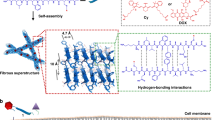
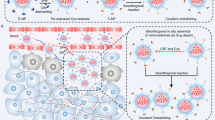
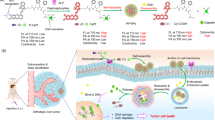
Abstract
In situ self-assembly — the in situ formation of complex materials via biochemical reactions of monomers — has enhanced the efficacy of drug delivery for cancer therapy and imaging. So far, nanomedicine has been confined to ex situ self-assembly, which is limited by poor deep-tumour penetration and poor blood circulation. By contrast, in situ self-assembly-based cancer treatments offer various advantages, including enhanced blood circulation of monomers, long-term drug delivery pharmacokinetics, low drug resistance and the ability to target deep tumours and organelles, which can result in disruption-mediated apoptosis and enable the imaging of cellular activity for effective cancer therapy and diagnosis. In this Review, we discuss the design of in situ self-assembled nanomedicines for cancer therapy and imaging based on various endogenous and exogenous stimuli in both the extracellular and the intracellular milieu. We also highlight the advantages of cancer treatment via multimodal dynamic transformations of nanostructures self-assembled in situ, including the ability to induce mechanical stress, deploy cancer-specific targeted therapies, obtain deep-tumour penetration and sustain prolonged drug retention time in the body. Finally, we discuss from a clinical viewpoint the challenges of in situ self-assembled nanomedicine and its potential to offer advanced alternatives to existing cancer therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Wyld, L., Audisio, R. A. & Poston, G. J. The evolution of cancer surgery and future perspectives. Nat. Rev. Clin. Oncol. 12, 115–124 (2015).
Sgouros, G., Bodei, L., McDevitt, M. R. & Nedrow, J. R. Radiopharmaceutical therapy in cancer: clinical advances and challenges. Nat. Rev. Drug Discov. 19, 589–608 (2020).
Alexander, J. L. et al. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat. Rev. Gastroenterol. Hepatol. 14, 356–365 (2017).
Helmink, B. A., Khan, M. A. W., Hermann, A., Gopalakrishnan, V. & Wargo, J. A. The microbiome, cancer, and cancer therapy. Nat. Med. 25, 377–388 (2019).
Price, J. M., Prabhakaran, A. & West, C. M. L. Predicting tumour radiosensitivity to deliver precision radiotherapy. Nat. Rev. Clin. Oncol. 20, 83–98 (2023).
Schaue, D. & McBride, W. H. Opportunities and challenges of radiotherapy for treating cancer. Nat. Rev. Clin. Oncol. 12, 527–540 (2015).
Roscoe, J. A. et al. Patient expectation is a strong predictor of severe nausea after chemotherapy. Cancer 101, 2701–2708 (2004).
Hesketh, P. J. Chemotherapy-induced nausea and vomiting. N. Engl. J. Med. 358, 2482–2494 (2008).
Dirix, P., Nuyts, S. & Van den Bogaert, W. Radiation-induced xerostomia in patients with head and neck cancer. Cancer 107, 2525–2534 (2006).
Lalla, R. V. et al. A systematic review of oral fungal infections in patients receiving cancer therapy. Support. Care Cancer 18, 985–992 (2010).
Kato, K. et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 20, 1506–1517 (2019).
Krishnan, V. & Mitragotri, S. Nanoparticles for topical drug delivery: potential for skin cancer treatment. Adv. Drug Deliv. Rev. 153, 87–108 (2020).
Gilhar, A., Keren, A. & Paus, R. JAK inhibitors and alopecia areata. Lancet 393, 318–319 (2019).
Di Maio, M., Basch, E., Bryce, J. & Perrone, F. Patient-reported outcomes in the evaluation of toxicity of anticancer treatments. Nat. Rev. Clin. Oncol. 13, 319–325 (2016).
Li, S. et al. Neoadjuvant therapy with immune checkpoint blockade, antiangiogenesis, and chemotherapy for locally advanced gastric cancer. Nat. Commun. 14, 8 (2023).
Vasan, N., Baselga, J. & Hyman, D. M. A view on drug resistance in cancer. Nature 575, 299–309 (2019).
Gottesman, M. M., Fojo, T. & Bates, S. E. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat. Rev. Cancer 2, 48–58 (2002).
Chi, Y. et al. CapG promotes resistance to paclitaxel in breast cancer through transactivation of PIK3R1/P50. Theranostics 9, 6840–6855 (2019).
Wang, P. et al. NIR-II nanoprobes in-vivo assembly to improve image-guided surgery for metastatic ovarian cancer. Nat. Commun. 9, 2898 (2018).
Rajan, S. S. et al. The mechanism of cancer drug addiction in ALK-positive T-cell lymphoma. Oncogene 39, 2103–2117 (2020).
Zhou, J. et al. Enzymatic self-assembly confers exceptionally strong synergism with NF-κB targeting for selective necroptosis of cancer cells. J. Am. Chem. Soc. 140, 2301–2308 (2018).
Li, P. et al. Site-selective in situ growth-induced self-assembly of protein–polymer conjugates into pH-responsive micelles for tumor microenvironment triggered fluorescence imaging. Biomacromolecules 19, 4472–4479 (2018).
Liu, Y. et al. In situ supramolecular polymerization-enhanced self-assembly of polymer vesicles for highly efficient photothermal therapy. Nat. Commun. 11, 1724 (2020).
Wu, G. et al. One-step in situ self-assembly of biodegradable films for long-term intravesical bladder cancer therapy. ACS Appl. Bio Mater. 5, 825–832 (2022).
Ma, B. et al. Self-assembled copper–amino acid nanoparticles for in situ glutathione “AND” H2O2 sequentially triggered chemodynamic therapy. J. Am. Chem. Soc. 141, 849–857 (2019).
Samperi, M., Pérez-García, L. & Amabilino, D. B. Quantification of energy of activation to supramolecular nanofibre formation reveals enthalpic and entropic effects and morphological consequence. Chem. Sci. 10, 10256–10266 (2019).
Yin, P., Choi, H. M. T., Calvert, C. R. & Pierce, N. A. Programming biomolecular self-assembly pathways. Nature 451, 318–322 (2008).
Dergham, M., Lin, S. & Geng, J. Supramolecular self-assembly in living cells. Angew. Chem. Int. Ed. 61, e202114267 (2022).
Du, X. W. et al. In situ generated D-peptidic nanofibrils as multifaceted apoptotic inducers to target cancer cells. Cell Death Dis. 8, e2614 (2017).
Zhang, Y. et al. Controlled intracellular polymerization for cancer treatment. JACS Au 2, 579–589 (2022).
Wu, J. et al. Programmable ROS-mediated cancer therapy via magneto-inductions. Adv. Sci. 7, 1902933 (2020).
He, H., Liu, S., Wu, D. & Xu, B. Enzymatically formed peptide assemblies sequestrate proteins and relocate inhibitors to selectively kill cancer cells. Angew. Chem. Int. Ed. 59, 16445–16450 (2020).
Fan, J. Q. et al. Binding-induced fibrillogenesis peptides recognize and block intracellular vimentin skeletonization against breast cancer. Nano Lett. 21, 6202–6210 (2021).
Li, G., Hu, X. W., Wu, X. & Zhang, Y. Microtubule-targeted self-assembly triggers prometaphase-metaphase oscillations suppressing tumor growth. Nano Lett. 21, 3052–3059 (2021).
Song, N. et al. In situ oxidation-regulated self-assembly of peptides into transformable scaffolds for cascade therapy. Nano Today 38, 101198 (2021).
Yang, Y. et al. Photodynamic therapy with liposomes encapsulating photosensitizers with aggregation-induced emission. Nano Lett. 19, 1821–1826 (2019).
Liu, B., Wu, R., Gong, S., Xiao, H. & Thayumanavan, S. In situ formation of polymeric nanoassemblies using an efficient reversible click reaction. Angew. Chem. Int. Ed. 59, 15135–15140 (2020).
Cheng, Z. F. et al. Self-assembly of pentapeptides into morphology-adaptable nanomedicines for enhanced combinatorial chemo-photodynamic therapy. Nano Today 33, 100878 (2020).
Li, M. et al. Proline isomerization-regulated tumor microenvironment-adaptable self-assembly of peptides for enhanced therapeutic efficacy. Nano Lett. 19, 7965–7976 (2019).
Kang, H. et al. Immunoregulation of macrophages by dynamic ligand presentation via ligand–cation coordination. Nat. Commun. 10, 1696 (2019).
Wang, X. et al. Tumor-microenvironment-activated in situ self-assembly of sequentially responsive biopolymer for targeted photodynamic therapy. Adv. Funct. Mater. 30, 2000229 (2020).
Cheng, G. et al. Programmed size-changeable nanotheranostic agents for enhanced imaging-guided chemo/photodynamic combination therapy and fast elimination. Adv. Mater. 33, 2100398 (2021).
Cheng, Q. et al. Supramolecular tropism driven aggregation of nanoparticles in situ for tumor-specific bioimaging and photothermal therapy. Small 17, 2101332 (2021).
Li, S. et al. Smart NIR-II croconaine dye-peptide for enhanced photo-sonotheranostics of hepatocellular carcinoma. Theranostics 12, 76–86 (2022).
She, J. et al. Thermo-triggered in situ chitosan-based gelation system for repeated and enhanced sonodynamic therapy post a single injection. Adv. Healthc. Mater. 10, 2001208 (2021).
Chen, Z., Chen, M., Zhou, K. & Rao, J. Pre-targeted imaging of protease activity through in situ assembly of nanoparticles. Angew. Chem. Int. Ed. 59, 7864–7870 (2020).
Hou, D.-Y. et al. An activated excretion-retarded tumor imaging strategy towards metabolic organs. Bioact. Mater. 14, 110–119 (2022).
Hu, Y. X. et al. Enzyme-mediated in situ self-assembly promotes in vivo bioorthogonal reaction for pretargeted multimodality imaging. Angew. Chem. Int. Ed. 60, 18082–18093 (2021).
He, H., Guo, J., Lin, X. & Xu, B. Enzyme-instructed assemblies enable mitochondria localization of histone H2B in cancer cells. Angew. Chem. Int. Ed. 59, 9330–9334 (2020).
Yang, K. et al. Enzyme-induced in vivo assembly of gold nanoparticles for imaging-guided synergistic chemo-photothermal therapy of tumor. Biomaterials 223, 119460 (2019).
Wang, Y. et al. Tailoring a near-infrared macrocyclization scaffold allows the control of in situ self-assembly for photoacoustic/PET bimodal imaging. Angew. Chem. Int. Ed. 61, e202200369 (2022).
Sun, Z. Y. et al. Controlled nano-bio interface of functional nanoprobes for in vivo monitoring enzyme activity in tumors. ACS Nano 13, 1153–1167 (2019).
Feng, H.-T., Lam, J. W. Y. & Tang, B. Z. Self-assembly of AIEgens. Coord. Chem. Rev. 406, 213142 (2020).
Yang, Z. et al. Hierarchical self-assembly of a pyrene-based discrete organoplatinum(II) double-metallacycle with triflate anions via hydrogen bonding and its tunable fluorescence emission. J. Am. Chem. Soc. 142, 13689–13694 (2020).
Ciesielski, A., El Garah, M., Masiero, S. & Samorì, P. Self-assembly of natural and unnatural nucleobases at surfaces and interfaces. Small 12, 83–95 (2016).
Nonappa & Ikkala, O. Hydrogen bonding directed colloidal self-assembly of nanoparticles into 2D crystals, capsids, and supracolloidal assemblies. Adv. Funct. Mater. 28, 1704328 (2018).
Zhang, Z. et al. Intra- and intermolecular self-assembly of a 20-nm-wide supramolecular hexagonal grid. Nat. Chem. 12, 468–474 (2020).
Walker, D. A., Browne, K. P., Kowalczyk, B. & Grzybowski, B. A. Self-assembly of nanotriangle superlattices facilitated by repulsive electrostatic interactions. Angew. Chem. Int. Ed. 49, 6760–6763 (2010).
Guo, T. et al. Intermolecular self-assembly of dopamine-conjugated carboxymethylcellulose and carbon nanotubes toward supertough filaments and multifunctional wearables. Chem. Eng. J. 416, 128981 (2021).
Chen, L.-J. & Yang, H.-B. Construction of stimuli-responsive functional materials via hierarchical self-assembly involving coordination interactions. Acc. Chem. Res. 51, 2699–2710 (2018).
Domoto, Y. & Fujita, M. Self-assembly of nanostructures with high complexity based on metal⋯unsaturated-bond coordination. Coord. Chem. Rev. 466, 214605 (2022).
Xu, J. et al. Wet and functional adhesives from one-step aqueous self-assembly of natural amino acids and polyoxometalates. Angew. Chem. Int. Ed. 56, 8731–8735 (2017).
Kang, H. et al. An in situ reversible heterodimeric nanoswitch controlled by metal-ion-ligand coordination regulates the mechanosensing and differentiation of stem cells. Adv. Mater. 30, 1803591 (2018).
Chen, Z. et al. Exploring the condensation reaction between aromatic nitriles and amino thiols to optimize in situ nanoparticle formation for the imaging of proteases and glycosidases in cells. Angew. Chem. Int. Ed. 59, 3272–3279 (2020).
Qi, G. et al. Enzyme-mediated intracellular polymerization of AIEgens for light-up tumor localization and theranostics. Adv. Mater. 34, 2106885 (2022).
Liu, J. & Liu, B. Living cell-mediated in-situ polymerization for biomedical applications. Prog. Polym. Sci. 129, 101545 (2022).
Bishop, K. J. M. Self-assembly across scales. Nat. Mater. 21, 501–502 (2022).
Nevers, D. R. et al. Mesophase formation stabilizes high-purity magic-sized clusters. J. Am. Chem. Soc. 140, 3652–3662 (2018).
Zhou, Z. X., Maxeiner, K., Ng, D. Y. W. & Weil, T. Polymer chemistry in living cells. Acc. Chem. Res. 55, 2998–3009 (2022).
O’Leary, L. E. R., Fallas, J. A., Bakota, E. L., Kang, M. K. & Hartgerink, J. D. Multi-hierarchical self-assembly of a collagen mimetic peptide from triple helix to nanofibre and hydrogel. Nat. Chem. 3, 821–828 (2011).
Levin, A. et al. Biomimetic peptide self-assembly for functional materials. Nat. Rev. Chem. 4, 615–634 (2020).
Luo, S. et al. Targeting self-assembly peptide for inhibiting breast tumor progression and metastasis. Biomaterials 249, 120055 (2020).
Guo, R. C. et al. In vivo self-assembly induced cell membrane phase separation for improved peptide drug internalization. Angew. Chem. Int. Ed. 60, 25128–25134 (2021).
Brito, A. et al. Inhibiting cancer metabolism by aromatic carbohydrate amphiphiles that act as antagonists of the glucose transporter GLUT1. Chem. Sci. 11, 3737–3744 (2020).
Yan, R. et al. Activatable NIR fluorescence/MRI bimodal probes for in vivo imaging by enzyme-mediated fluorogenic reaction and self-assembly. J. Am. Chem. Soc. 141, 10331–10341 (2019).
Feng, Z., Han, X., Wang, H., Tang, T. & Xu, B. Enzyme-instructed peptide assemblies selectively inhibit bone tumors. Chem 5, 2442–2449 (2019).
Wang, Q. et al. In situ supramolecular self-assembly of Pt(IV) prodrug to conquer cisplatin resistance. Adv. Funct. Mater. 31, 2101826 (2021).
Wang, Y. H. et al. Selective degradation of PD-L1 in cancer cells by enzyme-instructed self-assembly. Adv. Funct. Mater. 31, 2102505 (2021).
Zhao, X.-X. et al. In situ self-assembled nanofibers precisely target cancer-associated fibroblasts for improved tumor imaging. Angew. Chem. Int. Ed. 58, 15287–15294 (2019).
An, H.-W. et al. A near-infrared peptide probe with tumor-specific excretion-retarded effect for image-guided surgery of renal cell carcinoma. ACS Nano 14, 927–936 (2020).
Li, G. et al. Lipid-raft-targeted molecular self-assembly inactivates YAP to treat ovarian cancer. Nano Lett. 21, 747–755 (2021).
Wang, M. D. et al. Targeted in situ self-assembly augments peptide drug conjugate cell-entry efficiency. Biomaterials 278, 121139 (2021).
Wang, Z. et al. Addressable peptide self-assembly on the cancer cell membrane for sensitizing chemotherapy of renal cell carcinoma. Adv. Mater. 31, 1807175 (2019).
Mang, D., Roy, S. R., Zhang, Q., Hu, X. & Zhang, Y. Heparan sulfate-instructed self-assembly selectively inhibits cancer cell migration. ACS Appl. Bio Mater. 13, 17236–17242 (2021).
Roy, S. R. et al. Integrin and heparan sulfate dual-targeting peptide assembly suppresses cancer metastasis. ACS Appl. Mater. Interfaces 12, 19277–19284 (2020).
Wang, M.-D., Lv, G. T., An, H. W., Zhang, N. Y. & Wang, H. In situ self-assembly of bispecific peptide for cancer immunotherapy. Angew. Chem. Int. Ed. 61, e202113649 (2022).
Cao, Z. et al. Bioorthogonal in situ assembly of nanomedicines as drug depots for extracellular drug delivery. Nat. Commun. 13, 2038 (2022).
Cong, Y. et al. Microenvironment-induced in situ self-assembly of polymer-peptide conjugates that attack solid tumors deeply. Angew. Chem. Int. Ed. 58, 4632–4637 (2019).
Hu, B. et al. Acid-driven aggregation of selenol-functionalized zwitterionic gold nanoparticles improves the photothermal treatment efficacy of tumors. Mater. Chem. Front. 6, 775–782 (2022).
Zhang, R. et al. Acid-induced in vivo assembly of gold nanoparticles for enhanced photoacoustic imaging-guided photothermal therapy of tumors. Adv. Healthc. Mater. 9, 2000394 (2020).
Shi, Y. et al. Fe-doped polyoxometalate as acid-aggregated nanoplatform for NIR-II photothermal-enhanced chemodynamic therapy. Adv. Healthc. Mater. 9, 2000005 (2020).
Chen, H. et al. Smart self-assembly amphiphilic cyclopeptide-dye for near-infrared window-II imaging. Adv. Mater. 33, 2006902 (2021).
Zhang, K. et al. Peptide-based nanoparticles mimic fibrillogenesis of laminin in tumor vessels for precise embolization. ACS Nano 14, 7170–7180 (2020).
Chen, W. et al. Combined tumor environment triggered self-assembling peptide nanofibers and inducible multivalent ligand display for cancer cell targeting with enhanced sensitivity and specificity. Small 16, 2002780 (2020).
Zhang, P. et al. Quantitative mapping of glutathione within intracranial tumors through interlocked MRI signals of a responsive nanoprobe. Angew. Chem. Int. Ed. 60, 8130–8138 (2021).
Cui, L. et al. Reduction triggered in situ polymerization in living mice. J. Am. Chem. Soc. 142, 15575–15584 (2020).
Wang, Y. et al. Tumor vessel targeted self-assemble nanoparticles for amplification and prediction of the embolization effect in hepatocellular carcinoma. ACS Nano 14, 14907–14918 (2020).
Guillin, O. M., Vindry, C., Ohlmann, T. & Chavatte, L. Selenium, selenoproteins and viral infection. Nutrients 11, 2101 (2019).
Kim, Y. et al. Photoswitchable microgels for dynamic macrophage modulation. Adv. Mater. 34, 2205498 (2022).
Meng, Z. et al. Light-triggered in situ gelation to enable robust photodynamic-immunotherapy by repeated stimulations. Adv. Mater. 31, 1900927 (2019).
Wang, Q., Li, D., Xiao, J. Y., Guo, F. C. & Qi, L. M. Reversible self-assembly of gold nanorods mediated by photoswitchable molecular adsorption. Nano Res. 12, 1563–1569 (2019).
Lee, H. P. et al. Light-triggered in situ gelation of hydrogels using 2D molybdenum disulfide (MoS2) nanoassemblies as crosslink epicenter. Adv. Mater. 33, 2101238 (2021).
Liu, F.-H. et al. Near-infrared laser-driven in situ self-assembly as a general strategy for deep tumor therapy. Nano Lett. 18, 6577–6584 (2018).
Lee, S. et al. Magnetic control and real-time monitoring of stem cell differentiation by the ligand nanoassembly. Small 17, 2102892 (2021).
Cai, X. Q. et al. In situ pepsin-assisted needle assembly of magnetic-graphitic-nanocapsules for enhanced gastric retention and mucus penetration. Nano Today 36, 101032 (2021).
Wu, H. et al. Injectable thermosensitive magnetic nanoemulsion hydrogel for multimodal-imaging-guided accurate thermoablative cancer therapy. Nanoscale 9, 16175–16182 (2017).
Gong, D., Celi, N., Zhang, D. Y. & Cai, J. Magnetic biohybrid microrobot multimers based on chlorella cells for enhanced targeted drug delivery. ACS Appl. Mater. Interfaces 14, 6320–6330 (2022).
Yu, B., Choi, B., Li, W. & Kim, D.-H. Magnetic field boosted ferroptosis-like cell death and responsive MRI using hybrid vesicles for cancer immunotherapy. Nat. Commun. 11, 3637 (2020).
Cheng, D.-B. et al. Ultrasound-activated cascade effect for synergistic orthotopic pancreatic cancer therapy. iScience 23, 101144 (2020).
Zhu, J. et al. Ultrasound-triggered in situ gelation to overcome tumor hypoxia for enhanced photodynamic and sustained chemotherapy. Adv. Ther. 4, 2100052 (2021).
Zheng, D.-W. et al. Controllable gelation of artificial extracellular matrix for altering mass transport and improving cancer therapies. Nat. Commun. 11, 4907 (2020).
Son, S. et al. Cancer therapeutics based on diverse energy sources. Chem. Soc. Rev. 51, 8201–8215 (2022).
Chagri, S., Ng, D. Y. W. & Weil, T. Designing bioresponsive nanomaterials for intracellular self-assembly. Nat. Rev. Chem. 6, 320–338 (2022).
Du, W., Hu, X., Wei, W. & Liang, G. Intracellular peptide self-assembly: a biomimetic approach for in situ nanodrug preparation. Bioconjug. Chem. 29, 826–837 (2018).
Liu, J., Chen, W., Zhao, Z. & Xu, H. H. Reprogramming of mesenchymal stem cells derived from iPSCs seeded on biofunctionalized calcium phosphate scaffold for bone engineering. Biomaterials 34, 7862–7872 (2013).
Yi, M. et al. Enzyme responsive rigid-rod aromatics target “undruggable” phosphatases to kill cancer cells in a mimetic bone microenvironment. J. Am. Chem. Soc. 144, 13055–13059 (2022).
López-Otín, C. & Matrisian, L. M. Emerging roles of proteases in tumour suppression. Nat. Rev. Cancer 7, 800–808 (2007).
Chen, R., Jäättelä, M. & Liu, B. Lysosome as a central hub for rewiring pH homeostasis in tumors. Cancers 12, 2437 (2020).
Van Damme, P. et al. Caspase-specific and nonspecific in vivo protein processing during Fas-induced apoptosis. Nat. Methods 2, 771–777 (2005).
Sun, M., Wang, C., Lv, M., Fan, Z. & Du, J. Intracellular self-assembly of peptides to induce apoptosis against drug-resistant melanoma. J. Am. Chem. Soc. 144, 7337–7345 (2022).
An, H.-W. et al. A tumour-selective cascade activatable self-detained system for drug delivery and cancer imaging. Nat. Commun. 10, 4861 (2019).
He, H. et al. Enzymatic cleavage of branched peptides for targeting mitochondria. J. Am. Chem. Soc. 140, 1215–1218 (2018).
He, H., Lin, X., Guo, J., Wang, J. & Xu, B. Perimitochondrial enzymatic self-assembly for selective targeting the mitochondria of cancer cells. ACS Nano 14, 6947–6955 (2020).
Gao, Z. et al. β-Galactosidase responsive AIE fluorogene for identification and removal of senescent cancer cells. Sci. China Chem. 63, 398–403 (2020).
Ohkuma, S. & Poole, B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc. Natl Acad. Sci. USA 75, 3327–3331 (1978).
Geisow, M. J., D’Arcy Hart, P. & Young, M. R. Temporal changes of lysosome and phagosome pH during phagolysosome formation in macrophages: studies by fluorescence spectroscopy. J. Cell Biol. 89, 645–652 (1981).
Mindell, J. A. Lysosomal acidification mechanisms. Annu. Rev. Physiol. 74, 69–86 (2012).
Wang, J. et al. Intracellular condensates of oligopeptide for targeting lysosome and addressing multiple drug resistance of cancer. Adv. Mater. 34, 2104704 (2022).
Wang, J. et al. Nanolab in a cell: crystallization-induced in situ self-assembly for cancer theranostic amplification. J. Am. Chem. Soc. 144, 14388–14395 (2022).
Wang, Y. et al. Smart acid-activatable self-assembly of black phosphorous as photosensitizer to overcome poor tumor retention in photothermal therapy. Adv. Funct. Mater. 30, 2003338 (2020).
Panieri, E. & Santoro, M. M. ROS homeostasis and metabolism: a dangerous liason in cancer cells. Cell Death Dis. 7, e2253 (2016).
Tu, Y., Peng, F., White, P. B. & Wilson, D. A. Redox-sensitive stomatocyte nanomotors: destruction and drug release in the presence of glutathione. Angew. Chem. Int. Ed. 56, 7620–7624 (2017).
Yang, D.-S. et al. A redox-triggered bispecific supramolecular nanomedicine based on peptide self-assembly for high-efficacy and low-toxic cancer therapy. Adv. Funct. Mater. 30, 1904969 (2020).
Qiu, L. et al. Tumor microenvironment responsive “head-to-foot” self-assembly nanoplatform for positron emission tomography imaging in living subjects. ACS Nano 15, 18250–18259 (2021).
Yang, H., Yuan, B., Zhang, X. & Scherman, O. A. Supramolecular chemistry at interfaces: host–guest interactions for fabricating multifunctional biointerfaces. Acc. Chem. Res. 47, 2106–2115 (2014).
Boekhoven, J., Rubert Pérez, C. M., Sur, S., Worthy, A. & Stupp, S. I. Dynamic display of bioactivity through host–guest chemistry. Angew. Chem. Int. Ed. 52, 12077–12080 (2013).
Pluth, M. D. et al. Structural consequences of anionic host−cationic guest interactions in a supramolecular assembly. Inorg. Chem. 48, 111–120 (2009).
Alavi, S., Udachin, K. & Ripmeester, J. A. Effect of guest–host hydrogen bonding on the structures and properties of clathrate hydrates. Chem. Eur. J. 16, 1017–1025 (2010).
Hua, B., Zhou, J. & Yu, G. Hydrophobic interactions in the pillar[5]arene-based host–guest complexation and their application in the inhibition of acetylcholine hydrolysis. Tetrahedron Lett. 56, 986–989 (2015).
Zhao, X. et al. Intracellular self-assembly driven nucleus-targeted photo-immune stimulator with chromatin decompaction function for robust innate and adaptive antitumor immunity. Adv. Funct. Mater. 32, 2108883 (2022).
Shi, J. et al. An intracellular self-assembly-driven uninterrupted ROS generator augments 5-aminolevulinic-acid-based tumor therapy. Adv. Mater. 34, 2201049 (2022).
Ko, M. J., Hong, H., Choi, H., Kang, H. & Kim, D.-H. Multifunctional magnetic nanoparticles for dynamic imaging and therapy. Adv. NanoBiomed Res. 2, 2200053 (2022).
Sun, S., Liang, H.-W., Wang, H. & Zou, Q. Light-triggered self-assembly of peptide nanoparticles into nanofibers in living cells through molecular conformation changes and H-bond interactions. ACS Nano 16, 18978–18989 (2022).
Shi, H. & Sadler, P. J. How promising is phototherapy for cancer? Br. J. Cancer 123, 871–873 (2020).
Shao, J. et al. Biodegradable black phosphorus-based nanospheres for in vivo photothermal cancer therapy. Nat. Commun. 7, 12967 (2016).
Long, X. et al. Autophagy-targeted nanoparticles for effective cancer treatment: advances and outlook. NPG Asia Mater. 14, 71 (2022).
Zhang, Y. et al. Harnessing copper-palladium alloy tetrapod nanoparticle-induced pro-survival autophagy for optimized photothermal therapy of drug-resistant cancer. Nat. Commun. 9, 4236 (2018).
Yu, G. et al. A discrete organoplatinum(II) metallacage as a multimodality theranostic platform for cancer photochemotherapy. Nat. Commun. 9, 4335 (2018).
Chen, Q. et al. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat. Commun. 7, 13193 (2016).
Li, X., Lovell, J. F., Yoon, J. & Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 17, 657–674 (2020).
Zhang, Y. et al. Permission to enter cell by shape: nanodisk vs nanosphere. ACS Appl. Mater. Interfaces 4, 4099–4105 (2012).
Cho, E. C., Au, L., Zhang, Q. & Xia, Y. The effects of size, shape, and surface functional group of gold nanostructures on their adsorption and internalization by cells. Small 6, 517–522 (2010).
Mumcuoglu, D. et al. Cellular internalization of therapeutic oligonucleotides by peptide amphiphile nanofibers and nanospheres. ACS Appl. Mater. Interfaces 8, 11280–11287 (2016).
Huang, X., Teng, X., Chen, D., Tang, F. & He, J. The effect of the shape of mesoporous silica nanoparticles on cellular uptake and cell function. Biomaterials 31, 438–448 (2010).
Rao, A., Roy, S., Jain, V. & Pillai, P. P. Nanoparticle self-assembly: from design principles to complex matter to functional materials. ACS Appl. Mater. Interfaces 15, 25248–25274 (2023).
Wu, C., Zhang, R., Du, W., Cheng, L. & Liang, G. Alkaline phosphatase-triggered self-assembly of near-infrared nanoparticles for the enhanced photoacoustic imaging of tumors. Nano Lett. 18, 7749–7754 (2018).
Chang, Y. et al. Cationic vesicles based on amphiphilic pillar[5]arene capped with ferrocenium: a redox-responsive system for drug/siRNA co-delivery. Angew. Chem. Int. Ed. 53, 13126–13130 (2014).
Liu, S. et al. Facile fabrication of redox-responsive covalent organic framework nanocarriers for efficiently loading and delivering doxorubicin. Macromol. Rapid Commun. 41, 1900570 (2020).
Croissant, J. G. et al. Protein-gold clusters-capped mesoporous silica nanoparticles for high drug loading, autonomous gemcitabine/doxorubicin co-delivery, and in-vivo tumor imaging. J. Control. Release 229, 183–191 (2016).
Ye, D. et al. Bioorthogonal cyclization-mediated in situ self-assembly of small-molecule probes for imaging caspase activity in vivo. Nat. Chem. 6, 519–526 (2014).
Gambhir, S. S. Molecular imaging of cancer with positron emission tomography. Nat. Rev. Cancer 2, 683–693 (2002).
Shields, A. F. et al. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat. Med. 4, 1334–1336 (1998).
Hennrich, U. & Benešová, M. [68Ga]Ga-DOTA-TOC: the first FDA-approved 68Ga-radiopharmaceutical for PET imaging. Pharmaceuticals 13, 38 (2020).
Pienta, K. J. et al. A phase 2/3 prospective multicenter study of the diagnostic accuracy of prostate specific membrane antigen PET/CT with 18F-DCFPyL in prostate cancer patients (OSPREY). J. Urol. 206, 52–61 (2021).
Lin, J. et al. Stimuli-responsive macrocyclization scaffold allows in situ self-assembly of radioactive tracers for positron emission tomography imaging of enzyme activity. J. Am. Chem. Soc. 144, 7667–7675 (2022).
Zheng, X. et al. Successively activatable ultrasensitive probe for imaging tumour acidity and hypoxia. Nat. Biomed. Eng. 1, 0057 (2017).
Li, H. et al. Activity-based NIR enzyme fluorescent probes for the diagnosis of tumors and image-guided surgery. Angew. Chem. Int. Ed. 60, 17268–17289 (2021).
Huang, J., Li, J., Lyu, Y., Miao, Q. & Pu, K. Molecular optical imaging probes for early diagnosis of drug-induced acute kidney injury. Nat. Mater. 18, 1133–1143 (2019).
Huang, J. et al. Renal clearable polyfluorophore nanosensors for early diagnosis of cancer and allograft rejection. Nat. Mater. 21, 598–607 (2022).
Liu, D. et al. Xanthene-based NIR-II dyes for in vivo dynamic imaging of blood circulation. J. Am. Chem. Soc. 143, 17136–17143 (2021).
Wen, X. et al. Controlled sequential in situ self-assembly and disassembly of a fluorogenic cisplatin prodrug for cancer theranostics. Nat. Commun. 14, 800 (2023).
Shi, S. et al. Versatile pH-response micelles with high cell-penetrating helical diblock copolymers for photoacoustic imaging guided synergistic chemo-photothermal therapy. Theranostics 6, 2170–2182 (2016).
Zhang, J., Smaga, L. P., Satyavolu, N. S. R., Chan, J. & Lu, Y. DNA aptamer-based activatable probes for photoacoustic imaging in living mice. J. Am. Chem. Soc. 139, 17225–17228 (2017).
Pu, K. et al. Semiconducting polymer nanoparticles as photoacoustic molecular imaging probes in living mice. Nat. Nanotechnol. 9, 233–239 (2014).
Zhang, Y. M. et al. Enhanced radiosensitization by gold nanoparticles with acid-triggered aggregation in cancer radiotherapy. Adv. Sci. 6, 1801806 (2019).
Bourlinos, A. B. et al. Gd(III)-doped carbon dots as a dual fluorescent-MRI probe. J. Mater. Chem. 22, 23327–23330 (2012).
Wang, H. et al. Paramagnetic properties of metal-free boron-doped graphene quantum dots and their application for safe magnetic resonance imaging. Adv. Mater. https://doi.org/10.1002/adma.201605416 (2017).
Kelly, B. D. et al. Anticancer activity of the taxane nanoparticles, DEP® docetaxel and DEP® cabazitaxel. Cancer Res. 80, 1716 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/study/NCT03255343 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01861496 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02112656 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00617981 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00041808 (2005).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03458975 (2023).
Barenholz, Y. C. Doxil®—the first FDA-approved nano-drug: lessons learned. J. Control. Release 160, 117–134 (2012).
Mazzotta, E., Tavano, L. & Muzzalupo, R. Thermo-sensitive vesicles in controlled drug delivery for chemotherapy. Pharmaceutics 10, 150 (2018).
Suresh, D., Suresh, A. & Kannan, R. Engineering biomolecular systems: controlling the self-assembly of gelatin to form ultra-small bioactive nanomaterials. Bioact. Mater. 18, 321–336 (2022).
Wang, D. et al. Nucleoside analogue-based supramolecular nanodrugs driven by molecular recognition for synergistic cancer therapy. J. Am. Chem. Soc. 140, 8797–8806 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04649359 (2023).
Hu, L. et al. Structure-based programming of supramolecular assemblies in living cells for selective cancer cell inhibition. Angew. Chem. Int. Ed. 60, 21807–21816 (2021).
Yuan, Y. et al. Furin-mediated intracellular self-assembly of olsalazine nanoparticles for enhanced magnetic resonance imaging and tumour therapy. Nat. Mater. 18, 1376–1383 (2019).
Chen, J. et al. Furin-instructed intracellular gold nanoparticle aggregation for tumor photothermal therapy. Adv. Funct. Mater. 30, 2001566 (2020).
Li, J. et al. Supramolecular self-assembly-facilitated aggregation of tumor-specific transmembrane receptors for signaling activation and converting immunologically cold to hot tumors. Adv. Mater. 33, 2008518 (2021).
Zhou, Z. et al. In situ assembly of platinum (II)-metallopeptide nanostructures disrupts energy homeostasis and cellular metabolism. J. Am. Chem. Soc. 144, 12219–12228 (2022).
Allison, S. J. et al. Self-assembly of an anion receptor with metal-dependent kinase inhibition and potent in vitro anti-cancer properties. Nat. Commun. 12, 3898 (2021).
Gao, C. et al. In vivo hitchhiking of immune cells by intracellular self-assembly of bacteria-mimetic nanomedicine for targeted therapy of melanoma. Sci. Adv. 8, eabn1805 (2022).
Liang, G., Ren, H. & Rao, J. A biocompatible condensation reaction for controlled assembly of nanostructures in living cells. Nat. Chem. 2, 54–60 (2010).
Seo, J. et al. Light-directed trapping of metastable intermediates in a self-assembly process. Nat. Commun. 11, 6260 (2020).
Williams, R. J. et al. Enzyme-assisted self-assembly under thermodynamic control. Nat. Nanotechnol. 4, 19–24 (2009).
Acknowledgements
This work was supported by the National Research Foundation of Korea (no. RS-2023-00208427 to H.K.; and CRI project No. 2018R1A3B1052702 to J.S.K.).
Author information
Authors and Affiliations
Contributions
J.K. and S.L. contributed equally to this work. J.K., S.L., Y.K., K.P., H.K. and J.S.K. conceptualized the manuscript. J.K., S.L., Y.K., M.C., I.L., E.K., C.G.Y. and H.K. reviewed the relevant literature and wrote the manuscript. H.K. and J.S.K. edited and finalized the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Materials thanks Xiaoyuan Chen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, J., Lee, S., Kim, Y. et al. In situ self-assembly for cancer therapy and imaging. Nat Rev Mater 8, 710–725 (2023). https://doi.org/10.1038/s41578-023-00589-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41578-023-00589-3
This article is cited by
-
Selenopeptide nanomedicine ameliorates atherosclerosis by reducing monocyte adhesions and inflammations
Nano Research (2024)
-
Nanomaterial-based contrast agents for common disease imaging
Science China Materials (2024)