, V. M. & Werb, Z. The extracellular matrix: a dynamic niche in cancer progression. J. Cell Biol. 196, 395–406 (2012).
De Laporte, L., Rice, J. J., Tortelli, F. & Hubbell, J. A. Tenascin C promiscuously binds growth factors via its fifth fibronectin type III-like domain. PLoS ONE 8, e62076 (2013).
Hamidi, H. & Ivaska, J. Every step of the way: integrins in cancer progression and metastasis. Nat. Rev. Cancer 18, 533–548 (2018).
Schnittert, J., Bansal, R., Storm, G. & Prakash, J. Integrins in wound healing, fibrosis and tumor stroma: high potential targets for therapeutics and drug delivery. Adv. Drug Deliv. Rev. 129, 37–53 (2018).
Sun, Z., Costell, M. & Fassler, R. Integrin activation by talin, kindlin and mechanical forces. Nat. Cell Biol. 21, 25–31 (2019).
Horton, E. R. et al. The integrin adhesome network at a glance. J. Cell Sci. 129, 4159–4163 (2016).
Hahm, K. et al. αvβ6 integrin regulates renal fibrosis and inflammation in Alport mouse. Am. J. Pathol. 170, 110–125 (2007).
Henderson, N. C. et al. Targeting of αv integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat. Med. 19, 1617–1624 (2013).
Bandyopadhyay, A. & Raghavan, S. Defining the role of integrin αvβ6 in cancer. Curr. Drug Targets 10, 645–652 (2009).
Weston, B. S., Wahab, N. A. & Mason, R. M. CTGF mediates TGF-β-induced fibronectin matrix deposition by upregulating active α5β1 integrin in human mesangial cells. J. Am. Soc. Nephrol. 14, 601–610 (2003).
Lin, M. et al. Toll-like receptor 4 promotes tubular inflammation in diabetic nephropathy. J. Am. Soc. Nephrol. 23, 86–102 (2012).
Souza, A. C. et al. TLR4 mutant mice are protected from renal fibrosis and chronic kidney disease progression. Physiol. Rep. 3, e12558 (2015).
Ma, J. et al. TLR4 activation promotes podocyte injury and interstitial fibrosis in diabetic nephropathy. PLoS ONE 9, e97985 (2014).
Pushpakumar, S. et al. Toll-like receptor 4 deficiency reduces oxidative stress and macrophage mediated inflammation in hypertensive kidney. Sci. Rep. 7, 6349 (2017).
Kimura, T. et al. Tenascin-C accelerates adverse ventricular remodelling after myocardial infarction by modulating macrophage polarization. Cardiovasc. Res. 115, 614–624 (2019).
Bhattacharyya, S. et al. Tenascin-C drives persistence of organ fibrosis. Nat. Commun. 7, 11703 (2016).
Gondokaryono, S. P. et al. The extra domain A of fibronectin stimulates murine mast cells via Toll-like receptor 4. J. Leukoc. Biol. 82, 657–665 (2007).
Schaefer, L. et al. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J. Clin. Invest. 115, 2223–2233 (2005).
Ren, Q., Chen, J. & Liu, Y. LRP5 and LRP6 in Wnt signaling: similarity and divergence. Front. Cell Dev. Biol. 9, 670960 (2021).
Mo, H. et al. CXCR4 induces podocyte injury and proteinuria by activating β-catenin signaling. Theranostics 12, 767–781 (2022).
Zuo, Y. & Liu, Y. New insights into the role and mechanism of Wnt/β-catenin signalling in kidney fibrosis. Nephrology 23 (Suppl. 4), 38–43 (2018).
Zhou, L. & Liu, Y. Wnt/β-catenin signalling and podocyte dysfunction in proteinuric kidney disease. Nat. Rev. Nephrol. 11, 535–545 (2015).
Zhou, T. et al. Implication of dysregulation of the canonical wingless-type MMTV integration site (WNT) pathway in diabetic nephropathy. Diabetologia 55, 255–266 (2012).
Ren, S. et al. LRP-6 is a coreceptor for multiple fibrogenic signaling pathways in pericytes and myofibroblasts that are inhibited by DKK-1. Proc. Natl Acad. Sci. USA 110, 1440–1445 (2013).
Berendsen, A. D. et al. Modulation of canonical Wnt signaling by the extracellular matrix component biglycan. Proc. Natl Acad. Sci. USA 108, 17022–17027 (2011).
Aggelidakis, J. et al. Biglycan regulates MG63 osteosarcoma cell growth through a LPR6/β-catenin/IGFR-IR signaling axis. Front. Oncol. 8, 470 (2018).
Li, J. et al. Fibronectin type III domain containing four promotes differentiation of C2C12 through the Wnt/β-catenin signaling pathway. FASEB J. 34, 7759–7772 (2020).
Kim, K. A. et al. R-Spondin proteins: a novel link to β-catenin activation. Cell Cycle 5, 23–26 (2006).
Johnson, B. G. et al. Connective tissue growth factor domain 4 amplifies fibrotic kidney disease through activation of LDL receptor-related protein 6. J. Am. Soc. Nephrol. 28, 1769–1782 (2017).
Walraven, M. & Hinz, B. Therapeutic approaches to control tissue repair and fibrosis: extracellular matrix as a game changer. Matrix Biol. 71–72, 205–224 (2018).
Szeto, S. G. et al. YAP/TAZ are mechanoregulators of TGF-β-Smad signaling and renal fibrogenesis. J. Am. Soc. Nephrol. 27, 3117–3128 (2016).
Chen, H. et al. Mechanosensing by the alpha6-integrin confers an invasive fibroblast phenotype and mediates lung fibrosis. Nat. Commun. 7, 12564 (2016).
Htwe, S. S. et al. Role of Rho-associated coiled-coil forming kinase isoforms in regulation of stiffness-induced myofibroblast differentiation in lung fibrosis. Am. J. Respir. Cell Mol. Biol. 56, 772–783 (2017).
Sivaraman, K. & Shanthi, C. Matrikines for therapeutic and biomedical applications. Life Sci. 214, 22–33 (2018).
Farris, A. B. & Alpers, C. E. What is the best way to measure renal fibrosis?: A pathologist’s perspective. Kidney Int. Suppl. 4, 9–15 (2014).
Chen, Y. et al. Assessment of a computerized quantitative quality control tool for whole slide images of kidney biopsies. J. Pathol. 253, 268–278 (2021).
Bulow, R. D. & Boor, P. Extracellular matrix in kidney fibrosis: more than just a scaffold. J. Histochem. Cytochem. 67, 643–661 (2019).
Hwang, J. H. et al. Urinary periostin excretion predicts renal outcome in IgA nephropathy. Am. J. Nephrol. 44, 481–492 (2016).
Paunas, F. T. I. et al. Characterization of glomerular extracellular matrix in IgA nephropathy by proteomic analysis of laser-captured microdissected glomeruli. BMC Nephrol. 20, 410 (2019).
Yang, X. et al. Urinary matrix metalloproteinase 7 and prediction of IgA nephropathy progression. Am. J. Kidney Dis. 75, 384–393 (2020).
Nanthakumar, C. B. et al. Dissecting fibrosis: therapeutic insights from the small-molecule toolbox. Nat. Rev. Drug Discov. 14, 693–720 (2015).
Venning, F. A., Wullkopf, L. & Erler, J. T. Targeting ECM disrupts cancer progression. Front. Oncol. 5, 224 (2015).
Arpino, V., Brock, M. & Gill, S. E. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol. 44–46, 247–254 (2015).
Zhou, D. et al. Matrix metalloproteinase-7 is a urinary biomarker and pathogenic mediator of kidney fibrosis. J. Am. Soc. Nephrol. 28, 598–611 (2017).
Yuan, Q. et al. A Klotho-derived peptide protects against kidney fibrosis by targeting TGF-β signaling. Nat. Commun. 13, 438 (2022).
Sun, Y. et al. Single-cell landscape of the ecosystem in early-relapse hepatocellular carcinoma. Cell 184, 404–421.e16 (2021).
Wilson, P. C. & Humphreys, B. D. Single-cell genomics and gene editing: implications for nephrology. Nat. Rev. Nephrol. 15, 63–64 (2019).
Kuppe, C. et al. Decoding myofibroblast origins in human kidney fibrosis. Nature 589, 281–286 (2021).
Acknowledgements
The authors’ work was supported by the National Natural Science Foundation of China (NSFC) grant 81920108007, National Institutes of Health grant DK064005 and Bioland Laboratory grants 2018GZR110104001 and 2018GZR110102004. L.L. was supported by NSFC grant 82100785 and China Postdoctoral Science Foundation grant 2021M691471.
Author information
Authors and Affiliations
Contributions
Y.L. conceived the article and provided the outlines of the manuscript. L.L. researched data for the article. L.L. and Y.L. wrote the manuscript. L.L. and H.F. made the figures. All authors made substantial contributions to discussions of the content and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks Christos Chatziantoniou, Hui Yao Lan and Marta Ruiz-Ortega, who co-reviewed with Raul R. Rodrigues-Diez, for their contributions to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Proteolysis: https://www.sciencedirect.com/topics/medicine-and-dentistry/protein-degradation
Glossary
- Partial epithelial-to-mesenchymal transition
-
(Partial EMT). Tubular epithelial cells undergo a process of partial EMT in vivo in which they exhibit some phenotypic changes but stay within the tubular compartment. Partial EMT could be viewed as a transitional stage in which epithelial cells can further regress to cell-cycle arrest and senescence or return to normal epithelia.
- Extracellular vesicles
-
Lipid bilayer-encircled particles that are released from almost all cell types and carry a cargo of proteins, mRNAs, microRNAs, long non-coding RNAs, lipids and metabolites. Extracellular vesicles can mediate cell–cell communication and signal exchange and are thought to have important roles in regulating biological processes including embryogenesis, injury repair and regeneration and the pathogenesis of disease.
- Stem cell niche
-
A specific tissue microenvironment in which stem cells are present in an undifferentiated and self-renewable state.
- Kidney tissue scaffolds
-
(KTS). Decellularized extracellular matrix scaffold derived from normal or diseased kidneys that retains the critical structural, mechanical and physiological properties of renal structures.
- Matrikines
-
Peptides that are liberated by partial proteolysis of extracellular matrix proteins such as collagens, fibronectin, laminins, elastin and matricellular proteins. Matrikines are able to regulate biological processes and often have activities that are different from those of their parent proteins.
Rights and permissions
About this article
Cite this article
Li, L., Fu, H. & Liu, Y. The fibrogenic niche in kidney fibrosis: components and mechanisms. Nat Rev Nephrol 18, 545–557 (2022). https://doi.org/10.1038/s41581-022-00590-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-022-00590-z
This article is cited by
-
LATS2 degradation promoted fibrosis damage and rescued by vitamin K3 in lupus nephritis
Arthritis Research & Therapy (2024)
-
Epithelial cell states associated with kidney and allograft injury
Nature Reviews Nephrology (2024)
-
Engineered extracellular vesicle-encapsulated CHIP as novel nanotherapeutics for treatment of renal fibrosis
npj Regenerative Medicine (2024)
-
WNT-dependent interaction between inflammatory fibroblasts and FOLR2+ macrophages promotes fibrosis in chronic kidney disease
Nature Communications (2024)
-
Fibroblast-specific PRMT5 deficiency suppresses cardiac fibrosis and left ventricular dysfunction in male mice
Nature Communications (2024)