Abstract
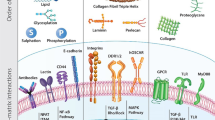
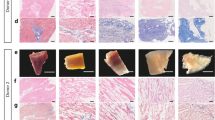
In tissue engineering and regenerative medicine, a biomaterial provides mechanical support and biochemical signals to encourage cell attachment and modulate cell behaviour. Nature’s template for a biomaterial is the extracellular matrix (ECM). The ECM contains intrinsic biochemical and mechanical cues that regulate cell phenotype and function in development, in homeostasis and in response to injury. The use of ECM-based materials in biomedical research has advanced from coating cell culture plates with purified ECM components to the design of ECM-mimicking biomaterials and the engineering of decellularized tissues aimed at recapitulating the dynamics, composition and structure of the ECM. In this Review, we highlight important matrix properties and functions in the context of tissue engineering and regenerative medicine, consider techniques such as proteomics for the investigation of matrix structure and composition and discuss different engineering strategies for the design of matrix-mimicking biomaterials. Tissue, whole organ and cell culture decellularization approaches are examined for their potential to preserve the tissue-specific biochemical composition and ultrastructure of the ECM and for the development of biomaterials that promote the formation of functional tissues in clinical applications. Finally, we investigate challenges and opportunities of ECM biomaterials for the design of organotypic models to study disease progression, for the ex vivo creation of engineered tissue and for the clinical translation of functional tissue reconstruction strategies in vivo.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Mecham, R. P. Overview of extracellular matrix. Curr. Protoc. Cell Biol. Chapter 57, 10.1.1–10.1.16 (2012).
Yannas, I. V., Burke, J. F., Orgill, D. P. & Skrabut, E. M. Wound tissue can utilize a polymeric template to synthesize a functional extension of skin. Science 215, 174–176 (1982).
Ozbek, S., Balasubramanian, P. G., Chiquet-Ehrismann, R., Tucker, R. P. & Adams, J. C. The evolution of extracellular matrix. Mol. Biol. Cell 21, 4300–4305 (2010).
Hynes, R. O. The evolution of metazoan extracellular matrix. J. Cell Biol. 196, 671–679 (2012).
Huxley-Jones, J., Robertson, D. L. & Boot-Handford, R. P. On the origins of the extracellular matrix in vertebrates. Matrix Biol. 26, 2–11 (2007).
Zagris, N. Extracellular matrix in development of the early embryo. Micron 32, 427–438 (2001).
Leivo, I., Vaheri, A., Timpl, R. & Wartiovaara, J. Appearance and distribution of collagens and laminin in the early mouse embryo. Dev. Biol. 76, 100–114 (1980).
Bonnans, C., Chou, J. & Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 15, 786–801 (2014).
Bissell, M. J., Hall, H. G. & Parry, G. How does the extracellular matrix direct gene expression? J. Theor. Biol. 99, 31–68 (1982).
Bornstein, P., McPherson, J. & Sage, H. in Pathobiology of the Endothelial Cell 1st edn (eds Nossel, H. L. & Vogel, H. J.) 215–228 (Academic Press, 1982).
Schultz, G. S., Davidson, J. M., Kirsner, R. S., Bornstein, P. & Herman, I. M. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen. 19, 134–148 (2011).
Yue, B. Biology of the extracellular matrix: an overview. J. Glaucoma 23, S20–23 (2014).
Neve, A., Cantatore, F. P., Maruotti, N., Corrado, A. & Ribatti, D. Extracellular matrix modulates angiogenesis in physiological and pathological conditions. Biomed. Res. Int. 2014, 756078 (2014).
Ahmed, M. & Ffrench-Constant, C. Extracellular matrix regulation of stem cell behavior. Curr. Stem Cell Rep. 2, 197–206 (2016).
Gattazzo, F., Urciuolo, A. & Bonaldo, P. Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim. Biophys. Acta 1840, 2506–2519 (2014).
Sottile, J. Regulation of angiogenesis by extracellular matrix. Biochim. Biophys. Acta 1654, 13–22 (2004).
Agrawal, V., Brown, B. N., Beattie, A. J., Gilbert, T. W. & Badylak, S. F. Evidence of innervation following extracellular matrix scaffold-mediated remodelling of muscular tissues. J. Tissue Eng. Regen. Med. 3, 590–600 (2009).
Schultz, G. S. & Wysocki, A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 17, 153–162 (2009).
Agren, M. S. & Werthen, M. The extracellular matrix in wound healing: a closer look at therapeutics for chronic wounds. Int. J. Low. Extrem. Wounds 6, 82–97 (2007).
Xue, M. & Jackson, C. J. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv. Wound Care 4, 119–136 (2015).
Wolfe, P. S., Sell, S. A. & Bowlin, G. L. in Tissue Engineering: From Lab to Clinic (eds Pallua, N. & Christoph Suscheck, C. V.) 41–67 (Springer, Berlin Heidelberg, 2011).
Cruz-Acuna, R. & Garcia, A. J. Synthetic hydrogels mimicking basement membrane matrices to promote cell-matrix interactions. Matrix Biol. 57–58, 324–333 (2017).
Zhu, J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials 31, 4639–4656 (2010).
Gao, C. et al. Current progress in bioactive ceramic scaffolds for bone repair and regeneration. Int. J. Mol. Sci. 15, 4714–4732 (2014).
Ruvinov, E. & Cohen, S. Alginate biomaterial for the treatment of myocardial infarction: progress, translational strategies, and clinical outlook:from ocean algae to patient bedside. Adv. Drug Deliv. Rev. 96, 54–76 (2016).
Baranwal, A. et al. Chitosan: An undisputed bio-fabrication material for tissue engineering and bio-sensing applications. Int. J. Biol Macromol. 110, 110–123 (2018).
Courtenay, J. C. et al. Surface modified cellulose scaffolds for tissue engineering. Cellulose 24, 253–267 (2017).
Wang, Y., Kim, H. J., Vunjak-Novakovic, G. & Kaplan, D. L. Stem cell-based tissue engineering with silk biomaterials. Biomaterials 27, 6064–6082 (2006).
Gomes, M. et al. in Handbook of Biopolymers and Biodegradable Plastics (ed. Ebnesajjad, S.) 385–425 (William Andrew Publishing, 2013).
Young, J. L., Holle, A. W. & Spatz, J. P. Nanoscale and mechanical properties of the physiological cell-ECM microenvironment. Exp. Cell Res. 343, 3–6 (2016).
Miller, R. T. Mechanical properties of basement membrane in health and disease. Matrix Biol. 57–58, 366–373 (2017).
Muiznieks, L. D. & Keeley, F. W. Molecular assembly and mechanical properties of the extracellular matrix: a fibrous protein perspective. Biochim. Biophys. Acta 1832, 866–875 (2013).
Stylianopoulos, T. et al. Diffusion of particles in the extracellular matrix: the effect of repulsive electrostatic interactions. Biophys. J. 99, 1342–1349 (2010).
Taipale, J. & Keski-Oja, J. Growth factors in the extracellular matrix. FASEB J. 11, 51–59 (1997).
Hynes, R. O. The extracellular matrix: not just pretty fibrils. Science 326, 1216–1219 (2009).
Kim, S. H., Turnbull, J. & Guimond, S. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J. Endocrinol. 209, 139–151 (2011).
Escobedo-Lucea, C. et al. Development of a human extracellular matrix for applications related with stem cells and tissue engineering. Stem Cell Rev. 8, 170–183 (2012).
Heino, J. & Kapyla, J. Cellular receptors of extracellular matrix molecules. Curr. Pharm. Des. 15, 1309–1317 (2009).
Campbell, I. D. & Humphries, M. J. Integrin structure, activation, and interactions. Cold Spring Harb. Perspect. Biol. 3, a004994 (2011).
Horton, E. R., Astudillo, P., Humphries, M. J. & Humphries, J. D. Mechanosensitivity of integrin adhesion complexes: role of the consensus adhesome. Exp. Cell Res. 343, 7–13 (2016).
Rozario, T. & DeSimone, D. W. The extracellular matrix in development and morphogenesis: a dynamic view. Dev. Biol. 341, 126–140 (2010).
Nelson, C. M. & Bissell, M. J. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu. Rev. Cell Dev. Biol. 22, 287–309 (2006).
Humphrey, J. D., Dufresne, E. R. & Schwartz, M. A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 15, 802–812 (2014).
Tottey, S. et al. The effect of source animal age upon extracellular matrix scaffold properties. Biomaterials 32, 128–136 (2011).
Kular, J. K., Basu, S. & Sharma, R. I. The extracellular matrix: structure, composition, age-related differences, tools for analysis and applications for tissue engineering. J. Tissue Eng. 5, 2041731414557112 (2014).
Chester, D. & Brown, A. C. The role of biophysical properties of provisional matrix proteins in wound repair. Matrix Biol. 60–61, 124–140 (2017).
Lampi, M. C. & Reinhart-King, C. A. Targeting extracellular matrix stiffness to attenuate disease: from molecular mechanisms to clinical trials. Sci. Transl Med. 10, eaao0475 (2018).
Diegelmann, R. F. & Evans, M. C. Wound healing: an overview of acute, fibrotic and delayed healing. Front. Biosci. 9, 283–289 (2004).
Kim, H. E. et al. Disruption of the myocardial extracellular matrix leads to cardiac dysfunction. J. Clin. Invest. 106, 857–866 (2000).
Bondeson, J., Wainwright, S., Hughes, C. & Caterson, B. The regulation of the ADAMTS4 and ADAMTS5 aggrecanases in osteoarthritis: a review. Clin. Exp. Rheumatol 26, 139–145 (2008).
Houghton, A. M. Matrix metalloproteinases in destructive lung disease. Matrix Biol. 44–46, 167–174 (2015).
Badylak, S. F. Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transpl. Immunol. 12, 367–377 (2004).
van der Rest, M. & Garrone, R. Collagen family of proteins. FASEB J. 5, 2814–2823 (1991).
Mouw, J. K., Ou, G. & Weaver, V. M. Extracellular matrix assembly: a multiscale deconstruction. Nat. Rev. Mol. Cell Biol. 15, 771–785 (2014).
Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 3, a004978 (2011).
Kowitsch, A., Zhou, G. & Groth, T. Medical application of glycosaminoglycans: a review. J. Tissue Eng. Regen. Med. 12, e23–e41 (2018).
Aumailley, M. The laminin family. Cell Adh. Migr. 7, 48–55 (2013).
Zollinger, A. J. & Smith, M. L. Fibronectin, the extracellular glue. Matrix Biol. 60–61, 27–37 (2017).
Midwood, K. S., Chiquet, M., Tucker, R. P. & Orend, G. Tenascin-C at a glance. J. Cell Sci. 129, 4321–4327 (2016).
Kanie, K. et al. Focused screening of ECM-selective adhesion peptides on cellulose-bound peptide microarrays. Bioengineering 3, 31 (2016).
Bellis, S. L. Advantages of RGD peptides for directing cell association with biomaterials. Biomaterials 32, 4205–4210 (2011).
Hubbell, J. A., Massia, S. P., Desai, N. P. & Drumheller, P. D. Endothelial cell-selective materials for tissue engineering in the vascular graft via a new receptor. Biotechnology 9, 568–572 (1991).
Gobin, A. S. & West, J. L. Val-ala-pro-gly, an elastin-derived non-integrin ligand: smooth muscle cell adhesion and specificity. J. Biomed. Mater. Res. A 67, 255–259 (2003).
Robins, S. P. Biochemistry and functional significance of collagen cross-linking. Biochem. Soc. Trans. 35, 849–852 (2007).
Hynes, R. O. & Naba, A. Overview of the matrisome — an inventory of extracellular matrix constituents and functions. Cold Spring Harb. Perspect. Biol. 4, a004903 (2012).
Naba, A. et al. The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol. Cell. Proteom. https://doi.org/10.1074/mcp.M111.014647 (2012).
Li, F. et al. Low-molecular-weight peptides derived from extracellular matrix as chemoattractants for primary endothelial cells. Endothelium 11, 199–206 (2004).
Davis, G. E., Bayless, K. J., Davis, M. J. & Meininger, G. A. Regulation of tissue injury responses by the exposure of matricryptic sites within extracellular matrix molecules. Am. J. Pathol. 156, 1489–1498 (2000).
Banerjee, P. & Shanthi, C. Cryptic peptides from collagen: a critical review. Protein Pept. Lett. 23, 664–672 (2016).
Sicari, B. M., Zhang, L., Londono, R. & Badylak, S. F. An assay to quantify chemotactic properties of degradation products from extracellular matrix. Methods Mol. Biol. 1202, 103–110 (2014).
Agrawal, V. et al. Recruitment of progenitor cells by an extracellular matrix cryptic peptide in a mouse model of digit amputation. Tissue Eng. Part A 17, 2435–2443 (2011).
Agrawal, V. et al. An isolated cryptic peptide influences osteogenesis and bone remodeling in an adult mammalian model of digit amputation. Tissue Eng. Part A 17, 3033–3044 (2011).
Ames, J. J. et al. Identification of an endogenously generated cryptic collagen epitope (XL313) that may selectively regulate angiogenesis by an integrin Yes-associated protein (YAP) mechano-transduction pathway. J. Biol. Chem. 291, 2731–2750 (2016).
Adair-Kirk, T. L. & Senior, R. M. Fragments of extracellular matrix as mediators of inflammation. Int. J. Biochem. Cell Biol. 40, 1101–1110 (2008).
Huleihel, L. et al. Matrix-bound nanovesicles within ECM bioscaffolds. Sci. Adv. 2, e1600502 (2016).
Faust, A. et al. Urinary bladder extracellular matrix hydrogels and matrix-bound vesicles differentially regulate central nervous system neuron viability and axon growth and branching. J. Biomater. Appl. 31, 1277–1295 (2017).
Huleihel, L. et al. Matrix-Bound Nanovesicles Recapitulate Extracellular Matrix Effects on Macrophage Phenotype. Tissue Eng. Part A 23, 1283–1294 (2017).
Calle, E. A. et al. Targeted proteomics effectively quantifies differences between native lung and detergent-decellularized lung extracellular matrices. Acta Biomater. 46, 91–100 (2016).
Hill, R. C., Calle, E. A., Dzieciatkowska, M., Niklason, L. E. & Hansen, K. C. Quantification of extracellular matrix proteins from a rat lung scaffold to provide a molecular readout for tissue engineering. Mol. Cell. Proteom. 14, 961–973 (2015).
Goddard, E. T. et al. Quantitative extracellular matrix proteomics to study mammary and liver tissue microenvironments. Int. J. Biochem. Cell Biol. 81, 223–232 (2016).
Naba, A. et al. The extracellular matrix: tools and insights for the “omics” era. Matrix Biol. 49, 10–24 (2016).
Glavey, S. V. et al. Proteomic characterization of human multiple myeloma bone marrow extracellular matrix. Leukemia 31, 2426–2434 (2017).
Naba, A. et al. Characterization of the extracellular matrix of normal and diseased tissues using proteomics. J. Proteome Res. 16, 3083–3091 (2017).
FitzGerald, J. F. & Kumar, A. S. Biologic versus synthetic mesh reinforcement: what are the pros and cons? Clin. Colon Rectal Surg. 27, 140–148 (2014).
Kao, W. J., Zhao, Q. H., Hiltner, A. & Anderson, J. M. Theoretical analysis of in vivo macrophage adhesion and foreign body giant cell formation on polydimethylsiloxane, low density polyethylene, and polyetherurethanes. J. Biomed. Mater. Res. 28, 73–79 (1994).
Klinge, U., Klosterhalfen, B., Muller, M. & Schumpelick, V. Foreign body reaction to meshes used for the repair of abdominal wall hernias. Eur. J. Surg. 165, 665–673 (1999).
Luttikhuizen, D. T., Harmsen, M. C. & Van Luyn, M. J. Cellular and molecular dynamics in the foreign body reaction. Tissue Eng. 12, 1955–1970 (2006).
Brown, B. N. et al. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater. 8, 978–987 (2012).
Allman, A. J. et al. Xenogeneic extracellular matrix grafts elicit a TH2-restricted immune response. Transplantation 71, 1631–1640 (2001).
Zhang, S. Fabrication of novel biomaterials through molecular self-assembly. Nat. Biotechnol. 21, 1171–1178 (2003).
Silva, G. A. et al. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science 303, 1352–1355 (2004).
Halstenberg, S., Panitch, A., Rizzi, S., Hall, H. & Hubbell, J. A. Biologically engineered protein-graft-poly(ethylene glycol) hydrogels: a cell adhesive and plasmin-degradable biosynthetic material for tissue repair. Biomacromolecules 3, 710–723 (2002).
Lutolf, M. P. et al. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics. Proc. Natl Acad. Sci. USA 100, 5413–5418 (2003).
Mahoney, M. J. & Saltzman, W. M. Transplantation of brain cells assembled around a programmable synthetic microenvironment. Nat. Biotechnol. 19, 934–939 (2001).
Liu, C. Y., Apuzzo, M. L. & Tirrell, D. A. Engineering of the extracellular matrix: working toward neural stem cell programming and neurorestoration — concept and progress report. Neurosurgery 52, 1154–1165 (2003).
Anderson, D. G., Levenberg, S. & Langer, R. Nanoliter-scale synthesis of arrayed biomaterials and application to human embryonic stem cells. Nat. Biotechnol. 22, 863–866 (2004).
Lutolf, M. P. et al. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat. Biotechnol. 21, 513–518 (2003).
Zisch, A. H. et al. Cell-demanded release of VEGF from synthetic, biointeractive cell ingrowth matrices for vascularized tissue growth. FASEB J. 17, 2260–2262 (2003).
Meran, L., Baulies, A. & Li, V. S. W. Intestinal stem cell niche: the extracellular matrix and cellular components. Stem Cells Int. 2017, 7970385 (2017).
Gjorevski, N. et al. Designer matrices for intestinal stem cell and organoid culture. Nature 539, 560–564 (2016).
Cruz-Acuna, R. et al. Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Nat. Cell Biol. 19, 1326–1335 (2017).
Lutolf, M. P. & Hubbell, J. A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 23, 47–55 (2005).
Drinnan, C. T., Zhang, G., Alexander, M. A., Pulido, A. S. & Suggs, L. J. Multimodal release of transforming growth factor-beta1 and the BB isoform of platelet derived growth factor from PEGylated fibrin gels. J. Control. Release 147, 180–186 (2010).
Baker, B. M. & Chen, C. S. Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. J. Cell Sci. 125, 3015–3024 (2012).
O’Neill, C., Jordan, P. & Ireland, G. Evidence for two distinct mechanisms of anchorage stimulation in freshly explanted and 3T3 Swiss mouse fibroblasts. Cell 44, 489–496 (1986).
Kane, R. S., Takayama, S., Ostuni, E., Ingber, D. E. & Whitesides, G. M. in The Biomaterials: Silver Jubilee Compendium (Williams, D. F.) 161–174 (Elsevier, 2006).
Whitesides, G. M., Ostuni, E., Takayama, S., Jiang, X. & Ingber, D. E. Soft lithography in biology and biochemistry. Annu. Rev. Biomed. Eng. 3, 335–373 (2001).
Di Cio, S., Boggild, T. M. L., Connelly, J., Sutherland, D. S. & Gautrot, J. E. Differential integrin expression regulates cell sensing of the matrix nanoscale geometry. Acta Biomater. 50, 280–292 (2017).
McWhorter, F. Y., Wang, T., Nguyen, P., Chung, T. & Liu, W. F. Modulation of macrophage phenotype by cell shape. Proc. Natl Acad. Sci. USA 110, 17253–17258 (2013).
Patel, N. R. et al. Cell elasticity determines macrophage function. PLoS ONE 7, e41024 (2012).
Hudlicka, O. What makes blood vessels grow? J. Physiol. 444, 1–24 (1991).
Chen, S., Kawazoe, N. & Chen, G. Biomimetic assembly of vascular endothelial cells and muscle cells in microgrooved collagen porous scaffolds. Tissue Eng. Part C Methods 23, 367–376 (2017).
Wells, R. G. The role of matrix stiffness in regulating cell behavior. Hepatology 47, 1394–1400 (2008).
Xie, S. A. et al. Matrix stiffness determines the phenotype of vascular smooth muscle cell in vitro and in vivo: role of DNA methyltransferase 1. Biomaterials 155, 203–216 (2018).
Qiu, Y. et al. A role for matrix stiffness in the regulation of cardiac side population cell function. Am. J. Physiol. Heart Circ. Physiol. 308, H990–997 (2015).
Kural, M. H. & Billiar, K. L. Regulating tension in three-dimensional culture environments. Exp. Cell Res. 319, 2447–2459 (2013).
Boudreau, N., Werb, Z. & Bissell, M. J. Suppression of apoptosis by basement membrane requires three-dimensional tissue organization and withdrawal from the cell cycle. Proc. Natl Acad. Sci. USA 93, 3509–3513 (1996).
Emerman, J. T., Burwen, S. J. & Pitelka, D. R. Substrate properties influencing ultrastructural differentiation of mammary epithelial cells in culture. Tissue Cell 11, 109–119 (1979).
Farmer, S. R., Ben-Ze’av, A., Benecke, B. J. & Penman, S. Altered translatability of messenger RNA from suspended anchorage-dependent fibroblasts: reversal upon cell attachment to a surface. Cell 15, 627–637 (1978).
Roskelley, C. D., Desprez, P. Y. & Bissell, M. J. Extracellular matrix-dependent tissue-specific gene expression in mammary epithelial cells requires both physical and biochemical signal transduction. Proc. Natl Acad. Sci. USA 91, 12378–12382 (1994).
Seeman, N. C. & Belcher, A. M. Emulating biology: building nanostructures from the bottom up. Proc. Natl Acad. Sci. USA 99 (Suppl. 2), 6451–6455 (2002).
Djalali, R., Chen, Y. F. & Matsui, H. Au nanowire fabrication from sequenced histidine-rich peptide. J. Am. Chem. Soc. 124, 13660–13661 (2002).
Hamad-Schifferli, K., Schwartz, J. J., Santos, A. T., Zhang, S. & Jacobson, J. M. Remote electronic control of DNA hybridization through inductive coupling to an attached metal nanocrystal antenna. Nature 415, 152–155 (2002).
Mao, C. et al. Viral assembly of oriented quantum dot nanowires. Proc. Natl Acad. Sci. USA 100, 6946–6951 (2003).
Aggeli, A. et al. Hierarchical self-assembly of chiral rod-like molecules as a model for peptide beta -sheet tapes, ribbons, fibrils, and fibers. Proc. Natl Acad. Sci. USA 98, 11857–11862 (2001).
Vaquette, C. & Cooper-White, J. A simple method for fabricating 3D multilayered composite scaffolds. Acta Biomater. 9, 4599–4608 (2013).
Sill, T. J. & von Recum, H. A. Electrospinning: applications in drug delivery and tissue engineering. Biomaterials 29, 1989–2006 (2008).
Nam, J., Huang, Y., Agarwal, S. & Lannutti, J. Materials selection and residual solvent retention in biodegradable electrospun fibers. J. Appl. Polym. Sci. 107, 1547–1554 (2008).
Khorshidi, S. et al. A review of key challenges of electrospun scaffolds for tissue-engineering applications. J. Tissue Eng. Regen. Med. 10, 715–738 (2016).
Du, J. et al. Prompt peripheral nerve regeneration induced by a hierarchically aligned fibrin nanofiber hydrogel. Acta Biomater. 55, 296–309 (2017).
Fleischer, S., Shapira, A., Feiner, R. & Dvir, T. Modular assembly of thick multifunctional cardiac patches. Proc. Natl Acad. Sci. USA 114, 1898–1903 (2017).
Thakkar, S., Fernandes, H. & Moroni, L. Decellularized extracellular matrix scaffolds for cartilage regeneration. Methods Mol. Biol. 1340, 133–151 (2015).
Bridge, J. C. et al. Adapting the electrospinning process to provide three unique environments for a tri-layered in vitro model of the airway wall. J. Vis. Exp. 101, e52986 (2015).
Jang, J., Park, J. Y., Gao, G. & Cho, D. W. Biomaterials-based 3D cell printing for next-generation therapeutics and diagnostics. Biomaterials 156, 88–106 (2018).
Murphy, S. V. & Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 32, 773–785 (2014).
Ji, S. & Guvendiren, M. Recent advances in bioink design for 3D bioprinting of tissues and organs. Front. Bioeng. Biotechnol. 5, 23 (2017).
Shah, A. M., Jung, H. & Skirboll, S. Materials used in cranioplasty: a history and analysis. Neurosurg. Focus 36, E19 (2014).
Zopf, D. A., Hollister, S. J., Nelson, M. E., Ohye, R. G. & Green, G. E. Bioresorbable airway splint created with a three-dimensional printer. N. Engl. J. Med. 368, 2043–2045 (2013).
Yi, H. G., Lee, H. & Cho, D. W. 3D printing of organs-on-chips. Bioengineering 4, 10 (2017).
Pati, F. et al. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 5, 3935 (2014).
Hinton, T. J. et al. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 1, e1500758 (2015).
Jang, J. et al. 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials 112, 264–274 (2017).
Lee, J. S. et al. Liver extracellular matrix providing dual functions of two-dimensional substrate coating and three-dimensional injectable hydrogel platform for liver tissue engineering. Biomacromolecules 15, 206–218 (2014).
Drury, J. L. & Mooney, D. J. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials 24, 4337–4351 (2003).
Kumar, A. C. & Erothu, H. in Biomedical Applications of Polymeric Materials and Composites (Francis, R. & Kumar, D. S.) 141–162 (Wiley-VCH, 2016).
Geckil, H., Xu, F., Zhang, X., Moon, S. & Demirci, U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine 5, 469–484 (2010).
Vega, S. L., Kwon, M. Y. & Burdick, J. A. Recent advances in hydrogels for cartilage tissue engineering. Eur. Cell. Mater. 33, 59–75 (2017).
Snyder, T. N., Madhavan, K., Intrator, M., Dregalla, R. C. & Park, D. A fibrin/hyaluronic acid hydrogel for the delivery of mesenchymal stem cells and potential for articular cartilage repair. J. Biol. Eng. 8, 10 (2014).
Brigham, M. D. et al. Mechanically robust and bioadhesive collagen and photocrosslinkable hyaluronic acid semi-interpenetrating networks. Tissue Eng. Part A 15, 1645–1653 (2009).
Guo, Y. et al. Hydrogels of collagen/chondroitin sulfate/hyaluronan interpenetrating polymer network for cartilage tissue engineering. J. Mater. Sci. Mater. Med. 23, 2267–2279 (2012).
Kleinman, H. K. & Martin, G. R. Matrigel: basement membrane matrix with biological activity. Semin. Cancer Biol. 15, 378–386 (2005).
Freytes, D. O., Martin, J., Velankar, S. S., Lee, A. S. & Badylak, S. F. Preparation and rheological characterization of a gel form of the porcine urinary bladder matrix. Biomaterials 29, 1630–1637 (2008).
Voytik-Harbin, S. L., Brightman, A. O., Waisner, B. Z., Robinson, J. P. & Lamar, C. H. Small intestinal submucosa: a tissue-derived extracellular matrix that promotes tissue-specific growth and differentiation of cells in vitro. Tissue Engineer. 4, 157–174 (1998).
Keane, T. J. et al. Restoring mucosal barrier function and modifying macrophage phenotype with an extracellular matrix hydrogel: potential therapy for ulcerative colitis. J. Crohns Colitis 11, 360–368 (2017).
Wu, Y. et al. Implantation of brain-derived extracellular matrix enhances neurological recovery after traumatic brain injury. Cell Transplant. 26, 1224–1234 (2017).
Ghuman, H. et al. Long-term retention of ECM hydrogel after implantation into a sub-acute stroke cavity reduces lesion volume. Acta Biomater. 63, 50–63 (2017).
Massensini, A. R. et al. Concentration-dependent rheological properties of ECM hydrogel for intracerebral delivery to a stroke cavity. Acta Biomater. 27, 116–130 (2015).
Keane, T. J. et al. Tissue-specific effects of esophageal extracellular matrix. Tissue Eng. Part A 21, 2293–2300 (2015).
Lindberg, K. & Badylak, S. F. Porcine small intestinal submucosa (SIS): a bioscaffold supporting in vitro primary human epidermal cell differentiation and synthesis of basement membrane proteins. Burns 27, 254–266 (2001).
Faulk, D. M. et al. ECM hydrogel coating mitigates the chronic inflammatory response to polypropylene mesh. Biomaterials 35, 8585–8595 (2014).
Saldin, L. T., Cramer, M. C., Velankar, S. S., White, L. J. & Badylak, S. F. Extracellular matrix hydrogels from decellularized tissues: Structure and function. Acta Biomater. 49, 1–15 (2017).
Tibbitt, M. W. & Anseth, K. S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 103, 655–663 (2009).
Ruedinger, F., Lavrentieva, A., Blume, C., Pepelanova, I. & Scheper, T. Hydrogels for 3D mammalian cell culture: a starting guide for laboratory practice. Appl. Microbiol. Biotechnol. 99, 623–636 (2015).
Wolf, M. T. et al. A hydrogel derived from decellularized dermal extracellular matrix. Biomaterials 33, 7028–7038 (2012).
Medberry, C. J. et al. Hydrogels derived from central nervous system extracellular matrix. Biomaterials 34, 1033–1040 (2013).
Ungerleider, J. L. et al. Extracellular matrix hydrogel promotes tissue remodeling, arteriogenesis, and perfusion in a rat hindlimb ischemia model. JACC Bas. Transl Sci. 1, 32–44 (2016).
Fu, Y. et al. Decellularization of porcine skeletal muscle extracellular matrix for the formulation of a matrix hydrogel: a preliminary study. J. Cell. Mol. Med. 20, 740–749 (2016).
Wu, J. et al. An injectable extracellular matrix derived hydrogel for meniscus repair and regeneration. Acta Biomater. 16, 49–59 (2015).
Keane, T. J. et al. Preparation and characterization of a biologic scaffold and hydrogel derived from colonic mucosa. J. Biomed. Mater. Res. B Appl. Biomater. 105, 291–306 (2017).
Uriel, S. et al. Extraction and assembly of tissue-derived gels for cell culture and tissue engineering. Tissue Eng. Part C Methods 15, 309–321 (2009).
Paduano, F., Marrelli, M., White, L. J., Shakesheff, K. M. & Tatullo, M. Odontogenic differentiation of human dental pulp stem cells on hydrogel scaffolds derived from decellularized bone extracellular matrix and collagen type I. PLoS ONE 11, e0148225 (2016).
Uriel, S. et al. The role of adipose protein derived hydrogels in adipogenesis. Biomaterials 29, 3712–3719 (2008).
Ungerleider, J. L. & Christman, K. L. Concise review: injectable biomaterials for the treatment of myocardial infarction and peripheral artery disease: translational challenges and progress. Stem Cells Transl Med. 3, 1090–1099 (2014).
Hernandez, M. J. & Christman, K. L. Designing acellular injectable biomaterial therapeutics for treating myocardial infarction and peripheral artery disease. JACC Bas. Transl Sci. 2, 212–226 (2017).
Wassenaar, J. W. et al. Evidence for mechanisms underlying the functional benefits of a myocardial matrix hydrogel for post-MI treatment. J. Am. Coll. Cardiol. 67, 1074–1086 (2016).
Poel, W. E. Preparation of acellular homogenates from muscle samples. Science 108, 390–391 (1948).
Meezan, E., Hjelle, J. T., Brendel, K. & Carlson, E. C. A simple, versatile, nondisruptive method for the isolation of morphologically and chemically pure basement membranes from several tissues. Life Sci. 17, 1721–1732 (1975).
Rojkind, M. et al. Connective tissue biomatrix: its isolation and utilization for long-term cultures of normal rat hepatocytes. J. Cell Biol. 87, 255–263 (1980).
Badylak, S. F. et al. The use of xenogeneic small intestinal submucosa as a biomaterial for Achilles tendon repair in a dog model. J. Biomed. Mater. Res. 29, 977–985 (1995).
Wainwright, D. J. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns 21, 243–248 (1995).
Ott, H. C. et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat. Med. 14, 213–221 (2008).
Scarritt, M. E., Pashos, N. C. & Bunnell, B. A. A review of cellularization strategies for tissue engineering of whole organs. Front. Bioeng. Biotechnol. 3, 43 (2015).
Keane, T. J., Swinehart, I. T. & Badylak, S. F. Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods 84, 25–34 (2015).
Crapo, P. M., Gilbert, T. W. & Badylak, S. F. An overview of tissue and whole organ decellularization processes. Biomaterials 32, 3233–3243 (2011).
Dearth, C. L. et al. The effect of terminal sterilization on the material properties and in vivo remodeling of a porcine dermal biologic scaffold. Acta Biomater. 33, 78–87 (2016).
Wong, M. L. & Griffiths, L. G. Immunogenicity in xenogeneic scaffold generation: antigen removal versus decellularization. Acta Biomater. 10, 1806–1816 (2014).
Wong, M. L., Wong, J. L., Vapniarsky, N. & Griffiths, L. G. In vivo xenogeneic scaffold fate is determined by residual antigenicity and extracellular matrix preservation. Biomaterials 92, 1–12 (2016).
Cissell, D. D., Hu, J. C., Griffiths, L. G. & Athanasiou, K. A. Antigen removal for the production of biomechanically functional, xenogeneic tissue grafts. J. Biomechan. 47, 1987–1996 (2014).
Matuska, A. M. & McFetridge, P. S. The effect of terminal sterilization on structural and biophysical properties of a decellularized collagen-based scaffold; implications for stem cell adhesion. J. Biomed. Mater. Res. B Appl. Biomater. 103, 397–406 (2015).
Keane, T. J. & Badylak, S. F. The host response to allogeneic and xenogeneic biological scaffold materials. J. Tissue Eng. Regen. Med. 9, 504–511 (2015).
Freytes, D. O., Tullius, R. S. & Badylak, S. F. Effect of storage upon material properties of lyophilized porcine extracellular matrix derived from the urinary bladder. J. Biomed. Mater. Res. B Appl. Biomater. 78, 327–333 (2006).
Freytes, D. O., Tullius, R. S., Valentin, J. E., Stewart-Akers, A. M. & Badylak, S. F. Hydrated versus lyophilized forms of porcine extracellular matrix derived from the urinary bladder. J. Biomed. Mater. Res. A 87, 862–872 (2008).
Burk, J. et al. Freeze-thaw cycles enhance decellularization of large tendons. Tissue Eng. Part C Methods 20, 276–284 (2014).
Sasaki, S. et al. In vivo evaluation of a novel scaffold for artificial corneas prepared by using ultrahigh hydrostatic pressure to decellularize porcine corneas. Mol. Vis. 15, 2022–2028 (2009).
Funamoto, S. et al. The use of high-hydrostatic pressure treatment to decellularize blood vessels. Biomaterials 31, 3590–3595 (2010).
Badylak, S. F., Lantz, G. C., Coffey, A. & Geddes, L. A. Small intestinal submucosa as a large diameter vascular graft in the dog. J. Surg. Res. 47, 74–80 (1989).
Gilbert, T. W. et al. Collagen fiber alignment and biaxial mechanical behavior of porcine urinary bladder derived extracellular matrix. Biomaterials 29, 4775–4782 (2008).
Hodde, J. et al. Effects of sterilization on an extracellular matrix scaffold: part I. Composition and matrix architecture. J. Mater. Sci. Mater. Med. 18, 537–543 (2007).
Reing, J. E. et al. The effects of processing methods upon mechanical and biologic properties of porcine dermal extracellular matrix scaffolds. Biomaterials 31, 8626–8633 (2010).
Cox, B. & Emili, A. Tissue subcellular fractionation and protein extraction for use in mass-spectrometry-based proteomics. Nat. Protoc. 1, 1872–1878 (2006).
Xu, C. C., Chan, R. W. & Tirunagari, N. A biodegradable, acellular xenogeneic scaffold for regeneration of the vocal fold lamina propria. Tissue Eng. 13, 551–566 (2007).
Flynn, L. E. The use of decellularized adipose tissue to provide an inductive microenvironment for the adipogenic differentiation of human adipose-derived stem cells. Biomaterials 31, 4715–4724 (2010).
Montoya, C. V. & McFetridge, P. S. Preparation of ex vivo-based biomaterials using convective flow decellularization. Tissue Eng. Part C Methods 15, 191–200 (2009).
Bolland, F. et al. Development and characterisation of a full-thickness acellular porcine bladder matrix for tissue engineering. Biomaterials 28, 1061–1070 (2007).
Petersen, T. H. et al. Tissue-engineered lungs for in vivo implantation. Science 329, 538–541 (2010).
Faulk, D. M., Wildemann, J. D. & Badylak, S. F. Decellularization and cell seeding of whole liver biologic scaffolds composed of extracellular matrix. J. Clin. Exp. Hepatol. 5, 69–80 (2015).
Sullivan, D. C. et al. Decellularization methods of porcine kidneys for whole organ engineering using a high-throughput system. Biomaterials 33, 7756–7764 (2012).
Sawada, K., Terada, D., Yamaoka, T., Kitamura, S. & Fujisato, T. Cell removal with supercritical carbon dioxide for acellular artificial tissue. J. Chem. Technol. Biot 83, 943–949 (2008).
Phillips, M., Maor, E. & Rubinsky, B. Nonthermal irreversible electroporation for tissue decellularization. J. Biomech. Eng. 132, 091003 (2010).
Sano, M. B. et al. Towards the creation of decellularized organ constructs using irreversible electroporation and active mechanical perfusion. Biomed. Eng. Online 9, 83 (2010).
White, L. J. et al. The impact of detergents on the tissue decellularization process: a ToF-SIMS study. Acta Biomater. 50, 207–219 (2017).
Keane, T. J., Londono, R., Turner, N. J. & Badylak, S. F. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials 33, 1771–1781 (2012).
Costa, A. et al. Mechanical strength versus degradation of a biologically-derived surgical mesh over time in a rodent full thickness abdominal wall defect. Biomaterials 108, 81–90 (2016).
Daly, K. A. et al. Damage associated molecular patterns within xenogeneic biologic scaffolds and their effects on host remodeling. Biomaterials 33, 91–101 (2012).
Badylak, S. F. Decellularized allogeneic and xenogeneic tissue as a bioscaffold for regenerative medicine: factors that influence the host response. Ann. Biomed. Engineer. 42, 1517–1527 (2014).
Londono, R. & Badylak, S. F. Biologic scaffolds for regenerative medicine: mechanisms of in vivo remodeling. Ann. Biomed. Engineer. 43, 577–592 (2015).
Parmaksiz, M., Dogan, A., Odabas, S., Elcin, A. E. & Elcin, Y. M. Clinical applications of decellularized extracellular matrices for tissue engineering and regenerative medicine. Biomed. Mater. 11, 022003 (2016).
Alicuben, E. T. & DeMeester, S. R. Onlay ventral hernia repairs using porcine non-cross-linked dermal biologic mesh. Hernia 18, 705–712 (2014).
Mase, V. J. Jr. et al. Clinical application of an acellular biologic scaffold for surgical repair of a large, traumatic quadriceps femoris muscle defect. Orthopedics 33, 511 (2010).
Badylak, S. F. et al. Esophageal preservation in five male patients after endoscopic inner-layer circumferential resection in the setting of superficial cancer: a regenerative medicine approach with a biologic scaffold. Tissue Eng. Part A 17, 1643–1650 (2011).
Bejjani, G. K., Zabramski, J. & Durasis Study, G. Safety and efficacy of the porcine small intestinal submucosa dural substitute: results of a prospective multicenter study and literature review. J. Neurosurg. 106, 1028–1033 (2007).
Salzberg, C. A. Nonexpansive immediate breast reconstruction using human acellular tissue matrix graft (AlloDerm). Ann. Plast. Surg. 57, 1–5 (2006).
Moroni, F. & Mirabella, T. Decellularized matrices for cardiovascular tissue engineering. Am. J. Stem Cells 3, 1–20 (2014).
Badylak, S. F. The extracellular matrix as a biologic scaffold material. Biomaterials 28, 3587–3593 (2007).
Badylak, S. F., Freytes, D. O. & Gilbert, T. W. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 5, 1–13 (2009).
Sicari, B. M. et al. The effect of source animal age upon the in vivo remodeling characteristics of an extracellular matrix scaffold. Biomaterials 33, 5524–5533 (2012).
Swinehart, I. T. & Badylak, S. F. Extracellular matrix bioscaffolds in tissue remodeling and morphogenesis. Dev. Dynam. 245, 351–360 (2016).
Turner, N. J., Badylak, J. S., Weber, D. J. & Badylak, S. F. Biologic scaffold remodeling in a dog model of complex musculoskeletal injury. J. Surg. Res. 176, 490–502 (2012).
Sicari, B. M. et al. A murine model of volumetric muscle loss and a regenerative medicine approach for tissue replacement. Tissue Eng. Part A 18, 1941–1948 (2012).
Agrawal, V. et al. Epimorphic regeneration approach to tissue replacement in adult mammals. Proc. Natl Acad. Sci. USA 107, 3351–3355 (2010).
Beattie, A. J., Gilbert, T. W., Guyot, J. P., Yates, A. J. & Badylak, S. F. Chemoattraction of progenitor cells by remodeling extracellular matrix scaffolds. Tissue Eng. Part A 15, 1119–1125 (2009).
Sarikaya, A. et al. Antimicrobial activity associated with extracellular matrices. Tissue Eng. 8, 63–71 (2002).
Brown, B. N., Ratner, B. D., Goodman, S. B., Amar, S. & Badylak, S. F. Macrophage polarization: an opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials 33, 3792–3802 (2012).
Brown, B. N., Valentin, J. E., Stewart-Akers, A. M., McCabe, G. P. & Badylak, S. F. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials 30, 1482–1491 (2009).
Badylak, S. F., Valentin, J. E., Ravindra, A. K., McCabe, G. P. & Stewart-Akers, A. M. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng. Part A 14, 1835–1842 (2008).
Dziki, J. L., Huleihel, L., Scarritt, M. E. & Badylak, S. F. Extracellular matrix bioscaffolds as immunomodulatory biomaterials. Tissue Eng. Part A 23, 1152–1159 (2017).
Aamodt, J. M. & Grainger, D. W. Extracellular matrix-based biomaterial scaffolds and the host response. Biomaterials 86, 68–82 (2016).
Sadtler, K. et al. Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells. Science 352, 366–370 (2016).
Agmon, G. & Christman, K. L. Controlling stem cell behavior with decellularized extracellular matrix scaffolds. Curr. Opin. Solid State Mater. Sci. 20, 193–201 (2016).
Plunkett, N. & O’Brien, F. J. Bioreactors in tissue engineering. Technol. Health Care 19, 55–69 (2011).
Badylak, S. F., Taylor, D. & Uygun, K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu. Rev. Biomed. Eng. 13, 27–53 (2011).
Allen, R. A. et al. Adrenal extracellular matrix scaffolds support adrenocortical cell proliferation and function in vitro. Tissue Eng. Part A 16, 3363–3374 (2010).
Sellaro, T. L. et al. Maintenance of human hepatocyte function in vitro by liver-derived extracellular matrix gels. Tissue Eng. Part A 16, 1075–1082 (2010).
Brennan, E. P., Tang, X. H., Stewart-Akers, A. M., Gudas, L. J. & Badylak, S. F. Chemoattractant activity of degradation products of fetal and adult skin extracellular matrix for keratinocyte progenitor cells. J. Tissue Eng. Regen. Med. 2, 491–498 (2008).
Crapo, P. M. et al. Biologic scaffolds composed of central nervous system extracellular matrix. Biomaterials 33, 3539–3547 (2012).
Zhang, Y. et al. Tissue-specific extracellular matrix coatings for the promotion of cell proliferation and maintenance of cell phenotype. Biomaterials 30, 4021–4028 (2009).
Cortiella, J. et al. Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng. Part A 16, 2565–2580 (2010).
Shojaie, S. et al. Acellular lung scaffolds direct differentiation of endoderm to functional airway epithelial cells: requirement of matrix-bound HS proteoglycans. Stem Cell Rep. 4, 419–430 (2015).
Schweinlin, M. et al. Development of an advanced primary human in vitro model of the small intestine. Tissue Eng. Part C Methods 22, 873–883 (2016).
Robertson, M. J., Soibam, B., O’Leary, J. G., Sampaio, L. C. & Taylor, D. A. Recellularization of rat liver: an in vitro model for assessing human drug metabolism and liver biology. PLoS ONE 13, e0191892 (2018).
Genovese, L. et al. Cellular localization, invasion, and turnover are differently influenced by healthy and tumor-derived extracellular matrix. Tissue Eng. Part A 20, 2005–2018 (2014).
Chen, H. J. et al. A recellularized human colon model identifies cancer driver genes. Nat. Biotechnol. 34, 845–851 (2016).
Lu, W. D. et al. Development of an acellular tumor extracellular matrix as a three-dimensional scaffold for tumor engineering. PLoS ONE 9, e103672 (2014).
Nietzer, S. et al. Mimicking metastases including tumor stroma: a new technique to generate a three-dimensional colorectal cancer model based on a biological decellularized intestinal scaffold. Tissue Eng. Part C Methods 22, 621–635 (2016).
Hussein, K. H., Park, K. M., Ghim, J. H., Yang, S. R. & Woo, H. M. Three dimensional culture of HepG2 liver cells on a rat decellularized liver matrix for pharmacological studies. J. Biomed. Mater. Res. B Appl. Biomater. 104, 263–273 (2016).
Fitzpatrick, L. E. & McDevitt, T. C. Cell-derived matrices for tissue engineering and regenerative medicine applications. Biomater. Sci. 3, 12–24 (2015).
Lu, H. et al. Cultured cell-derived extracellular matrix scaffolds for tissue engineering. Biomaterials 32, 9658–9666 (2011).
Pei, M. et al. Modulation of in vitro microenvironment facilitates synovium-derived stem cell-based nucleus pulposus tissue regeneration. Spine 37, 1538–1547 (2012).
Syedain, Z. H., Meier, L. A., Bjork, J. W., Lee, A. & Tranquillo, R. T. Implantable arterial grafts from human fibroblasts and fibrin using a multi-graft pulsed flow-stretch bioreactor with noninvasive strength monitoring. Biomaterials 32, 714–722 (2011).
Lu, H., Hoshiba, T., Kawazoe, N. & Chen, G. Autologous extracellular matrix scaffolds for tissue engineering. Biomaterials 32, 2489–2499 (2011).
Lu, H., Hoshiba, T., Kawazoe, N. & Chen, G. Comparison of decellularization techniques for preparation of extracellular matrix scaffolds derived from three-dimensional cell culture. J. Biomed. Mater. Res. A 100, 2507–2516 (2012).
Ruff, S. M. et al. clickECM: Development of a cell-derived extracellular matrix with azide functionalities. Acta Biomater. 52, 159–170 (2017).
Marinkovic, M. et al. One size does not fit all: developing a cell-specific niche for in vitro study of cell behavior. Matrix Biol. 52–54, 426–441 (2016).
Prewitz, M. C. et al. Tightly anchored tissue-mimetic matrices as instructive stem cell microenvironments. Nat. Methods 10, 788–794 (2013).
Xiao, W. et al. Brain-mimetic 3D culture platforms allow investigation of cooperative effects of extracellular matrix features on therapeutic resistance in glioblastoma. Cancer Res. https://doi.org/10.1158/0008-5472.CAN-17-2429 (2017).
Devarasetty, M., Skardal, A., Cowdrick, K., Marini, F. & Soker, S. Bioengineered submucosal organoids for in vitro modeling of colorectal cancer. Tissue Eng. Part A 23, 1026–1041 (2017).
Syedain, Z. H., Meier, L. A., Lahti, M. T., Johnson, S. L. & Tranquillo, R. T. Implantation of completely biological engineered grafts following decellularization into the sheep femoral artery. Tissue Eng. Part A 20, 1726–1734 (2014).
Quint, C. et al. Decellularized tissue-engineered blood vessel as an arterial conduit. Proc. Natl Acad. Sci. USA 108, 9214–9219 (2011).
Weber, B. et al. Off-the-shelf human decellularized tissue-engineered heart valves in a non-human primate model. Biomaterials 34, 7269–7280 (2013).
McAllister, T. N. et al. Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: a multicentre cohort study. Lancet 373, 1440–1446 (2009).
Wystrychowski, W. et al. Case study: first implantation of a frozen, devitalized tissue-engineered vascular graft for urgent hemodialysis access. J. Vasc. Access 12, 67–70 (2011).
L’Heureux, N., McAllister, T. N. & de la Fuente, L. M. Tissue-engineered blood vessel for adult arterial revascularization. N. Engl. J. Med. 357, 1451–1453 (2007).
Zhang, W. et al. Cell-derived extracellular matrix: basic characteristics and current applications in orthopedic tissue engineering. Tissue Eng. Part B Rev. 22, 193–207 (2016).
Wainwright, D. et al. Clinical evaluation of an acellular allograft dermal matrix in full-thickness burns. J. Burn Care Rehabil. 17, 124–136 (1996).
Vlodavsky, I. Preparation of extracellular matrices produced by cultured corneal endothelial and PF-HR9 endodermal cells. Curr. Protoc. Cell Biol. 1, 10.4.1–10.4.14 (2001).
Elkins, R. C., Dawson, P. E., Goldstein, S., Walsh, S. P. & Black, K. S. Decellularized human valve allografts. Ann. Thorac. Surg. 71, S428–S432 (2001).
Zeltinger, J., Landeen, L. K., Alexander, H. G., Kidd, I. D. & Sibanda, B. Development and characterization of tissue-engineered aortic valves. Tissue Eng. 7, 9–22 (2001).
Hudson, T. W., Liu, S. Y. & Schmidt, C. E. Engineering an improved acellular nerve graft via optimized chemical processing. Tissue Eng. 10, 1346–1358 (2004).
Goncalves, A. C., Griffiths, L. G., Anthony, R. V. & Orton, E. C. Decellularization of bovine pericardium for tissue-engineering by targeted removal of xenoantigens. J. Heart Valve Dis. 14, 212–217 (2005).
Flynn, L., Semple, J. L. & Woodhouse, K. A. Decellularized placental matrices for adipose tissue engineering. J. Biomed. Mater. Res. A 79, 359–369 (2006).
Chen, X. D., Dusevich, V., Feng, J. Q., Manolagas, S. C. & Jilka, R. L. Extracellular matrix made by bone marrow cells facilitates expansion of marrow-derived mesenchymal progenitor cells and prevents their differentiation into osteoblasts. J. Bone Miner. Res. 22, 1943–1956 (2007).
Whitlock, P. W., Smith, T. L., Poehling, G. G., Shilt, J. S. & Van Dyke, M. A naturally derived, cytocompatible, and architecturally optimized scaffold for tendon and ligament regeneration. Biomaterials 28, 4321–4329 (2007).
Stankus, J. J., Freytes, D. O., Badylak, S. F. & Wagner, W. R. Hybrid nanofibrous scaffolds from electrospinning of a synthetic biodegradable elastomer and urinary bladder matrix. J. Biomater. Sci. Polym. Ed 19, 635–652 (2008).
Jungebluth, P. et al. Structural and morphologic evaluation of a novel detergent-enzymatic tissue-engineered tracheal tubular matrix. J. Thorac Cardiovasc. Surg. 138, 586–593 (2009).
Uygun, B. E. et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat. Med. 16, 814–820 (2010).
Hashimoto, Y. et al. Preparation and characterization of decellularized cornea using high-hydrostatic pressurization for corneal tissue engineering. Biomaterials 31, 3941–3948 (2010).
Nichols, J. E. et al. Giving new life to old lungs: methods to produce and assess whole human paediatric bioengineered lungs. J. Tissue Eng. Regen. Med. 11, 2136–2152 (2017).
Dejardin, L. M., Arnoczky, S. P., Ewers, B. J., Haut, R. C. & Clarke, R. B. Tissue-engineered rotator cuff tendon using porcine small intestine submucosa: histologic and mechanical evaluation in dogs. Am. J. Sports Med. 29, 175–184 (2001).
Dopirak, R., Bond, J. L. & Snyder, S. J. Arthroscopic total rotator cuff replacement with an acellular human dermal allograft matrix. Int. J. Shoulder Surg. 1, 7 (2007).
Dziki, J. et al. An acellular biologic scaffold treatment for volumetric muscle loss: results of a 13-patient cohort study. NPJ Regen.Med. 1, 16008 (2016).
Knoll, L. D. Use of small intestinal submucosa graft for the surgical management of Peyronie’s disease. J. Urol. 178, 2474–2478 (2007).
Leventhal, D. D. & Pribitkin, E. A. Static facial suspension with Surgisis ES (Enhanced Strength) sling. Laryngoscope 118, 20–23 (2008).
Gholami, G. A., Saberi, A., Kadkhodazadeh, M., Amid, R. & Karami, D. Comparison of the clinical outcomes of connective tissue and acellular dermal matrix in combination with double papillary flap for root coverage: a 6-month trial. Dental Res. J. 10, 506 (2013).
Butterfield, J. L. 440 Consecutive immediate, implant-based, single-surgeon breast reconstructions in 281 patients: a comparison of early outcomes and costs between SurgiMend fetal bovine and AlloDerm human cadaveric acellular dermal matrices. Plast. Reconstr. Surg. 131, 940–951 (2013).
Gerdisch, M. W., Shea, R. J. & Barron, M. D. Clinical experience with CorMatrix extracellular matrix in the surgical treatment of mitral valve disease. J. Thorac. Cardiovasc. Surg. 148, 1370–1378 (2014).
Brown, J. W., Ruzmetov, M., Eltayeb, O., Rodefeld, M. D. & Turrentine, M. W. Performance of SynerGraft decellularized pulmonary homograft in patients undergoing a Ross procedure. Ann. Thorac. Surg. 91, 416–423 (2011).
Scholl, F. G., Boucek, M. M., Chan, K.-C., Valdes-Cruz, L. & Perryman, R. Preliminary experience with cardiac reconstruction using decellularized porcine extracellular matrix scaffold: human applications in congenital heart disease. World J. Pediatr. Congenital Heart Surg. 1, 132–136 (2010).
Dharmapuram, A., Ramadoss, N., Verma, S., Gouthami, V. & Rao, I. Preliminary experience with the use of an extracellular matrix to augment the native pulmonary valve during repair of tetralogy of fallot. World J. Pediatr. Congenital Heart Surg. 8, 174–181 (2017).
Roth, J., Brathwaite, C., Hacker, K., Fisher, K. & King, J. Complex ventral hernia repair with a human acellular dermal matrix. Hernia 19, 247–252 (2015).
Ladowski, J. M. & Ladowski, J. S. Retrospective analysis of bovine pericardium (Vascu-Guard) for patch closure in carotid endarterectomies. Ann. Vascular Surg. 25, 646–650 (2011).
Lecheminant, J. & Field, C. Porcine urinary bladder matrix: a retrospective study and establishment of protocol. J. Wound Care 21, 476–482 (2012).
O’Connor, L. et al. Efficacy of anal fistula plug in closure of Crohn’s anorectal fistulas. Dis. Colon Rectum 49, 1569–1573 (2006).
Wen, H. et al. Randomized controlled trial of minimally invasive surgery using acellular dermal matrix for complex anorectal fistula. World J. Gastroenterol. 16, 3279 (2010).
Barret, J. P., Dziewulski, P., McCauley, R. L., Herndon, D. N. & Desai, M. H. Dural reconstruction of a class IV calvarial burn with decellularized human dermis. Burns 25, 459–462 (1999).
Juhasz, I. et al. Long-term followup of dermal substitution with acellular dermal implant in burns and postburn scar corrections. Dermatol. Res. Pract. 2010, 210150 (2010).
Mostow, E. N., Haraway, G. D., Dalsing, M., Hodde, J. P. & King, D. Effectiveness of an extracellular matrix graft (OASIS Wound Matrix) in the treatment of chronic leg ulcers: a randomized clinical trial. J. Vasc. Surg. 41, 837–843 (2005).
Arunkalaivanan, A. & Barrington, J. Randomized trial of porcine dermal sling (Pelvicol™ implant) versus tension-free vaginal tape (TVT) in the surgical treatment of stress incontinence: a questionnaire-based study. Int. Urogynecol. J. 14, 17–23 (2003).
Armitage, S., Seman, E. I. & Keirse, M. J. Use of Surgisis for treatment of anterior and posterior vaginal prolapse. Obstet. Gynecol. Int. 2012, 376251 (2012).
Author information
Authors and Affiliations
Contributions
G.S.H., J.L.D. and S.F.B. researched data for the article and made substantial contributions to discussion of content. G.S.H., J.L.D. and S.F.B. wrote the article and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hussey, G.S., Dziki, J.L. & Badylak, S.F. Extracellular matrix-based materials for regenerative medicine. Nat Rev Mater 3, 159–173 (2018). https://doi.org/10.1038/s41578-018-0023-x
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41578-018-0023-x