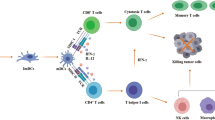
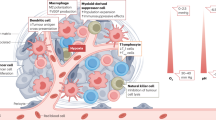
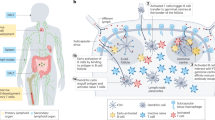
Abstract
Immunotherapy has emerged as an eminent and effective modality in the treatment of cancer. However, current cancer immunotherapies lack spatial and temporal control, resulting in systemic side effects and suboptimal patient outcomes. Responsive biomaterials have proven to be powerful tools for controlling cancer immunotherapies by providing precise control over the delivery and kinetics of immunotherapeutic cargoes. Here, we discuss biological barriers to cancer immunotherapy and how biomaterial-based strategies that respond to different stimuli — both internal and external — can be used to increase the therapeutic efficacy while reducing the toxicity of cancer immunotherapies. We examine the use of biomaterials that respond to physiological stimuli (pH, enzymes and redox potential) and exogenous energetic stimuli (light, magnetism and ultrasound) and expand upon the use of these strategies in propagating three key approaches in cancer immunotherapy: cancer vaccines, T cell-based therapy and therapies involving sustained delivery.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Zhu, Y. et al. STING: a master regulator in the cancer-immunity cycle. Mol. Cancer 18, 152 (2019).
Garland, K. M., Sheehy, T. L. & Wilson, J. T. Chemical and biomolecular strategies for STING pathway activation in cancer immunotherapy. Chem. Rev. 122, 5977–6039 (2022).
Lesterhuis, W. J., Haanen, J. B. A. G. & Punt, C. J. A. Cancer immunotherapy — revisited. Nat. Rev. Drug Discov. 10, 591–600 (2011).
Tanaka, A. & Sakaguchi, S. Regulatory T cells in cancer immunotherapy. Cell Res. 27, 109–118 (2017).
Colombo, M. P. & Piconese, S. Regulatory T-cell inhibition versus depletion: the right choice in cancer immunotherapy. Nat. Rev. Cancer 7, 880–887 (2007).
Nishikawa, H. & Sakaguchi, S. Regulatory T cells in cancer immunotherapy. Curr. Opin. Immunol. 27, 1–7 (2014).
Gong, N., Sheppard, N. C., Billingsley, M. M., June, C. H. & Mitchell, M. J. Nanomaterials for T-cell cancer immunotherapy. Nat. Nanotechnol. 16, 25–36 (2021).
Jackson, H. J., Rafiq, S. & Brentjens, R. J. Driving CAR T-cells forward. Nat. Rev. Clin. Oncol. 13, 370–383 (2016).
June, C. H., O’Connor, R. S., Kawalekar, O. U., Ghassemi, S. & Milone, M. C. CAR T cell immunotherapy for human cancer. Science 359, 1361–1365 (2018).
Shah, N. N. & Fry, T. J. Mechanisms of resistance to CAR T cell therapy. Nat. Rev. Clin. Oncol. 16, 372–385 (2019).
Mellman, I., Coukos, G. & Dranoff, G. Cancer immunotherapy comes of age. Nature 480, 480–489 (2011).
Chabner, B. A. & Roberts, T. G. Chemotherapy and the war on cancer. Nat. Rev. Cancer 5, 65–72 (2005).
Blattman, J. N. & Greenberg, P. D. Cancer immunotherapy: a treatment for the masses. Science 305, 200–205 (2004).
Javier, R. T. & Butel, J. S. The history of tumor virology. Cancer Res. 68, 7693–7706 (2008).
Topalian, S. L., Drake, C. G. & Pardoll, D. M. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27, 450–461 (2015).
Wei, S. C., Duffy, C. R. & Allison, J. P. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 8, 1069–1086 (2018).
Ledford, H., Else, H. & Warren, M. Cancer immunologists scoop medicine Nobel Prize. Nature 562, 20–21 (2018).
Fedorov, V. D., Themeli, M. & Sadelain, M. PD-1- and CTLA-4-based Inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci. Transl Med. 5, 215ra172 (2013).
Zitvogel, L., Rusakiewicz, S., Routy, B., Ayyoub, M. & Kroemer, G. Immunological off-target effects of imatinib. Nat. Rev. Clin. Oncol. 13, 431–446 (2016).
Irvine, D. J. & Dane, E. L. Enhancing cancer immunotherapy with nanomedicine. Nat. Rev. Immunol. 20, 321–334 (2020).
Martin, J. D., Cabral, H., Stylianopoulos, T. & Jain, R. K. Improving cancer immunotherapy using nanomedicines: progress, opportunities and challenges. Nat. Rev. Clin. Oncol. 17, 251–266 (2020).
Riley, R. S., June, C. H., Langer, R. & Mitchell, M. J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 18, 175–196 (2019).
Chao, Y. & Liu, Z. Biomaterials tools to modulate the tumour microenvironment in immunotherapy. Nat. Rev. Bioeng. 1, 125–138 (2023).
Bo, Y. & Wang, H. Biomaterial‐based in situ cancer vaccines. Adv. Mater. 17, e2210452 (2023).
Huang, P. et al. Nano-, micro-, and macroscale drug delivery systems for cancer immunotherapy. Acta Biomater. 85, 1–26 (2019).
Viswanath, D. I., Liu, H.-C., Huston, D. P., Chua, C. Y. X. & Grattoni, A. Emerging biomaterial-based strategies for personalized therapeutic in situ cancer vaccines. Biomaterials 280, 121297 (2022).
Weber, J. S. & Mulé, J. J. Cancer immunotherapy meets biomaterials. Nat. Biotechnol. 33, 44–45 (2015).
Yan, S. et al. Improving cancer immunotherapy outcomes using biomaterials. Angew. Chem. Int. Ed. 132, 17484–17495 (2020).
Curvello, R., Kast, V., Ordóñez-Morán, P., Mata, A. & Loessner, D. Biomaterial-based platforms for tumour tissue engineering. Nat. Rev. Mater. 8, 314–330 (2023).
De Angelis, B. et al. Stimuli-responsive nanoparticle-assisted immunotherapy: a new weapon against solid tumours. J. Mater. Chem. B 8, 1823–1840 (2020).
Li, L., Yang, Z. & Chen, X. Recent advances in stimuli-responsive platforms for cancer immunotherapy. Acc. Chem. Res. 53, 2044–2054 (2020).
Pacifici, N., Bolandparvaz, A. & Lewis, J. S. Stimuli‐responsive biomaterials for vaccines and immunotherapeutic applications. Adv. Ther. 3, 2000129 (2020).
Qin, J. et al. Stimuli-responsive hydrogels for cancer immunotherapy. Polym. Chem. 14, 793–802 (2023).
Zhao, Y., Guo, Y. & Tang, L. Engineering cancer vaccines using stimuli-responsive biomaterials. Nano Res. 11, 5355–5371 (2018).
Lu, Y., Aimetti, A. A., Langer, R. & Gu, Z. Bioresponsive materials. Nat. Rev. Mater. 2, 16075 (2016).
Mahoney, K. M., Rennert, P. D. & Freeman, G. J. Combination cancer immunotherapy and new immunomodulatory targets. Nat. Rev. Drug Discov. 14, 561–584 (2015).
Nam, J. et al. Cancer nanomedicine for combination cancer immunotherapy. Nat. Rev. Mater. 4, 398–414 (2019).
Sang, W., Zhang, Z., Dai, Y. & Chen, X. Recent advances in nanomaterial-based synergistic combination cancer immunotherapy. Chem. Soc. Rev. 48, 3771–3810 (2019).
Smyth, M. J., Ngiow, S. F., Ribas, A. & Teng, M. W. L. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat. Rev. Clin. Oncol. 13, 143–158 (2016).
Quail, D. F. & Joyce, J. A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 19, 1423–1437 (2013).
Finger, E. C. & Giaccia, A. J. Hypoxia, inflammation, and the tumor microenvironment in metastatic disease. Cancer Metastasis Rev. 29, 285–293 (2010).
Tsai, M.-J., Chang, W.-A., Huang, M.-S. & Kuo, P.-L. Tumor microenvironment: a new treatment target for cancer. ISRN Biochem. 2014, 1–8 (2014).
Yang, M., Li, J., Gu, P. & Fan, X. The application of nanoparticles in cancer immunotherapy: targeting tumor microenvironment. Bioact. Mater. 6, 1973–1987 (2021).
Peng, S., Xiao, F., Chen, M. & Gao, H. Tumor‐microenvironment‐responsive nanomedicine for enhanced cancer immunotherapy. Adv. Sci. 9, 2103836 (2022).
Manchun, S., Dass, C. R. & Sriamornsak, P. Targeted therapy for cancer using pH-responsive nanocarrier systems. Life Sci. 90, 381–387 (2012).
Yan, Y. & Ding, H. pH-responsive nanoparticles for cancer immunotherapy: a brief review. Nanomaterials 10, 1613 (2020).
Li, Z., Huang, J. & Wu, J. pH-sensitive nanogels for drug delivery in cancer therapy. Biomater. Sci. 9, 574–589 (2021).
Zhou, T. et al. A nanogel of on-site tunable pH-response for efficient anticancer drug delivery. Acta Biomater. 9, 4546–4557 (2013).
Oberli, M. A. et al. Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano Lett. 17, 1326–1335 (2017).
Gu, Z., Da Silva, C., Van Der Maaden, K., Ossendorp, F. & Cruz, L. Liposome-based drug delivery systems in cancer immunotherapy. Pharmaceutics 12, 1054 (2020).
Trimaille, T., Lacroix, C. & Verrier, B. Self-assembled amphiphilic copolymers as dual delivery system for immunotherapy. Eur. J. Pharm. Biopharm. 142, 232–239 (2019).
Zhu, G., Zhang, F., Ni, Q., Niu, G. & Chen, X. Efficient nanovaccine delivery in cancer immunotherapy. ACS Nano 11, 2387–2392 (2017).
Ma, X. et al. Bioengineered nanogels for cancer immunotherapy. Chem. Soc. Rev. 51, 5136–5174 (2022).
Guo, R.-C. et al. Recent progress of therapeutic peptide based nanomaterials: from synthesis and self-assembly to cancer treatment. Biomater. Sci. 8, 6175–6189 (2020).
Ni, K., Luo, T., Nash, G. T. & Lin, W. Nanoscale metal–organic frameworks for cancer immunotherapy. Acc. Chem. Res. 53, 1739–1748 (2020).
Alsaiari, S. K. et al. Sustained and targeted delivery of checkpoint inhibitors by metal–organic frameworks for cancer immunotherapy. Sci. Adv. 7, eabe7174 (2021).
Li, J. et al. Hybrid nanomaterials for cancer immunotherapy. Adv. Sci. 10, 2204932 (2023).
Wang, C., Ye, Y., Hu, Q., Bellotti, A. & Gu, Z. Tailoring biomaterials for cancer immunotherapy: emerging trends and future outlook. Adv. Mater. 29, 1606036 (2017).
Nuhn, L. et al. pH-degradable imidazoquinoline-ligated nanogels for lymph node-focused immune activation. Proc. Natl Acad. Sci. USA 113, 8098–8103 (2016).
Jacobson, M. E., Wang-Bishop, L., Becker, K. W. & Wilson, J. T. Delivery of 5′-triphosphate RNA with endosomolytic nanoparticles potently activates RIG-I to improve cancer immunotherapy. Biomater. Sci. 7, 547–559 (2019).
Shae, D. et al. Endosomolytic polymersomes increase the activity of cyclic dinucleotide STING agonists to enhance cancer immunotherapy. Nat. Nanotechnol. 14, 269–278 (2019).
Luo, M. et al. A STING-activating nanovaccine for cancer immunotherapy. Nat. Nanotechnol. 12, 648–654 (2017).
Li, S. et al. Prolonged activation of innate immune pathways by a polyvalent STING agonist. Nat. Biomed. Eng. 5, 455–466 (2021).
Ahmad, A., Khan, J. M. & Haque, S. Strategies in the design of endosomolytic agents for facilitating endosomal escape in nanoparticles. Biochimie 160, 61–75 (2019).
Marušič, N. et al. Increased efficiency of charge-mediated fusion in polymer/lipid hybrid membranes. Proc. Natl Acad. Sci. USA 119, e2122468119 (2022).
Yuba, E., Harada, A., Sakanishi, Y., Watarai, S. & Kono, K. A liposome-based antigen delivery system using pH-sensitive fusogenic polymers for cancer immunotherapy. Biomaterials 34, 3042–3052 (2013).
Kunisawa, J. et al. Sendai virus fusion protein-mediates simultaneous induction of MHC class I/II-dependent mucosal and systemic immune responses via the nasopharyngeal-associated lymphoreticular tissue immune system. J. Immunol. 167, 1406–1412 (2001).
Yavlovich, A., Smith, B., Gupta, K., Blumenthal, R. & Puri, A. Light-sensitive lipid-based nanoparticles for drug delivery: design principles and future considerations for biological applications. Mol. Membr. Biol. 27, 364–381 (2010).
Zong, Y., Lin, Y., Wei, T. & Cheng, Q. Lipid nanoparticle (LNP) enables mRNA delivery for cancer therapy. Adv. Mater. https://doi.org/10.1002/adma.202303261 (2023).
Huang, T. et al. Lipid nanoparticle-based mRNA vaccines in cancers: current advances and future prospects. Front. Immunol. 13, 922301 (2022).
Liu, C. et al. mRNA-based cancer therapeutics. Nat. Rev. Cancer 23, 526–543 (2023).
Zhang, H. et al. Delivery of mRNA vaccine with a lipid-like material potentiates antitumor efficacy through Toll-like receptor 4 signaling. Proc. Natl Acad. Sci. USA 118, e2005191118 (2021).
Miao, L. et al. Delivery of mRNA vaccines with heterocyclic lipids increases anti-tumor efficacy by STING-mediated immune cell activation. Nat. Biotechnol. 37, 1174–1185 (2019).
Islam, M. A. et al. Adjuvant-pulsed mRNA vaccine nanoparticle for immunoprophylactic and therapeutic tumor suppression in mice. Biomaterials 266, 120431 (2021).
Lee, K. et al. Adjuvant incorporated lipid nanoparticles for enhanced mRNA-mediated cancer immunotherapy. Biomater. Sci. 8, 1101–1105 (2020).
Yan, J. et al. Nanomaterials‐mediated co‐stimulation of toll‐like receptors and CD40 for antitumor immunity. Adv. Mater. 34, 2207486 (2022).
Chen, Q. et al. In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat. Nanotechnol. 14, 89–97 (2019).
Huppertsberg, A. et al. Squaric ester-based, pH-degradable nanogels: modular nanocarriers for safe, systemic administration of toll-like receptor 7/8 agonistic immune modulators. J. Am. Chem. Soc. 143, 9872–9883 (2021).
Li, D. et al. Progress and perspective of microneedle system for anti-cancer drug delivery. Biomaterials 264, 120410 (2021).
Wang, C., Ye, Y., Hochu, G. M., Sadeghifar, H. & Gu, Z. Enhanced cancer immunotherapy by microneedle patch-assisted delivery of anti-PD1 antibody. Nano Lett. 16, 2334–2340 (2016).
Duong, H. T. T. et al. Smart vaccine delivery based on microneedle arrays decorated with ultra-pH-responsive copolymers for cancer immunotherapy. Biomaterials 185, 13–24 (2018).
Li, M., Zhao, G., Su, W.-K. & Shuai, Q. Enzyme-responsive nanoparticles for anti-tumor drug delivery. Front. Chem. 8, 647 (2020).
Shahriari, M. et al. Enzyme responsive drug delivery systems in cancer treatment. J. Control. Release 308, 172–189 (2019).
Wang, D. et al. Engineering nanoparticles to locally activate T cells in the tumor microenvironment. Sci. Immunol. 4, eaau6584 (2019).
Xing, F. Cancer associated fibroblasts (CAFs) in tumor microenvironment. Front. Biosci. 15, 166 (2010).
Ji, T. et al. Transformable peptide nanocarriers for expeditious drug release and effective cancer therapy via cancer-associated fibroblast activation. Angew. Chem. Int. Ed. 55, 1050–1055 (2016).
Sun, M. et al. Fibroblast activation protein‐α responsive peptide assembling prodrug nanoparticles for remodeling the immunosuppressive microenvironment and boosting cancer immunotherapy. Small 18, 2106296 (2022).
Wei, L., Zhao, Y., Hu, X. & Tang, L. Redox-responsive polycondensate neoepitope for enhanced personalized cancer vaccine. ACS Cent. Sci. 6, 404–412 (2020).
Estrela, J. M., Ortega, A. & Obrador, E. Glutathione in cancer biology and therapy. Crit. Rev. Clin. Lab. Sci. 43, 143–181 (2006).
Li, L. et al. Burst release of encapsulated annexin A5 in tumours boosts cytotoxic T-cell responses by blocking the phagocytosis of apoptotic cells. Nat. Biomed. Eng. 4, 1102–1116 (2020).
Wang, N. et al. Vaccination of TLR7/8 agonist‐conjugated antigen nanoparticles for cancer immunotherapy. Adv. Healthc. Mater. 4, e2300249 (2023).
Kuai, R., Ochyl, L. J., Bahjat, K. S., Schwendeman, A. & Moon, J. J. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat. Mater. 16, 489–496 (2017).
Lv, M. et al. Redox-responsive hyperbranched poly(amido amine) and polymer dots as a vaccine delivery system for cancer immunotherapy. J. Mater. Chem. B 5, 9532–9545 (2017).
Yang, B., Gao, J., Pei, Q., Xu, H. & Yu, H. Engineering prodrug nanomedicine for cancer immunotherapy. Adv. Sci. 7, 2002365 (2020).
Qian, S. et al. IDO as a drug target for cancer immunotherapy: recent developments in IDO inhibitors discovery. RSC Adv. 6, 7575–7581 (2016).
Yan, P. et al. A redox‐responsive nanovaccine combined with a2a receptor antagonist for cancer immunotherapy. Adv. Healthc. Mater. 10, 2101222 (2021).
Wang, K.-S. et al. A redox-responsive prodrug nanogel of TLR7/8 agonist for improved cancer immunotherapy. Chinese J. Polym. Sci. 41, 32–39 (2023).
Mi, Y. et al. Abstract 2866: neoantigen nanovaccine improves personalized cancer immunotherapy. Cancer Res. 80, 2866–2866 (2020).
Ben-Akiva, E. et al. Biodegradable lipophilic polymeric mRNA nanoparticles for ligand-free targeting of splenic dendritic cells for cancer vaccination. Proc. Natl Acad. Sci. USA 120, e2301606120 (2023).
Chen, J. et al. Lipid nanoparticle-mediated lymph node-targeting delivery of mRNA cancer vaccine elicits robust CD8 + T cell response. Proc. Natl Acad. Sci. USA 119, e2207841119 (2022).
Wang, F. et al. Tumour sensitization via the extended intratumoural release of a STING agonist and camptothecin from a self-assembled hydrogel. Nat. Biomed. Eng. 4, 1090–1101 (2020).
Yang, Y. & Sun, W. Recent advances in redox-responsive nanoparticles for combined cancer therapy. Nanoscale Adv. 4, 3504–3516 (2022).
Li, D., Zhang, R., Liu, G., Kang, Y. & Wu, J. Redox‐responsive self‐assembled nanoparticles for cancer therapy. Adv. Healthc. Mater. 9, 2000605 (2020).
Jeong, S., Choi, Y. & Kim, K. Engineering therapeutic strategies in cancer immunotherapy via exogenous delivery of toll-like receptor agonists. Pharmaceutics 13, 1374 (2021).
Kang, W., Liu, Y. & Wang, W. Light-responsive nanomedicine for cancer immunotherapy. Acta Pharm. Sin. B 13, 2346–2368 (2023).
Day, N. B., Wixson, W. C. & Shields, C. W. Magnetic systems for cancer immunotherapy. Acta Pharm. Sin. B 11, 2172–2196 (2021).
Unga, J. & Hashida, M. Ultrasound induced cancer immunotherapy. Adv. Drug Deliv. Rev. 72, 144–153 (2014).
Chen, H. & Zhao, Y. Applications of light-responsive systems for cancer theranostics. ACS Appl. Mater. Interfaces 10, 21021–21034 (2018).
Wang, X., Xiao, X., Feng, Y., Li, J. & Zhang, Y. A photoresponsive antibody–siRNA conjugate for activatable immunogene therapy of cancer. Chem. Sci. 13, 5345–5352 (2022).
Song, Y., Chen, Y., Li, P. & Dong, C.-M. Photoresponsive polypeptide-glycosylated dendron amphiphiles: UV-triggered polymersomes, OVA release, and in vitro enhanced uptake and immune response. Biomacromolecules 21, 5345–5357 (2020).
Yang, G., Liu, J., Wu, Y., Feng, L. & Liu, Z. Near-infrared-light responsive nanoscale drug delivery systems for cancer treatment. Coord. Chem. Rev. 320–321, 100–117 (2016).
Chu, H., Zhao, J., Mi, Y., Di, Z. & Li, L. NIR-light-mediated spatially selective triggering of anti-tumor immunity via upconversion nanoparticle-based immunodevices. Nat. Commun. 10, 2839 (2019).
Chen, G., Qiu, H., Prasad, P. N. & Chen, X. Upconversion nanoparticles: design, nanochemistry, and applications in theranostics. Chem. Rev. 114, 5161–5214 (2014).
Liu, Y., Meng, X. & Bu, W. Upconversion-based photodynamic cancer therapy. Coord. Chem. Rev. 379, 82–98 (2019).
Tian, G., Zhang, X., Gu, Z. & Zhao, Y. Recent advances in upconversion nanoparticles-based multifunctional nanocomposites for combined cancer therapy. Adv. Mater. 27, 7692–7712 (2015).
Wang, C., Cheng, L. & Liu, Z. Drug delivery with upconversion nanoparticles for multi-functional targeted cancer cell imaging and therapy. Biomaterials 32, 1110–1120 (2011).
Lee, H. P. & Gaharwar, A. K. Light‐responsive inorganic biomaterials for biomedical applications. Adv. Sci. 7, 2000863 (2020).
Zhang, X., Wang, S., Cheng, G., Yu, P. & Chang, J. Light-responsive nanomaterials for cancer therapy. Engineering 13, 18–30 (2022).
Yan, B. et al. Engineering magnetic nano-manipulators for boosting cancer immunotherapy. J. Nanobiotechnol. 20, 547 (2022).
Li, K., Nejadnik, H. & Daldrup-Link, H. E. Next-generation superparamagnetic iron oxide nanoparticles for cancer theranostics. Drug Discov. Today 22, 1421–1429 (2017).
Ito, A., Honda, H. & Kobayashi, T. Cancer immunotherapy based on intracellular hyperthermia using magnetite nanoparticles: a novel concept of ‘heat-controlled necrosis’ with heat shock protein expression. Cancer Immunol. Immunother. 55, 320–328 (2006).
Ksienski, D. et al. Pembrolizumab for advanced nonsmall cell lung cancer: efficacy and safety in everyday clinical practice. Lung Cancer 133, 110–116 (2019).
Chen, Q. et al. Novel targeted pH-responsive drug delivery systems based on PEGMA-modified bimetallic Prussian blue analogs for breast cancer chemotherapy. RSC Adv. 13, 1684–1700 (2023).
Morrow, M., Waters, J. & Morris, E. MRI for breast cancer screening, diagnosis, and treatment. Lancet 378, 1804–1811 (2011).
Li, T. et al. Bioinspired magnetic nanocomplexes amplifying STING activation of tumor-associated macrophages to potentiate cancer immunotherapy. Nano Today 43, 101400 (2022).
Chiang, C.-S. et al. Combination of fucoidan-based magnetic nanoparticles and immunomodulators enhances tumour-localized immunotherapy. Nat. Nanotechnol. 13, 746–754 (2018).
Mejías, R. et al. Dimercaptosuccinic acid-coated magnetite nanoparticles for magnetically guided in vivo delivery of interferon gamma for cancer immunotherapy. Biomaterials 32, 2938–2952 (2011).
Zhu, J., Wang, J. & Li, Y. Recent advances in magnetic nanocarriers for tumor treatment. Biomed. Pharmacother. 159, 114227 (2023).
Sharma, D., Leong, K. X. & Czarnota, G. J. Application of ultrasound combined with microbubbles for cancer therapy. Int. J. Mol. Sci. 23, 4393 (2022).
Tu, J., Zhang, H., Yu, J., Liufu, C. & Chen, Z. Ultrasound-mediated microbubble destruction: a new method in cancer immunotherapy. Onco Targets Ther. 11, 5763–5775 (2018).
Wang, S., Hu, X., Wei, W. & Ma, G. Transformable vesicles for cancer immunotherapy. Adv. Drug Deliv. Rev. 179, 113905 (2021).
Seo, H. S., Wang, C.-P. J., Park, W. & Park, C. G. Short review on advances in hydrogel-based drug delivery strategies for cancer immunotherapy. Tissue Eng. Regen. Med. 19, 263–280 (2022).
Li, X. et al. Cancer immunotherapy based on image-guided STING activation by nucleotide nanocomplex-decorated ultrasound microbubbles. Nat. Nanotechnol. 17, 891–899 (2022).
Meng, Z. et al. Ultrasound-mediated remotely controlled nanovaccine delivery for tumor vaccination and individualized cancer immunotherapy. Nano Lett. 21, 1228–1237 (2021).
Zhou, L.-Q., Li, P., Cui, X.-W. & Dietrich, C. F. Ultrasound nanotheranostics in fighting cancer: advances and prospects. Cancer Lett. 470, 204–219 (2020).
Zhang, N., Wang, J., Foiret, J., Dai, Z. & Ferrara, K. W. Synergies between therapeutic ultrasound, gene therapy and immunotherapy in cancer treatment. Adv. Drug Deliv. Rev. 178, 113906 (2021).
Saxena, M., Van Der Burg, S. H., Melief, C. J. M. & Bhardwaj, N. Therapeutic cancer vaccines. Nat. Rev. Cancer 21, 360–378 (2021).
Sahin, U. & Türeci, Ö. Personalized vaccines for cancer immunotherapy. Science 359, 1355–1360 (2018).
Finn, O. J. Cancer vaccines: between the idea and the reality. Nat. Rev. Immunol. 3, 630–641 (2003).
Duong, H. T. T. et al. Microneedle arrays coated with charge reversal pH-sensitive copolymers improve antigen presenting cells-homing DNA vaccine delivery and immune responses. J. Control. Release 269, 225–234 (2018).
Liu, K. et al. A novel multifunctional vaccine platform with dendritic cell-targeting and pH-responsive for cancer immunotherapy: antigen-directed biomimetic fabrication of a cabbage-like mannatide-zinc-antigen hybrid microparticles. Chem. Eng. J. 426, 130867 (2021).
Shih, T.-Y., Najibi, A. J., Bartlett, A. L., Li, A. W. & Mooney, D. J. Ultrasound-triggered release reveals optimal timing of CpG-ODN delivery from a cryogel cancer vaccine. Biomaterials 279, 121240 (2021).
Vasdekis, A. E., Scott, E. A., O’Neil, C. P., Psaltis, D. & Hubbell, J. A. Precision intracellular delivery based on optofluidic polymersome rupture. ACS Nano 6, 7850–7857 (2012).
Gao, Y., Zhao, Q., Xiao, M., Huang, X. & Wu, X. A versatile photothermal vaccine based on acid-responsive glyco-nanoplatform for synergistic therapy of cancer. Biomaterials 273, 120792 (2021).
Ni, Q. et al. Nanomaterials with changeable physicochemical property for boosting cancer immunotherapy. J. Control. Release 342, 210–227 (2022).
Wang, J. et al. In situ phase transitional polymeric vaccines for improved immunotherapy. Natl Sci. Rev. 9, nwab159 (2022).
Liu, S. et al. A DNA nanodevice-based vaccine for cancer immunotherapy. Nat. Mater. 20, 421–430 (2021).
Reddy, S. T. et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat. Biotechnol. 25, 1159–1164 (2007).
Wang, Y. et al. In situ manipulation of dendritic cells by an autophagy-regulative nanoactivator enables effective cancer immunotherapy. ACS Nano 13, 7568–7577 (2019).
Ghiringhelli, F. et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1β-dependent adaptive immunity against tumors. Nat. Med. 15, 1170–1178 (2009).
Gong, N. et al. Proton-driven transformable nanovaccine for cancer immunotherapy. Nat. Nanotechnol. 15, 1053–1064 (2020).
Xia, Y. et al. Exploiting the pliability and lateral mobility of Pickering emulsion for enhanced vaccination. Nat. Mater. 17, 187–194 (2018).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Mai, D., June, C. H. & Sheppard, N. C. In vivo gene immunotherapy for cancer. Sci. Transl Med. 14, eabo3603 (2022).
Zhu, G. et al. Intertwining DNA–RNA nanocapsules loaded with tumor neoantigens as synergistic nanovaccines for cancer immunotherapy. Nat. Commun. 8, 1482 (2017).
Khattak, A. et al. Abstract CT001: a personalized cancer vaccine, mRNA-4157, combined with pembrolizumab versus pembrolizumab in patients with resected high-risk melanoma: efficacy and safety results from the randomized, open-label phase 2 mRNA-4157-P201/Keynote-942 trial. Cancer Res. 83, CT001 (2023).
Rosenberg, S. A., Restifo, N. P., Yang, J. C., Morgan, R. A. & Dudley, M. E. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat. Rev. Cancer 8, 299–308 (2008).
Farhood, B., Najafi, M. & Mortezaee, K. CD8+ cytotoxic T lymphocytes in cancer immunotherapy: a review. J. Cell. Physiol. 234, 8509–8521 (2019).
Li, D. et al. Genetically engineered T cells for cancer immunotherapy. Signal Transduct. Target. Ther. 4, 35 (2019).
Miliotou, A. N. & Papadopoulou, L. C. CAR T-cell therapy: a new era in cancer immunotherapy. Curr. Pharm. Biotechnol. 19, 5–18 (2018).
Chen, Y.-J., Abila, B. & Mostafa Kamel, Y. CAR-T: what is next? Cancers 15, 663 (2023).
Liu, Y., Sperling, A. S., Smith, E. L. & Mooney, D. J. Optimizing the manufacturing and antitumour response of CAR T therapy. Nat. Rev. Bioeng. 1, 271–285 (2023).
Perica, K. et al. Magnetic field-induced T cell receptor clustering by nanoparticles enhances T cell activation and stimulates antitumor activity. ACS Nano 8, 2252–2260 (2014).
Smith, T. T. et al. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat. Nanotechnol. 12, 813–820 (2017).
Moffett, H. F. et al. Hit-and-run programming of therapeutic cytoreagents using mRNA nanocarriers. Nat. Commun. 8, 389 (2017).
Dunn, Z. S., Mac, J. & Wang, P. T cell immunotherapy enhanced by designer biomaterials. Biomaterials 217, 119265 (2019).
Tang, L. et al. Enhancing T cell therapy through TCR-signaling-responsive nanoparticle drug delivery. Nat. Biotechnol. 36, 707–716 (2018).
Shang, Q. et al. Local scaffold-assisted delivery of immunotherapeutic agents for improved cancer immunotherapy. Adv. Drug Deliv. Rev. 185, 114308 (2022).
Abdou, P. et al. Advances in engineering local drug delivery systems for cancer immunotherapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 12, e1632 (2020).
Li, J. et al. Implantable and injectable biomaterial scaffolds for cancer immunotherapy. Front. Bioeng. Biotechnol. 8, 612950 (2020).
Leach, D. G., Young, S. & Hartgerink, J. D. Advances in immunotherapy delivery from implantable and injectable biomaterials. Acta Biomater. 88, 15–31 (2019).
Lu, X. et al. Engineered PLGA microparticles for long-term, pulsatile release of STING agonist for cancer immunotherapy. Sci. Transl Med. 12, eaaz6606 (2020).
Khongorzul, P., Ling, C. J., Khan, F. U., Ihsan, A. U. & Zhang, J. Antibody–drug conjugates: a comprehensive review. Mol. Cancer Res. 18, 3–19 (2020).
Weiner, L. M., Murray, J. C. & Shuptrine, C. W. Antibody-based immunotherapy of cancer. Cell 148, 1081–1084 (2012).
Weiner, L. M., Surana, R. & Wang, S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat. Rev. Immunol. 10, 317–327 (2010).
Chia, C. S. B. A patent review on FDA‐approved antibody–drug conjugates, their linkers and drug payloads. ChemMedChem 17, e202200032 (2022).
Wang, F. et al. Supramolecular prodrug hydrogelator as an immune booster for checkpoint blocker-based immunotherapy. Sci. Adv. 6, eaaz8985 (2020).
Wang, C. et al. In situ formed reactive oxygen species-responsive scaffold with gemcitabine and checkpoint inhibitor for combination therapy. Sci. Transl Med. 10, eaan3682 (2018).
Ye, Y. et al. A melanin-mediated cancer immunotherapy patch. Sci. Immunol. 2, eaan5692 (2017).
Yu, S. et al. Injectable bioresponsive gel depot for enhanced immune checkpoint blockade. Adv. Mater. 30, 1801527 (2018).
Ruan, H. et al. A dual‐bioresponsive drug‐delivery depot for combination of epigenetic modulation and immune checkpoint blockade. Adv. Mater. 31, 1806957 (2019).
Berraondo, P. et al. Cytokines in clinical cancer immunotherapy. Br. J. Cancer 120, 6–15 (2019).
Xue, D., Hsu, E., Fu, Y.-X. & Peng, H. Next-generation cytokines for cancer immunotherapy. Antib. Ther. 4, 123–133 (2021).
Chao, Y., Chen, Q. & Liu, Z. Smart injectable hydrogels for cancer immunotherapy. Adv. Funct. Mater. 30, 1902785 (2020).
Erfani, A., Diaz, A. E. & Doyle, P. S. Hydrogel-enabled, local administration and combinatorial delivery of immunotherapies for cancer treatment. Mater. Today 65, 227–243 (2023).
Falcone, N. et al. Peptide hydrogels as immunomaterials and their use in cancer immunotherapy delivery. Adv. Healthc. Mater. 16, e2301096 (2023).
Zhang, J. et al. Immunostimulant hydrogel for the inhibition of malignant glioma relapse post-resection. Nat. Nanotechnol. 16, 538–548 (2021).
Jia, F. et al. Stabilizing RNA nanovaccines with transformable hyaluronan dynamic hydrogel for durable cancer immunotherapy. Adv. Funct. Mater. 33, 2204636 (2023).
Grosskopf, A. K. et al. Delivery of CAR-T cells in a transient injectable stimulatory hydrogel niche improves treatment of solid tumors. Sci. Adv. 8, eabn8264 (2022).
Agarwalla, P. et al. Bioinstructive implantable scaffolds for rapid in vivo manufacture and release of CAR-T cells. Nat. Biotechnol. 40, 1250–1258 (2022).
Kennedy, L. B. & Salama, A. K. S. A review of cancer immunotherapy toxicity. CA Cancer J. Clin. 70, 86–104 (2020).
Postow, M. A., Sidlow, R. & Hellmann, M. D. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 378, 158–168 (2018).
Kalbasi, A. & Ribas, A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat. Rev. Immunol. 20, 25–39 (2020).
Flugel, C. L. et al. Overcoming on-target, off-tumour toxicity of CAR T cell therapy for solid tumours. Nat. Rev. Clin. Oncol. 20, 49–62 (2023).
Gohil, S. H., Iorgulescu, J. B., Braun, D. A., Keskin, D. B. & Livak, K. J. Applying high-dimensional single-cell technologies to the analysis of cancer immunotherapy. Nat. Rev. Clin. Oncol. 18, 244–256 (2021).
Xu, J. et al. Near-infrared-triggered photodynamic therapy with multitasking upconversion nanoparticles in combination with checkpoint blockade for immunotherapy of colorectal cancer. ACS Nano 11, 4463–4474 (2017).
Ruan, S., Huang, Y., He, M. & Gao, H. Advanced biomaterials for cell‐specific modulation and restore of cancer immunotherapy. Adv. Sci. 9, 2200027 (2022).
Pan, H., Zheng, M., Ma, A., Liu, L. & Cai, L. Cell/bacteria‐based bioactive materials for cancer immune modulation and precision therapy. Adv. Mater. 33, 2100241 (2021).
Rodrigues, D. B., Reis, R. L. & Pirraco, R. P. How are natural-based polymers shaping the future of cancer immunotherapy — a review. Polym. Rev. https://doi.org/10.1080/15583724.2023.2234462 (2023).
Wong, K. H., Lu, A., Chen, X. & Yang, Z. Natural ingredient-based polymeric nanoparticles for cancer treatment. Molecules 25, 3620 (2020).
Munir, M. U. Nanomedicine penetration to tumor: challenges, and advanced strategies to tackle this issue. Cancers 14, 2904 (2022).
Zhang, Y. et al. Strategies to improve tumor penetration of nanomedicines through nanoparticle design. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 11, e1519 (2019).
Burugu, S., Dancsok, A. R. & Nielsen, T. O. Emerging targets in cancer immunotherapy. Semin. Cancer Biol. 52, 39–52 (2018).
Milling, L., Zhang, Y. & Irvine, D. J. Delivering safer immunotherapies for cancer. Adv. Drug Deliv. Rev. 114, 79–101 (2017).
Morris, E. C., Neelapu, S. S., Giavridis, T. & Sadelain, M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat. Rev. Immunol. 22, 85–96 (2022).
Gajewski, T. F. et al. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Curr. Opin. Immunol. 25, 268–276 (2013).
Boucher, Y., Baxter, L. T. & Jain, R. K. Interstitial pressure gradients in tissue-isolated and subcutaneous tumors: implications for therapy. Cancer Res. 50, 4478–4484 (1990).
Han, H. et al. Progress on the pathological tissue microenvironment barrier-modulated nanomedicine. Adv. Drug Deliv. Rev. 200, 115051 (2023).
Ribas, A. Clinical development of the anti-CTLA-4 antibody tremelimumab. Semin. Oncol. 37, 450–454 (2010).
Linsley, P. S. & Nadler, S. G. The clinical utility of inhibiting CD28‐mediated costimulation. Immunol. Rev. 229, 307–321 (2009).
McKinlay, C. J. et al. Charge-altering releasable transporters (CARTs) for the delivery and release of mRNA in living animals. Proc. Natl Acad. Sci. USA 114, E448–E456 (2017).
June, C. H. et al. Engineered T cells for cancer therapy. Cancer Immunol. Immunother. 63, 969–975 (2014).
Zheng, P.-P., Kros, J. M. & Wang, G. Elusive neurotoxicity in T cell-boosting anticancer therapies. Trends Immunol. 40, 274–278 (2019).
Acknowledgements
M.J.M. acknowledges support from the NIH Director’s New Innovator Award (DP2 TR002776), the American Cancer Society (no. RSG-22-122-01-ET) and an NSF CAREER Award (CBET-2145491). A.S.T. and R.M.H. acknowledge support from the National Science Foundation Graduate Research Fellowship (Award 1845298). D.M. acknowledges support from the Norman and Selma Kron Research Fellowship and the Robert Wood Johnson Foundation Health Policy Research Scholars.
Author information
Authors and Affiliations
Contributions
L.X., A.S.T. and M.J.M. conceived and outlined the general manuscript. L.X., A.S.T., D.M. and R.M.H. wrote the initial manuscript with contributions from X.H., N.G., K.W., N.C.S., C.H.J. and M.J.M. All authors edited the manuscript and figures and approved the final version for submission.
Corresponding author
Ethics declarations
Competing interests
N.C.S. holds equity in Tmunity Therapeutics. C.H.J. has received financial support from Novartis and has patents related to CAR therapy with royalties paid from Novartis to the University of Pennsylvania. C.H.J. is a scientific adviser for Alaunos, BluesphereBio, Cabaletta, Carisma, Cartography, Cellares, Cellcarta, Celldex, Danaher, Decheng, ImmuneSensor, Poseida, Verismo, Viracta and WIRB-Copernicus group and is a co-founder and holds equity in Capstan Therapeutics and Tmunity Therapeutics. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Materials thanks Christopher Jewell, James Moon, who co-reviewed with Yujin Kim, and Omid Veiseh for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Clinical trials: https://www.clinicaltrials.gov/
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xue, L., Thatte, A.S., Mai, D. et al. Responsive biomaterials: optimizing control of cancer immunotherapy. Nat Rev Mater 9, 100–118 (2024). https://doi.org/10.1038/s41578-023-00617-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41578-023-00617-2