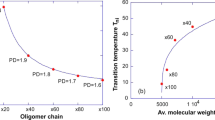
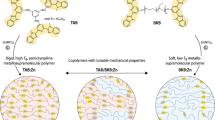
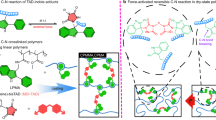
Abstract
Mechanically interlocked polymers (MIPs), such as polyrotaxanes and polycatenanes, are polymer architectures that incorporate a mechanical bond. In a polyrotaxane, the mechanical bond is the result of a linear dumbbell component threaded through a ring, while in a polycatenane, it is the consequence of interlocked ring components. The interlocked nature of these architectures can result in high degrees of conformational freedom and mobility of their components, which can give rise to unique property profiles. In recent years, the synthesis and studies of a range of MIPs has allowed researchers to build an initial understanding of how incorporating mechanical bonds within a polymer structure impacts its material properties. This Review focuses on the understanding of these structure–property relationships with an outlook towards their applications, specifically focusing on four main classes of MIPs: polyrotaxanes, slide-ring gels, daisy-chain polymers and polycatenanes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Schill, G. & Zollenkopf, H. Rotaxan-verbindungen, 1. Liebigs Ann. 721, 53–74 (1969).
Wasserman, E. The preparation of interlocking rings: a catenane. J. Am. Chem. Soc. 82, 4433–4434 (1960).
Dietrich-Buchecker, C. O. & Sauvage, J.-P. A synthetic molecular trefoil knot. Angew. Chem. Int. Ed. 28, 189–192 (1989).
Chichak, K. S. Molecular borromean rings. Science 304, 1308–1312 (2004).
Richards, V. Molecular machines. Nat. Chem. 8, 1090–1090 (2016).
Sauvage, J.-P. From chemical topology to molecular machines (Nobel Lecture). Angew. Chem. Int. Ed. 56, 11080–11093 (2017).
Feringa, B. L. The art of building small: from molecular switches to motors (Nobel Lecture). Angew. Chem. Int. Ed. 56, 11060–11078 (2017).
Stoddart, J. F. Mechanically interlocked molecules (MIMs)—molecular shuttles, switches, and machines (Nobel Lecture). Angew. Chem. Int. Ed. 56, 11094–11125 (2017).
Cotí, K. K. et al. Mechanised nanoparticles for drug delivery. Nanoscale 1, 16–39 (2009).
Garcia-Rio, L., Otero-Espinar, F. J., Luzardo-Alvarez, A. & Blanco-Mendez, J. Cyclodextrin based rotaxanes, polyrotaxanes and polypseudorotaxanes and their biomedical applications. Curr. Top. Med. Chem. 14, 478–493 (2014).
Zhang, Y. M., Liu, Y. H. & Liu, Y. Cyclodextrin-based multistimuli-responsive supramolecular assemblies and their biological functions. Adv. Mater. 32, 1806158 (2019).
Zhang, J. & Ma, P. X. Cyclodextrin-based supramolecular systems for drug delivery: Recent progress and future perspective. Adv. Drug Deliv. Rev. 65, 1215–1233 (2013).
Neal, E. A. & Goldup, S. M. Chemical consequences of mechanical bonding in catenanes and rotaxanes: isomerism, modification, catalysis and molecular machines for synthesis. Chem. Commun. 50, 5128–5142 (2014).
Evans, N. H. & Beer, P. D. Progress in the synthesis and exploitation of catenanes since the Millennium. Chem. Soc. Rev. 43, 4658–4683 (2014).
Leigh, D. A., Marcos, V. & Wilson, M. R. Rotaxane catalysts. ACS Catal. 4, 4490–4497 (2014).
Beves, J. E., Blight, B. A., Campbell, C. J., Leigh, D. A. & McBurney, R. T. Strategies and tactics for the metal-directed synthesis of rotaxanes, knots, catenanes, and higher order links. Angew. Chem. Int. Ed. 50, 9260–9327 (2011).
Hubin, T. J. & Busch, D. H. Template routes to interlocked molecular structures and orderly molecular entanglements. Coord. Chem. Rev. 200–202, 5–52 (2000).
Niu, Z. & Gibson, H. W. Polycatenanes. Chem. Rev. 109, 6024–6046 (2009).
Fang, L. et al. Mechanically bonded macromolecules. Chem. Soc. Rev. 39, 17–29 (2010).
Arunachalam, M. & Gibson, H. W. Recent developments in polypseudorotaxanes and polyrotaxanes. Prog. Polym. Sci. 39, 1043–1073 (2014).
Takata, T., Kihara, N. & Furusho, Y. Polyrotaxanes and polycatenanes: recent advances in syntheses and applications of polymers comprising of interlocked structures. Adv. Polym. Sci. 171, 1–75 (2004).
Huang, F. H. & Gibson, H. W. Polypseudorotaxanes and polyrotaxanes. Prog. Polym. Sci. 30, 982–1018 (2005).
Harrison, I. T. & Harrison, S. Synthesis of a stable complex of a macrocycle and a threaded chain. J. Am. Chem. Soc. 89, 5723–5724 (1967).
Wenz, G. & Keller, B. Threading cyclodextrin rings on polymer chains. Angew. Chem. Int. Ed. Engl. 31, 197–199 (1992).
Harada, A. The molecular necklace: a rotaxane containing many threaded α-cyclodextrins. Nature 356, 325–327 (1992).
Fleury, G. et al. Synthesis and characterization of high molecular weight polyrotaxanes: Towards the control over a wide range of threaded α-cyclodextrins. Soft Matter 1, 378–385 (2005).
Miyake, K. et al. Formation process of cyclodextrin necklace−analysis of hydrogen bonding on a molecular level. J. Am. Chem. Soc. 125, 5080–5085 (2003).
Inomata, A. et al. Crystallinity and cooperative motions of cyclic molecules in partially threaded solid-state polyrotaxanes. Macromolecules 43, 4660–4666 (2010).
Kato, K., Mizusawa, T., Yokoyama, H. & Ito, K. Polyrotaxane glass: peculiar mechanics attributable to the isolated dynamics of different components. J. Phys. Chem. Lett. 6, 4043–4048 (2015).
Mayumi, K. & Ito, K. Structure and dynamics of polyrotaxane and slide-ring materials. Polymer 51, 959–967 (2010).
Zhao, C. et al. Sliding mode of cyclodextrin in polyrotaxane and slide-ring gel. J. Phys. Condens. Matter 17, S2841–S2846 (2005).
Yasuda, Y. et al. Molecular dynamics of polyrotaxane in solution investigated by quasi-elastic neutron scattering and molecular dynamics simulation: sliding motion of rings on polymer. J. Am. Chem. Soc. 141, 9655–9663 (2019).
Yasuda, Y. et al. Sliding dynamics of ring on polymer in rotaxane: a coarse-grained molecular dynamics simulation study. Macromolecules 52, 3787–3793 (2019).
Mayumi, K. et al. Concentration-induced conformational change in linear polymer threaded into cyclic molecules. Macromolecules 41, 6480–6485 (2008).
Yamada, S., Sanada, Y., Tamura, A., Yui, N. & Sakurai, K. Chain architecture and flexibility of α-cyclodextrin/PEG polyrotaxanes in dilute solutions. Polym. J. 47, 464–467 (2015).
Gibson, H. W., Liu, S., Gong, C., Ji, Q. & Joseph, E. Studies of the formation of poly(ester rotaxane)s from diacid chlorides, diols, and crown ethers and their properties. Macromolecules 30, 3711–3727 (1997).
Gong, C. & Gibson, H. W. Controlling microstructure in polymeric molecular shuttles: Solvent-induced localization of macrocycles in poly(urethane/crown ether) rotaxanes. Angew. Chem. Int. Ed. Engl. 36, 2331–2333 (1997).
Shen, Y. X., Xie, D. & Gibson, H. W. Polyrotaxanes Based on polyurethane backbones and crown ether cyclics. 1. Synthesis. J. Am. Chem. Soc. 116, 537–548 (1994).
Gong, C., Glass, T. E. & Gibson, H. W. Poly(urethane/crown ether rotaxane)s with solvent switchable microstructures. Macromolecules 31, 308–313 (1998).
Gong, C., Ji, Q., Subramaniam, C. & Gibson, H. W. Main chain polyrotaxanes by threading crown ethers onto a preformed polyurethane: preparation and properties. Macromolecules 31, 1814–1818 (1998).
Gong, C. & Gibson, H. W. Synthesis and characterization of a polyester/crown ether rotaxane derived from a difunctional blocking group. Macromolecules 29, 7029–7033 (1996).
Gong, C. G. & Gibson, H. W. Polyrotaxanes and related structures: Synthesis and properties. Curr. Opin. Solid State Mater. Sci. 2, 647–652 (1997).
Gong, C. & Gibson, H. W. Dethreading during the preparation of polyrotaxanes. Macromol. Chem. Phys. 198, 2321–2332 (1997).
Chen, Z. et al. Effect of component mobility on the properties of macromolecular [2]rotaxanes. Angew. Chem. Int. Ed. 55, 2778–2781 (2016).
Uenuma, S. et al. Drastic change of mechanical properties of polyrotaxane bulk: ABA–BAB sequence change depending on ring position. ACS Macro Lett. 8, 140–144 (2019).
Uenuma, S. et al. Self-assembled structure of polyrotaxane consisting of β-cyclodextrin and poly(ethylene oxide)-block-poly(propylene oxide)-block-poly(ethylene oxide) triblock copolymer in bulk system. Chem. Lett. 45, 991–993 (2016).
Lin, Q., Li, L., Tang, M., Hou, X. & Ke, C. Rapid macroscale shape morphing of 3D-printed polyrotaxane monoliths amplified from pH-controlled nanoscale ring motions. J. Mater. Chem. C 6, 11956–11960 (2018).
Lin, Q., Hou, X. & Ke, C. Ring shuttling controls macroscopic motion in a three-dimensional printed polyrotaxane monolith. Angew. Chem. Int. Ed. Engl. 56, 4452–4457 (2017).
Lin, Q., Tang, M. & Ke, C. Thermo-responsive 3D-printed polyrotaxane monolith. Polym. Chem. 11, 304–308 (2020).
Kato, K., Nemoto, K., Mayumi, K., Yokoyama, H. & Ito, K. Ductile glass of polyrotaxane toughened by stretch-induced intramolecular phase separation. ACS Appl. Mater. Interfaces 9, 32436–32440 (2017).
Kato, K., Mizusawa, T., Yokoyama, H. & Ito, K. Effect of topological constraint and confined motions on the viscoelasticity of polyrotaxane glass with different interactions between rings. J. Phys. Chem. C 121, 1861–1869 (2017).
Kato, K., Ohara, A., Yokoyama, H. & Ito, K. Prolonged glass transition due to topological constraints in polyrotaxanes. J. Am. Chem. Soc. 141, 12502–12506 (2019).
Cardin, D. J. Encapsulated conducting polymers. Adv. Mater. 14, 553–563 (2002).
Mayumi, K., Ito, K. & Kato, K. Polyrotaxane and Slide-Ring Materials (Royal Society of Chemistry, 2015).
Frampton, M. J. & Anderson, H. L. Insulated molecular wires. Angew. Chem. Int. Ed. Engl. 46, 1028–1064 (2007).
Terao, J., Tang, A., Michels, J. J., Krivokapic, A. & Anderson, H. L. Synthesis of poly(para-phenylenevinylene) rotaxanes by aqueous Suzuki coupling. Chem. Commun. 56–57 (2004).
Cacialli, F. et al. Cyclodextrin-threaded conjugated polyrotaxanes as insulated molecular wires with reduced interstrand interactions. Nat. Mater. 1, 160–164 (2002).
Taylor, P. N. et al. Insulated molecular wires: synthesis of conjugated polyrotaxanes by Suzuki coupling in water. Angew. Chem. Int. Ed. Engl. 39, 3456–3460 (2000).
Michels, J. J. et al. Synthesis of conjugated polyrotaxanes. Chem. Eur. J. 9, 6167–6176 (2003).
van den Boogaard, M. et al. Synthesis of insulated single-chain semiconducting polymers based on polythiophene, polyfluorene, and β-cyclodextrin. Chem. Mater. 16, 4383–4385 (2004).
Ikeda, T., Higuchi, M. & Kurth, D. G. From thiophene [2]rotaxane to polythiophene polyrotaxane. J. Am. Chem. Soc. 131, 9158–9159 (2009).
Farcas, A. et al. Molecular wire formation from poly[2,7-(9,9-dioctylfluorene)-alt-(5,5′-bithiophene/cucurbit[7]uril)] polyrotaxane copolymer. Eur. Polym. J. 62, 124–129.
Belosludov, R. V., Mizuseki, H., Ichinoseki, K. & Kawazoe, Y. Theoretical study on inclusion complex of polyaniline covered by cyclodextrins for molecular device. Jpn. J. Appl. Phys. 41, 2739–2741 (2002).
Belosludov, R. V. et al. Molecular enamel wires for electronic devices: Theoretical study. Jpn. J. Appl. Phys. 42, 2492–2494 (2003).
Brovelli, S. et al. Tuning intrachain versus interchain photophysics via control of the threading ratio of conjugated polyrotaxanes. Nano Lett. 8, 4546–4551 (2008).
Oddy, F. E. et al. Influence of cyclodextrin size on fluorescence quenching in conjugated polyrotaxanes by methyl viologen in aqueous solution. J. Mater. Chem. 19, 2846–2852 (2009).
Farcas, A. et al. Cucurbit[7]uril-threaded poly(3,4-ethylenedioxythiophene): a novel processable conjugated polyrotaxane. Eur. J. Org. Chem. 2019, 3442–3450 (2019).
Terao, J. & Tsuji, Y. New synthetic methods of π-conjugated inclusion complexes with high conductivity. J. Incl. Phenom. Macrocycl. Chem. 80, 165–175 (2014).
Grigoras, M. & Stafie, L. Electrically insulated molecular wires. Supramol. Chem. 22, 237–248 (2010).
Terao, J. et al. Insulated molecular wire with highly conductive π-conjugated polymer core. J. Am. Chem. Soc. 131, 18046–18047 (2009).
Terao, J. et al. Design principle for increasing charge mobility of π-conjugated polymers using regularly localized molecular orbitals. Nat. Commun. 4, 1691 (2013).
Wankar, J. et al. Recent advances in host–guest self-assembled cyclodextrin carriers: implications for responsive drug delivery and biomedical engineering. Adv. Funct. Mater. 30, 1909049 (2020).
Tamura, A. & Yui, N. Threaded macromolecules as a versatile framework for biomaterials. Chem. Commun. 50, 13433–13446 (2014).
Li, J. J., Zhao, F. & Li, J. Polyrotaxanes for applications in life science and biotechnology. Appl. Microbiol. Biotechnol. 90, 427–443 (2011).
Loethen, S., Kim, J.-M. & Thompson, D. H. Biomedical applications of cyclodextrin based polyrotaxanes. Polym. Rev. 47, 383–418 (2007).
Arisaka, Y. & Yui, N. Polyrotaxane-based biointerfaces with dynamic biomaterial functions. J. Mater. Chem. B 7, 2123–2129 (2019).
Yui, N. & Ooya, T. Molecular mobility of interlocked structures exploiting new functions of advanced biomaterials. Chem. Eur. J. 12, 6730–6737 (2006).
Patel, P., Pol, A., Jain, R. & Dandekar, P. in Encyclopedia of Biomedical Polymers and Polymeric Biomaterials (ed. Mishra, M.) (CRC Press, 2015).
Ooya, T. et al. Effects of polyrotaxane structure on polyion complexation with DNA. Sci. Technol. Adv. Mater. 5, 363–369 (2004).
Ooya, T. et al. Biocleavable polyrotaxane-plasmid DNA polyplex for enhanced gene delivery. J. Am. Chem. Soc. 128, 3852–3853 (2006).
Mammen, M., Choi, S.-K. & Whitesides, G. M. Polyvalent interactions in biological systems: implications for design and use of mMultivalent ligands and inhibitors. Angew. Chem. Int. Ed. Engl. 37, 2754–2794 (1998).
Seo, J.-H. et al. Inducing rapid cellular response on RGD-binding threaded macromolecular surfaces. J. Am. Chem. Soc. 135, 5513–5516 (2013).
Ooya, T., Eguchi, M. & Yui, N. Supramolecular design for multivalent interaction: maltose mobility along polyrotaxane enhanced binding with concanavalin A. J. Am. Chem. Soc. 125, 13016–13017 (2003).
Sluysmans, D. & Stoddart, J. F. The burgeoning of mechanically interlocked molecules in chemistry. Trends Chem. 1, 185–197 (2019).
Berná, J. et al. Macroscopic transport by synthetic molecular machines. Nat. Mater. 4, 704–710 (2005).
Sun, X. et al. Towards the self-assembly of polyrotaxanes. Macromol. Symp. 77, 191–207 (1994).
Zhang, W. et al. Folding of a donor–acceptor polyrotaxane by using noncovalent bonding interactions. Proc. Natl Acad. Sci. USA 105, 6514–6519 (2008).
Zhu, Z. et al. Synthesis and solution-state dynamics of donor–acceptor oligorotaxane foldamers. Chem. Sci. 4, 1470–1483 (2013).
Basu, S. et al. Donor–acceptor oligorotaxanes made to order. Chem. Eur. J. 17, 2107–2119 (2011).
Zhu, Z. et al. Oligomeric pseudorotaxanes adopting infinite-chain lattice superstructures. Angew. Chem. Int. Ed. Engl. 51, 7231–7235 (2012).
Sluysmans, D. et al. Synthetic oligorotaxanes exert high forces when folding under mechanical load. Nat. Nanotechnol. 13, 209–213 (2018).
Sluysmans, D., Devaux, F., Bruns, C. J., Stoddart, J. F. & Duwez, A.-S. Dynamic force spectroscopy of synthetic oligorotaxane foldamers. Proc. Natl Acad. Sci. USA 115, 9362–9366 (2018).
Pezzato, C. et al. An efficient artificial molecular pump. Tetrahedron 73, 4849–4857 (2017).
Qiu, Y. et al. A molecular dual pump. J. Am. Chem. Soc. 141, 17472–17476 (2019).
Qiu, Y. et al. A precise polyrotaxane synthesizer. Science 368, 1247–1253 (2020).
Whittaker, A. K. in Modern Magnetic Resonance (ed. Webb, G. A.) 583–589 (Springer, 2006).
Okumura, Y. & Ito, K. The polyrotaxane gel: a topological gel by figure-of-eight cross-links. Adv. Mater. 13, 485–487 (2001).
Ito, K. Slide-ring materials using topological supramolecular architecture. Curr. Opin. Solid State Mater. Sci. 14, 28–34 (2010).
Karino, T., Okumura, Y., Ito, K. & Shibayama, M. SANS studies on spatial inhomogeneities of slide-ring gels. Macromolecules 37, 6177–6182 (2004).
Liu, C., Kadono, H., Yokoyama, H., Mayumi, K. & Ito, K. Crack propagation resistance of slide-ring gels. Polymer 181, 121782 (2019).
Kato, K., Ikeda, Y. & Ito, K. Direct determination of cross-link density and its correlation with the elastic modulus of a gel with slidable cross-links. ACS Macro Lett. 8, 700–704 (2019).
Jiang, L. et al. Highly stretchable and instantly recoverable slide-ring gels consisting of enzymatically synthesized polyrotaxane with low host coverage. Chem. Mater. 30, 5013–5019 (2018).
Wang, Z. et al. Highly stretchable and compressible shape memory hydrogels based on polyurethane network and supramolecular interaction. Mater. Today Commun. 17, 246–251 (2018).
Kato, K., Karube, K., Nakamura, N. & Ito, K. The effect of ring size on the mechanical relaxation dynamics of polyrotaxane gels. Polym. Chem. 6, 2241–2248 (2015).
Liu, C. et al. Direct observation of large deformation and fracture behavior at the crack tip of slide-ring gel. J. Electrochem. Soc. 166, B3143–B3147 (2019).
Higgs, P. G. & Ball, R. C. Trapped entanglements in rubbers. A unification of models. Europhys. Lett. 8, 357–361 (1989).
Kość, M. “Belt-loop” model of chain entanglement. Colloid Polym. Sci. 266, 105–113 (1988).
Adolf, D. Origins of entanglement effects in rubber elasticity. Macromolecules 21, 228–230 (1988).
Kholodenko, A. L. & Vilgis, T. A. Some geometrical and topological problems in polymer physics. Phys. Rep. 298, 251–370 (1998).
Edwards, S. F. & Vilgis, T. The effect of entanglements in rubber elasticity. Polymer 27, 483–492 (1986).
Graessley, W. W. & Pearson, D. S. Stress–strain behavior in polymer networks containing nonlocalized junctions. J. Chem. Phys. 66, 3363–3370 (1977).
Ziabicki, A. Contribution of entrapped entanglements to equilibrium elasticity of rubber networks. Colloid Polym. Sci. 254, 1–5 (1976).
Marrucci, G. A mechanical model for rubbers containing entanglements. Rheol. Acta 18, 193–198 (1979).
Ito, K. Novel cross-linking concept of polymer network: synthesis, structure, and properties of slide-ring gels with freely movable junctions. Polym. J. 39, 489–499 (2007).
Yasuda, Y. et al. Molecular dynamics simulation and theoretical model of elasticity in slide-ring gels. ACS Macro Lett. 9, 1280–1285 (2020).
Kato, K., Yasuda, T. & Ito, K. Viscoelastic properties of slide-ring gels reflecting sliding dynamics of partial chains and entropy of ring components. Macromolecules 46, 310–316 (2013).
Fleury, G., Schlatter, G., Brochon, C. & Hadziioannou, G. From high molecular weight precursor polyrotaxanes to supramolecular sliding networks. The ‘sliding gels’. Polymer 46, 8494–8501 (2005).
Rubinstein, M. & Colby, R. H. Polymer Physics (Oxford Univ. Press, 2003).
Koga, T. & Tanaka, F. Elastic properties of polymer networks with sliding junctions. Eur. Phys. J. E 17, 225–229 (2005).
Zhang, Z. et al. Designing the slide-ring polymer network with both good mechanical and damping properties via molecular dynamics simulation. Polymers 10, 964 (2018).
Gavrilov, A. A. & Potemkin, I. I. Adaptive structure of gels and microgels with sliding cross-links: enhanced softness, stretchability and permeability. Soft Matter 14, 5098–5105 (2018).
Karino, T. et al. SANS studies on deformation mechanism of slide-ring gel. Macromolecules 38, 6161–6167 (2005).
Karino, T., Shibayama, M., Okumura, Y. & Ito, K. SANS study on pulley effect of slide-ring gel. Phys. B Condens. Matter 385–386, 807–809 (2006).
Shinohara, Y. et al. Small-angle X-ray scattering study of the pulley effect of slide-ring gels. Macromolecules 39, 7386–7391 (2006).
Kato, K., Yasuda, T. & Ito, K. Peculiar elasticity and strain hardening attributable to counteracting entropy of chain and ring in slide-ring gels. Polymer 55, 2614–2619 (2014).
Mayumi, K., Tezuka, M., Bando, A. & Ito, K. Mechanics of slide-ring gels: novel entropic elasticity of a topological network formed by ring and string. Soft Matter 8, 8179–8183 (2012).
de Gennes, P.-G. Sliding gels. Phys. A 271, 231–237 (1999).
Tonks, L. The complete equation of state of one, two and three-dimensional gases of hard elastic spheres. Phys. Rev. 50, 955–963 (1936).
Sevick, E. M. & Williams, D. R. M. Piston-rotaxanes as molecular shock absorbers. Langmuir 26, 5864–5868 (2010).
Gao, Y., Williams, D. R. M. & Sevick, E. M. Dynamics of molecular shock-absorbers: Energy dissipation and the fluctuation theorem. Soft Matter 7, 5739–5744 (2011).
Pinson, M. B., Sevick, E. M. & Williams, D. R. M. Mobile rings on a polyrotaxane lead to a yield force. Macromolecules 46, 4191–4197 (2013).
Boesten, R. J. J., Sevick, E. M. & Williams, D. R. M. Piston rotaxane monolayers: shear swelling and nanovalve behavior. Macromolecules 43, 7244–7249 (2010).
Sevick, E., Williams, D., Sevick, E. M. & Williams, D. R. M. A piston-rotaxane with two potential stripes: force transitions and yield stresses. Molecules 18, 13398–13409 (2013).
Metzler, R., Kantor, Y. & Kardar, M. Force-extension relations for polymers with sliding links. Phys. Rev. E 66, 022102 (2002).
Ito, K. Novel entropic elasticity of polymeric materials: why is slide-ring gel so soft? Polym. J. 44, 38–41 (2012).
Müller, T., Sommer, J.-U. & Lang, M. Tendomers – force sensitive bis-rotaxanes with jump-like deformation behavior. Soft Matter 15, 3671–3679 (2019).
Kato, K., Okabe, Y., Okazumi, Y. & Ito, K. A significant impact of host–guest stoichiometry on the extensibility of polyrotaxane gels. Chem. Commun. 51, 16180–16183 (2015).
Murakami, T., Schmidt, B. V. K. J., Brown, H. R. & Hawker, C. J. Structural versatility in slide-ring gels: influence of co-threaded cyclodextrin spacers. J. Polym. Sci. A Polym. Chem. 55, 1156–1165 (2017).
Liu, C., Kadono, H., Yokoyama, H., Mayumi, K. & Ito, K. Crack propagation resistance of slide-ring gels. Polymer 181, 121782 (2019).
Araki, J., Kataoka, T. & Ito, K. Preparation of a “sliding graft copolymer”, an organic solvent-soluble polyrotaxane containing mobile side chains, and its application for a crosslinked elastomeric supramolecular film. Soft Matter 4, 245–249 (2008).
Kato, K., Hori, A. & Ito, K. An efficient synthesis of low-covered polyrotaxanes grafted with poly(ε-caprolactone) and the mechanical properties of its cross-linked elastomers. Polymer 147, 67–73 (2018).
Li, X. et al. Highly toughened polylactide with novel sliding graft copolymer by in situ reactive compatibilization, crosslinking and chain extension. Polymer 55, 4313–4323 (2014).
Fleury, G., Schlatter, G., Brochon, C. & Hadziioannou, G. Unveiling the sliding motion in topological networks: influence of the swelling solvent on the relaxation dynamics. Adv. Mater. 18, 2847–2851 (2006).
Fleury, G. et al. Topological polymer networks with sliding cross-link points: The “sliding gels”. Relationship between their molecular structure and the viscoelastic as well as the swelling properties. Macromolecules 40, 535–543 (2007).
Sawada, J., Aoki, D., Otsuka, H. & Takata, T. A guiding principle for strengthening crosslinked polymers: synthesis and application of mobility-controlling rotaxane crosslinkers. Angew. Chem. Int. Ed. Engl. 58, 2765–2768 (2019).
Koyama, Y. Synthesis of topologically crosslinked polymers with rotaxane-crosslinking points. Polym. J. 46, 315–322 (2014).
Sawada, J., Aoki, D., Uchida, S., Otsuka, H. & Takata, T. Synthesis of vinylic macromolecular rotaxane cross-linkers endowing network polymers with toughness. ACS Macro Lett. 4, 598–601 (2015).
Iijima, K., Aoki, D., Otsuka, H. & Takata, T. Synthesis of rotaxane cross-linked polymers with supramolecular cross-linkers based on γ-CD and PTHF macromonomers: The effect of the macromonomer structure on the polymer properties. Polymer 128, 392–396 (2017).
Tan, S., Blencowe, A., Ladewig, K. & Qiao, G. G. A novel one-pot approach towards dynamically cross-linked hydrogels. Soft Matter 9, 5239–5250 (2013).
Sawada, J., Aoki, D., Sun, Y., Nakajima, K. & Takata, T. Effect of coexisting covalent cross-links on the properties of rotaxane-cross-linked polymers. ACS Appl. Polym. Mater. 2, 1061–1064 (2020).
Noda, Y., Hayashi, Y. & Ito, K. From topological gels to slide-ring materials. J. Appl. Polym. Sci. 131, 40509 (2014).
Minato, K. et al. Mechanical properties of supramolecular elastomers prepared from polymer-grafted polyrotaxane. Polymer 128, 386–391 (2017).
Koyanagi, K., Takashima, Y., Yamaguchi, H. & Harada, A. Movable cross-linked polymeric materials from bulk polymerization of reactive polyrotaxane cross-linker with acrylate monomers. Macromolecules 50, 5695–5700 (2017).
Shi, C.-Y. et al. An ultrastrong and highly stretchable polyurethane elastomer enabled by a zipper-like ring-sliding effect. Adv. Mater. 32, 2000345 (2020).
Oku, T., Furusho, Y. & Takata, T. A concept for recyclable cross-linked polymers: Topologically networked polyrotaxane capable of undergoing reversible assembly and disassembly. Angew. Chem. Int. Ed. Engl. 43, 966–969 (2004).
Bilig, T. et al. Polyrotaxane networks formed via rotaxanation utilizing dynamic covalent chemistry of disulfide. Macromolecules 41, 8496–8503 (2008).
Kohsaka, Y., Nakazono, K., Koyama, Y., Asai, S. & Takata, T. Size-complementary rotaxane cross-linking for the stabilization and degradation of a supramolecular network. Angew. Chem. Int. Ed. Engl. 50, 4872–4875 (2011).
Sugihara, N. et al. Ion-conductive and elastic slide-ring gel Li electrolytes swollen with ionic liquid. Electrochim. Acta 229, 166–172 (2017).
Zhuo, Y. et al. An ultra-durable icephobic coating by a molecular pulley. Soft Matter 15, 3607–3611 (2019).
Choi, S., Kwon, T., Coskun, A. & Choi, J. W. Highly elastic binders integrating polyrotaxanes for silicon microparticle anodes in lithium ion batteries. Science 357, 279–283 (2017).
Yoo, D.-J. et al. Highly elastic polyrotaxane binders for mechanically stable lithium hosts in lithium-metal batteries. Adv. Mater. 31, 1901645 (2019).
Rowan, S. J., Cantrill, S. J., Cousins, G. R. L., Sanders, J. K. M. & Stoddart, J. F. Dynamic covalent chemistry. Angew. Chem. Int. Ed. Engl. 41, 898–952 (2002).
Nakahata, M., Mori, S., Takashima, Y., Yamaguchi, H. & Harada, A. Self-healing materials formed by cross-linked polyrotaxanes with reversible bonds. Chem 1, 766–775 (2016).
Zheng, S. Y. et al. Slide-ring cross-links mediated tough metallosupramolecular hydrogels with superior self-recoverability. Macromolecules 52, 6748–6755 (2019).
Sood, N., Bhardwaj, A., Mehta, S. & Mehta, A. Stimuli-responsive hydrogels in drug delivery and tissue engineering. Drug Deliv. 23, 748–770 (2016).
Imran, A. Bin, Seki, T. & Takeoka, Y. Recent advances in hydrogels in terms of fast stimuli responsiveness and superior mechanical performance. Polym. J. 42, 839–851 (2010).
Koetting, M. C., Peters, J. T., Steichen, S. D. & Peppas, N. A. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Mater. Sci. Eng. R Rep. 93, 1–49 (2015).
Sakai, T. et al. Photoresponsive slide-ring gel. Adv. Mater. 19, 2023–2025 (2007).
Haq, M. A., Su, Y. & Wang, D. Mechanical properties of PNIPAM based hydrogels: A review. Mater. Sci. Eng. C 70, 842–855 (2017).
Imran, A. Bin, Seki, T., Ito, K. & Takeoka, Y. Poly(N-isopropylacrylamide) gel prepared using a hydrophilic polyrotaxane-based movable cross-linker. Macromolecules 43, 1975–1980 (2010).
Bin Imran, A. et al. Extremely stretchable thermosensitive hydrogels by introducing slide-ring polyrotaxane cross-linkers and ionic groups into the polymer network. Nat. Commun. 5, 5124 (2014).
Ohmori, K. et al. Molecular weight dependency of polyrotaxane-cross-linked polymer gel extensibility. Chem. Commun. 52, 13757–13759 (2016).
Kobayashi, Y., Zheng, Y., Takashima, Y., Yamaguchi, H. & Harada, A. Physical and adhesion properties of supramolecular hydrogels cross-linked by movable cross-linking molecule and host-guest interactions. Chem. Lett. 47, 1387–1390 (2018).
Ikura, R. et al. Preparation of hydrophilic polymeric materials with movable cross-linkers and their mechanical property. Polymer 196, 122465 (2020).
Yasumoto, A. et al. Highly responsive hydrogel prepared using poly(N-isopropylacrylamide)-grafted polyrotaxane as a building block designed by reversible deactivation radical polymerization and click chemistry. Macromolecules 50, 364–374 (2017).
Seo, J., Yui, N. & Seo, J.-H. Development of a supramolecular accelerator simultaneously to increase the cross-linking density and ductility of an epoxy resin. Chem. Eng. J. 356, 303–311 (2019).
Wang, X.-S. et al. Relaxation and reinforcing effects of polyrotaxane in an epoxy resin matrix. Macromolecules 39, 1046–1052 (2006).
Levita, G., Petris, De, Marchetti, S., Lazzeri, A. & Crosslink, A. Density and fracture toughness of epoxy resins. J. Mater. Sci. 26, 2348–2352 (1991).
Ohtsuka, K. & Zhao, C. Properties of bismaleimide resin modified with polyrotaxane as a stress relaxation material. Polym. Int. 67, 1112–1117 (2018).
Li, X. et al. Miscibility, intramolecular specific interactions and mechanical properties of a DGEBA based epoxy resin toughened with a sliding graft copolymer. Chinese J. Polym. Sci. 33, 433–443 (2015).
Seo, J., Moon, S. W., Kang, H., Choi, B.-H. & Seo, J.-H. Foldable and extremely scratch-resistant hard coating materials from molecular necklace-like cross-linkers. ACS Appl. Mater. Interfaces 11, 27306–27317 (2019).
Pruksawan, S., Samitsu, S., Yokoyama, H. & Naito, M. Homogeneously dispersed polyrotaxane in epoxy adhesive and its improvement in the fracture toughness. Macromolecules 52, 2464–2475 (2019).
Wang, P., Gao, Z., Yuan, M., Zhu, J. & Wang, F. Mechanically linked poly[2]rotaxanes constructed from the benzo-21-crown-7/secondary ammonium salt recognition motif. Polym. Chem. 7, 3664–3668 (2016).
Zhang, M. et al. Preparation of a daisy chain via threading-followed-by-polymerization. Macromolecules 44, 9629–9634 (2011).
Sasabe, H. et al. Synthesis of poly[2]rotaxane by Sonogashira polycondensation. J. Polym. Sci. A Polym. Chem. 45, 4154–4160 (2007).
Rotzler, J. & Mayor, M. Molecular daisy chains. Chem. Soc. Rev. 42, 44–62 (2013).
Bruns, C. J. & Stoddart, J. F. Rotaxane-based molecular muscles. Acc. Chem. Res. 47, 2186–2199 (2014).
Clark, P. G., Day, M. W. & Grubbs, R. H. Switching and extension of a [c2]daisy-chain dimer polymer. J. Am. Chem. Soc. 131, 13631–13633 (2009).
Fang, L. et al. Acid-base actuation of [c2]daisy chains. J. Am. Chem. Soc. 131, 7126–7134 (2009).
Hmadeh, M. et al. On the thermodynamic and kinetic investigations of a [c2]daisy chain polymer. J. Mater. Chem. 20, 3422–3430 (2010).
Guidry, E. N., Li, J., Stoddart, J. F. & Grubbs, R. H. Bifunctional [c2]daisy-chains and their incorporation into mechanically interlocked polymers. J. Am. Chem. Soc. 129, 8944–8945 (2007).
Mariani, G. et al. Integration of molecular machines into supramolecular materials: Actuation between equilibrium polymers and crystal-like gels. Nanoscale 9, 18456–18466 (2017).
Goujon, A. et al. Controlled sol–gel transitions by actuating molecular machine based supramolecular polymers. J. Am. Chem. Soc. 139, 4923–4928 (2017).
Xia, D. & Xue, M. A supramolecular polymer gel with dual-responsiveness constructed by crown ether based molecular recognition. Polym. Chem. 5, 5591–5597 (2014).
Bruns, C. J. & Stoddart, J. F. Supramolecular polymers: Molecular machines muscle up. Nat. Nanotechnol. 8, 9–10 (2013).
Zhao, Y. L., Zhang, R. Q., Minot, C., Hermann, K. & Van Hove, M. A. Revealing highly unbalanced energy barriers in the extension and contraction of the muscle-like motion of a [c2]daisy chain. Phys. Chem. Chem. Phys. 17, 18318–18326 (2015).
Du, G., Moulin, E., Jouault, N., Buhler, E. & Giuseppone, N. Muscle-like supramolecular polymers: Integrated motion from thousands of molecular machines. Angew. Chem. Int. Ed. Engl. 51, 12504–12508 (2012).
Goujon, A. et al. Bistable [c2] daisy chain rotaxanes as reversible muscle-like actuators in mechanically active gels. J. Am. Chem. Soc. 139, 14825–14828 (2017).
Iwaso, K., Takashima, Y. & Harada, A. Fast response dry-type artificial molecular muscles with [c2]daisy chains. Nat. Chem. 8, 625–632 (2016).
Ikejiri, S., Takashima, Y., Osaki, M., Yamaguchi, H. & Harada, A. Solvent-free photoresponsive artificial muscles rapidly driven by molecular machines. J. Am. Chem. Soc. 140, 17308–17315 (2018).
Geerts, Y., Muscat, D. & Müllen, K. Synthesis of oligo[2]catenanes. Macromol. Chem. Phys. 196, 3425–3435 (1995).
Muscat, D., Witte, A., Köhler, W., Müllen, K. & Geerts, Y. Synthesis of a novel poly[2]-catenane containing rigid catenanes. Macromol. Rapid Commun. 18, 233–241 (1997).
Muscat, D. et al. Synthesis and characterization of poly[2]-catenanes containing rigid catenane segments. Macromolecules 32, 1737–1745 (1999).
Fustin, C.-A. et al. Mechanically linked polycarbonate. J. Am. Chem. Soc. 125, 2200–2207 (2003).
Fustin, C.-A., Bailly, C., Clarkson, G. J., Galow, T. H. & Leigh, D. A. Solution and solid-state properties of mechanically linked polycarbonates. Macromolecules 37, 66–70 (2004).
Fustin, C.-A. et al. Mechanically linked poly(ethylene terephthalate). Macromolecules 37, 7884–7892 (2004).
Van Quaethem, A., Lussis, P., Leigh, D. A., Duwez, A.-S. & Fustin, C.-A. Probing the mobility of catenane rings in single molecules. Chem. Sci. 5, 1449–1452 (2014).
Xing, H., Li, Z., Wu, Z. L. & Huang, F. Catenane crosslinked mechanically adaptive polymer gel. Macromol. Rapid Commun. 39, 1700361 (2018).
Ahamed, B. N., Van Velthem, P., Robeyns, K. & Fustin, C.-A. Influence of a single catenane on the solid-state properties of mechanically linked polymers. ACS Macro Lett. 6, 468–472 (2017).
Xing, H. et al. Mechanochemistry of an interlocked poly[2]catenane: from single molecule to bulk gel. CCS Chem. 1, 513–523 (2019).
Wang, W. & Xing, H. A novel supramolecular polymer network based on a catenane-type crosslinker. Polym. Chem. 9, 2087–2091 (2018).
Gan, Y., Dong, D. & Hogen-Esch, T. E. Synthesis and characterization of a catenated polystyrene−poly(2-vinylpyridine) block copolymer. Macromolecules 35, 6799–6803 (2002).
Bunha, A. K., Mangadlao, J., Felipe, M. J., Pangilinan, K. & Advincula, R. Catenated PS-PMMA block copolymers via supramolecularly templated ATRP initiator approach. Macromol. Rapid Commun. 33, 1214–1219 (2012).
Bunha, A. et al. Polymeric catenanes synthesized via ‘click’ chemistry and atom transfer radical coupling. Chem. Commun. 51, 7528–7531 (2015).
Cao, P.-F., Mangadlao, J. D., de Leon, A., Su, Z. & Advincula, R. C. Catenated poly(ε-caprolactone) and poly(L-lactide) via ring-expansion strategy. Macromolecules 48, 3825–3833 (2015).
Ishikawa, K., Yamamoto, T., Asakawa, M. & Tezuka, Y. Effective synthesis of polymer catenanes by cooperative electrostatic/hydrogen-bonding self-assembly and covalent fixation. Macromolecules 43, 168–176 (2010).
Ohta, Y., Nakamura, M., Matsushita, Y. & Takano, A. Synthesis, separation and characterization of knotted ring polymers. Polymer 53, 466–470 (2012).
Weidmann, J.-L. et al. Poly[2]catenanes and cyclic oligo[2]catenanes containing alternating topological and covalent bonds: synthesis and characterization. Chem. Eur. J. 5, 1841–1851 (2002).
Wu, Q. et al. Poly[n]catenanes: Synthesis of molecular interlocked chains. Science 358, 1434–1439 (2017).
Pakula, T. & Jeszka, K. Simulation of single complex macromolecules. 1. Structure and dynamics of catenanes. Macromolecules 32, 6821–6830 (1999).
Rauscher, P. M., Rowan, S. J. & de Pablo, J. J. Topological effects in isolated poly[n]catenanes: molecular dynamics simulations and rouse mode analysis. ACS Macro Lett. 7, 938–943 (2018).
Amabilino, D. B., Ashton, P. R., Reder, A. S., Spencer, N. & Stoddart, J. F. Olympiadane. Angew. Chem. Int. Ed. Engl. 33, 1286–1290 (1994).
Iwamoto, H. et al. Synthesis of linear [5]catenanes via olefin metathesis dimerization of pseudorotaxanes composed of a [2]catenane and a secondary ammonium salt. Chem. Commun. 52, 319–322 (2016).
Amabilino, D. B. et al. The five-stage self-assembly of a branched heptacatenane. Angew. Chem. Int. Ed. Engl. 36, 2070–2072 (1997).
Watanabe, N., Ikari, Y., Kihara, N. & Takata, T. Bridged polycatenane. Macromolecules 37, 6663–6666 (2004).
Brereton, M. G. The statistical mechanics of a concatenated polymer chain. J. Phys. A Math. Gen. 34, 5131 (2001).
Ahmadian Dehaghani, Z., Chubak, I., Likos, C. N. & Ejtehadi, M. R. Effects of topological constraints on linked ring polymers in solvents of varying quality. Soft Matter 16, 3029–3038 (2020).
Rauscher, P. M., Schweizer, K. S., Rowan, S. J. & de Pablo, J. J. Thermodynamics and structure of poly[n]catenane melts. Macromolecules 53, 3390–3408 (2020).
Rauscher, P. M., Schweizer, K. S., Rowan, S. J. & de Pablo, J. J. Dynamics of poly[n]catenane melts. J. Chem. Phys. 152, 214901 (2020).
de Gennes, P.-G. Scaling Concepts in Polymer Physics (Cornell Univ. Press, 1979).
Raphaël, E., Gay, C. & de Gennes, P.-G. Progressive construction of an “Olympic” gel. J. Stat. Phys. 89, 111–118 (1997).
Pickett, G. T. DNA-origami technique for olympic gels. Europhys. Lett. 76, 616–622 (2006).
Fischer, J., Lang, M. & Sommer, J.-U. The formation and structure of Olympic gels. J. Chem. Phys. 143, 243114 (2015).
Vilgis, T. A. & Otto, M. Elasticity of entangled polymer loops: Olympic gels. Phys. Rev. E 56, R1314–R1317 (1997).
Lang, M., Fischer, J., Werner, M. & Sommer, J.-U. Swelling of Olympic gels. Phys. Rev. Lett. 112, 238001 (2014).
Lang, M., Fischer, J., Werner, M. & Sommer, J.-U. Olympic gels: concatenation and swelling. Macromol. Symp. 358, 140–147 (2015).
Endo, K., Shiroi, T., Murata, N., Kojima, G. & Yamanaka, T. Synthesis and characterization of poly(1,2-dithiane). Macromolecules 37, 3143–3150 (2004).
Kim, Y. S. et al. Gelation of the genome by topoisomerase II targeting anticancer agents. Soft Matter 9, 1656–1663 (2013).
Arakawa, R., Watanabe, T., Fukuo, T. & Endo, K. Determination of cyclic structure for polydithiane using electrospray ionization mass spectrometry. J. Polym. Sci. A Polym. Chem. 38, 4403–4406 (2000).
Endo, K. & Yamanaka, T. Copolymerization of lipoic acid with 1,2-dithiane and characterization of the copolymer as an interlocked cyclic polymer. Macromolecules 39, 4038–4043 (2006).
Borst, P. & Hoeijmakers, J. H. J. Kinetoplast DNA. Plasmid 2, 20–40 (1979).
Riou, G. & Delain, E. Electron microscopy of the circular kinetoplastic DNA from Trypanosoma cruzi: occurrence of catenated forms. Proc. Natl Acad. Sci. USA 62, 210–217 (1969).
Cavalcanti, D. P., Gonçalves, D. L., Costa, L. T. & de Souza, W. The structure of the kinetoplast DNA network of Crithidia fasciculata revealed by atomic force microscopy. Micron 42, 553–559 (2011).
Renger, H. C. & Wolstenholme, D. R. Form and structure of kinetoplast DNA of Crithidia. J. Cell Biol. 54, 346–364 (1972).
Krasnow, M. & Cozzarelli, N. Catenation of DNA rings by topoisomerases. Mechanism of control by spermidine. J. Biol. Chem. 257, 2687–2693 (1982).
Waldeck, W., Theobald, M. & Zentgraf, H. Catenation of DNA by eucaryotic topoisomerase II associated with simian virus 40 minichromosomes. EMBO J. 2, 1255–1261 (1983).
Krajina, B. A., Zhu, A., Heilshorn, S. C. & Spakowitz, A. J. Active DNA olympic hydrogels driven by topoisomerase activity. Phys. Rev. Lett. 121, 148001 (2018).
Imholt, L. et al. Grafted polyrotaxanes as highly conductive electrolytes for lithium metal batteries. J. Power Sources 409, 148–158 (2019).
Acknowledgements
This work was funded by National Science Foundation (NSF) grant number CHE-1903603. P.M.R. thanks the NSF for the award of a Graduate Research Fellowship, grant number 1746045.
Author information
Authors and Affiliations
Contributions
All authors contributed to the writing, reviewing and editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hart, L.F., Hertzog, J.E., Rauscher, P.M. et al. Material properties and applications of mechanically interlocked polymers. Nat Rev Mater 6, 508–530 (2021). https://doi.org/10.1038/s41578-021-00278-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41578-021-00278-z
This article is cited by
-
A magnetically powered nanomachine with a DNA clutch
Nature Nanotechnology (2024)
-
Topological Confinement in Reversibly Interlocked Polymer Networks
Chinese Journal of Polymer Science (2024)
-
An approach to MOFaxanes by threading ultralong polymers through metal–organic framework microcrystals
Nature Communications (2023)
-
Topological Catenation Enhances Elastic Modulus of Single Linear Polycatenane
Chinese Journal of Polymer Science (2023)
-
Mechanically interlocked networks cross-linked by a molecular necklace
Nature Communications (2022)