Abstract
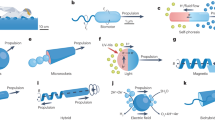
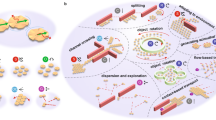
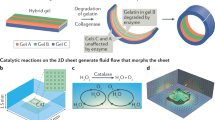
Microorganisms can move in complex media, respond to the environment and self-organize. The field of microrobotics strives to achieve these functions in mobile robotic systems of sub-millimetre size. However, miniaturization of traditional robots and their control systems to the microscale is not a viable approach. A promising alternative strategy in developing microrobots is to implement sensing, actuation and control directly in the materials, thereby mimicking biological matter. In this Review, we discuss design principles and materials for the implementation of robotic functionalities in microrobots. We examine different biological locomotion strategies, and we discuss how they can be artificially recreated in magnetic microrobots and how soft materials improve control and performance. We show that smart, stimuli-responsive materials can act as on-board sensors and actuators and that ‘active matter’ enables autonomous motion, navigation and collective behaviours. Finally, we provide a critical outlook for the field of microrobotics and highlight the challenges that need to be overcome to realize sophisticated microrobots, which one day might rival biological machines.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Dusenbery, D. B. Minimum size limit for useful locomotion by free-swimming microbes. Proc. Natl Acad. Sci. USA 94, 10949–10954 (1997).
Feynman, R. P. There’s plenty of room at the bottom. Engineer. Sci. 23, 22–36 (1960).
Nelson, B. J., Kaliakatsos, I. K. & Abbott, J. J. Microrobots for minimally invasive medicine. Annu. Rev. Biomed. Eng. 12, 55–85 (2010).
Li, J., Esteban-Fernández de Ávila, B., Gao, W., Zhang, L. & Wang, J. Micro/nanorobots for biomedicine: delivery, surgery, sensing, and detoxification. Sci. Robot. 2, eaam6431 (2017).
Purcell, E. M. Life at low Reynolds number. Am. J. Phys. 45, 3–11 (1977).
Palagi, S., Walker, D., Qiu, T. & Fischer, P. in Microbiorobotics 2nd edn (eds Kim, M., Julius, A. A. & Cheang, U. K.) 133–162 (Elsevier, 2017).
Qiu, T. et al. Swimming by reciprocal motion at low Reynolds number. Nat. Commun. 5, 5119 (2014).
Venugopalan, P. L. et al. Conformal cytocompatible ferrite coatings facilitate the realization of a nanovoyager in human blood. Nano Lett. 14, 1968–1975 (2014).
Lauga, E. & Powers, T. R. The hydrodynamics of swimming microorganisms. Rep. Prog. Phys. 72, 96601 (2009).
Behkam, B. & Sitti, M. Design methodology for biomimetic propulsion of miniature swimming robots. J. Dynam. Syst. Meas. Control 128, 36–43 (2006).
Fischer, P. & Ghosh, A. Magnetically actuated propulsion at low Reynolds numbers: towards nanoscale control. Nanoscale 3, 557–563 (2011).
Jarrell, K. F. & McBride, M. J. The surprisingly diverse ways that prokaryotes move. Nat. Rev. Microbiol. 6, 466–476 (2008).
Bray, D. Cell Movements: From Molecules to Motility. 2nd edn (Garland Science, 2001).
Walker, D., Kübler, M., Morozov, K. I., Fischer, P. & Leshansky, A. M. Optimal length of low Reynolds number nanopropellers. Nano Lett. 15, 4412–4416 (2015).
Zhang, L. et al. Artificial bacterial flagella: fabrication and magnetic control. Appl. Phys. Lett. 94, 64103–64107 (2009).
Ghosh, A. & Fischer, P. Controlled propulsion of artificial magnetic nanostructured propellers. Nano Lett. 9, 2243–2245 (2009).
Schamel, D. et al. Nanopropellers and their actuation in complex viscoelastic media. ACS Nano 8, 8794–8801 (2014).
Li, J. et al. Template electrosynthesis of tailored-made helical nanoswimmers. Nanoscale 6, 9415–9420 (2014).
Tottori, S. et al. Magnetic helical micromachines: fabrication, controlled swimming, and cargo transport. Adv. Mater. 24, 811–816 (2012).
Gao, W. et al. Bioinspired helical microswimmers based on vascular plants. Nano Lett. 14, 305–310 (2014).
Yan, X. et al. Multifunctional biohybrid magnetite microrobots for imaging-guided therapy. Sci. Robot. 2, eaaq1155 (2017).
Walker, D., Käsdorf, B. T., Jeong, H.-H., Lieleg, O. & Fischer, P. Enzymatically active biomimetic micropropellers for the penetration of mucin gels. Sci. Adv. 1, e1500501 (2015).
Huang, H. W., Chao, Q., Sakar, M. S. & Nelson, B. J. Optimization of tail geometry for the propulsion of soft microrobots. IEEE Robot. Autom. Lett. 2, 727–732 (2017).
Maier, A. M. et al. Magnetic propulsion of microswimmers with DNA-based flagellar bundles. Nano Lett. 16, 906–910 (2016).
Ishijima, S. Mechanical constraint converts planar waves into helices on tunicate and sea urchin sperm flagella. Cell Struct. Funct. 37, 13–19 (2012).
Abbott, J. J. et al. How should microrobots swim? Int. J. Robot. Res. 28, 1434–1447 (2009).
Lagomarsino, M. C., Capuani, F. & Lowe, C. P. A simulation study of the dynamics of a driven filament in an Aristotelian fluid. J. Theor. Biol. 224, 215–224 (2003).
Pak, O. S., Gao, W., Wang, J. & Lauga, E. High-speed propulsion of flexible nanowire motors: theory and experiments. Soft Matter 7, 8169–8181 (2011).
Khalil, I. S. M., Tabak, A. F., Klingner, A. & Sitti, M. Magnetic propulsion of robotic sperms at low-Reynolds number. Appl. Phys. Lett. 109, 033701 (2016).
Williams, B. J., Anand, S. V., Rajagopalan, J. & Saif, M. T. A. A self-propelled biohybrid swimmer at low Reynolds number. Nat. Commun. 5, 3081 (2014).
Dreyfus, R. et al. Microscopic artificial swimmers. Nature 437, 862–865 (2005).
Roper, M. et al. Do magnetic micro-swimmers move like eukaryotic cells? Proc. R. Soc. A Math. Phys. Engineer. Sci. 464, 877–904 (2008).
Li, T. et al. Magnetically propelled fish-like nanoswimmers. Small 12, 6098–6105 (2016).
Diller, E., Zhuang, J., Zhan Lum, G., Edwards, M. R. & Sitti, M. Continuously distributed magnetization profile for millimeter-scale elastomeric undulatory swimming. Appl. Phys. Lett. 104, 174101 (2014).
Hu, W., Lum, G. Z., Mastrangeli, M. & Sitti, M. Small-scale soft-bodied robot with multimodal locomotion. Nature 554, 81 (2018).
Evans, B. A. et al. Magnetically actuated nanorod arrays as biomimetic cilia. Nano Lett. 7, 1428–1434 (2007).
Shields, A. R. et al. Biomimetic cilia arrays generate simultaneous pumping and mixing regimes. Proc. Natl Acad. Sci. USA 107, 15670–15675 (2010).
Elgeti, J. & Gompper, G. Emergence of metachronal waves in cilia arrays. Proc. Natl Acad. Sci. USA 110, 4470–4475 (2013).
Yan, X., Wang, F., Zheng, B. & Huang, F. Stimuli-responsive supramolecular polymeric materials. Chem. Soc. Rev. 41, 6042–6065 (2012).
Zeng, H., Wasylczyk, P., Wiersma, D. S. & Priimagi, A. Light robots: bridging the gap between microrobotics and photomechanics in soft materials. Adv. Mater. https://doi.org/10.1002/adma.201703554 (2017).
Ohm, C., Brehmer, M. & Zentel, R. Liquid crystalline elastomers as actuators and sensors. Adv. Mater. 22, 3366–3387 (2010).
Palagi, S. et al. Structured light enables biomimetic swimming and versatile locomotion of photoresponsive soft microrobots. Nat. Mater. 15, 647–653 (2016).
Huang, H.-W., Sakar, M. S., Petruska, A. J., Pane, S. & Nelson, B. J. Soft micromachines with programmable motility and morphology. Nat. Commun. 7, 12263 (2016).
Wang, W. et al. Thermo-driven microcrawlers fabricated via a microfluidic approach. J. Phys. D Appl. Phys. 46, 114007 (2013).
Mourran, A., Zhang, H., Vinokur, R. & Möller, M. Soft microrobots employing nonequilibrium actuation via plasmonic heating. Adv. Mater. 29, 1604825 (2017).
Govorov, A. O. & Richardson, H. H. Generating heat with metal nanoparticles. Nano Today 2, 30–38 (2007).
Camacho-Lopez, M., Finkelmann, H., Palffy-Muhoray, P. & Shelley, M. Fast liquid-crystal elastomer swims into the dark. Nat. Mater. 3, 307–310 (2004).
Zeng, H. et al. Light-fueled microscopic walkers. Adv. Mater. 27, 3883–3887 (2015).
Palima, D. & Glückstad, J. Gearing up for optical microrobotics: micromanipulation and actuation of synthetic microstructures by optical forces. Laser Photon. Rev. 7, 478–494 (2013).
Blake, J. R. A spherical envelope approach to ciliary propulsion. J. Fluid Mech. 46, 199–208 (1971).
Childress, S. Mechanics of Swimming and Flying. Vol. 2 (Cambridge Univ. Press, 1981).
Palagi, S., Jager, E. W. H., Mazzolai, B. & Beccai, L. Propulsion of swimming microrobots inspired by metachronal waves in ciliates: from biology to material specifications. Bioinspir. Biomimet. 8, 46004 (2013).
Palagi, S. et al. in 2016 International Conference on Manipulation, Automation and Robotics at Small Scales (MARSS) (Paris, France, 2016).
Palagi, S. et al. in 2017 International Conference on Manipulation, Automation and Robotics at Small Scales (MARSS) (Montreal, Canada, 2017).
Magdanz, V., Guix, M., Hebenstreit, F. & Schmidt, O. G. Dynamic polymeric microtubes for the remote-controlled capture, guidance, and release of sperm cells. Adv. Mater. 28, 4084–4089 (2016).
Breger, J. C. et al. Self-folding thermo-magnetically responsive soft microgrippers. ACS Appl. Mater. Interfaces 7, 3398–3405 (2015).
Iacovacci, V. et al. Untethered magnetic millirobot for targeted drug delivery. Biomed. Microdevices 17, 1–12 (2015).
Tabatabaei, S. N., Lapointe, J. & Martel, S. Shrinkable hydrogel-based magnetic microrobots for interventions in the vascular network. Adv. Robot. 25, 1049–1067 (2011).
Fusco, S. et al. Shape-switching microrobots for medical applications: the influence of shape in drug delivery and locomotion. ACS Appl. Mater. Interfaces 7, 6803–6811 (2015).
Fusco, S. et al. Chitosan electrodeposition for microrobotic drug delivery. Adv. Healthc. Mater. 2, 1037–1044 (2013).
Li, H., Go, G., Ko, S. Y., Park, J.-O. & Park, S. Magnetic actuated pH-responsive hydrogel-based soft micro-robot for targeted drug delivery. Smart Mater. Struct. 25, 027001 (2016).
Yoshida, R. Self-oscillating polymer gel as novel biomimetic materials exhibiting spatiotemporal structure. Colloid. Polym. Sci. 289, 475–487 (2011).
Maeda, S., Hara, Y., Sakai, T., Yoshida, R. & Hashimoto, S. Self-walking gel. Adv. Mater. 19, 3480–3484 (2007).
Piovanelli, M., Fujie, T., Mazzolai, B. & Beccai, L. in 2012 4th IEEE RAS & EMBS International Conference on Biomedical Robotics and Biomechatronics (BioRob) 612–616 (Rome, Italy, 2012).
Lämmermann, T. & Sixt, M. Mechanical modes of ‘amoeboid’ cell migration. Curr. Opin. Cell Biol. 21, 636–644 (2009).
Onoda, M., Ueki, T., Tamate, R., Shibayama, M. & Yoshida, R. Amoeba-like self-oscillating polymeric fluids with autonomous sol-gel transition. Nat. Commun. 8, 15862 (2017).
Yi, J., Schmidt, J., Chien, A. & Montemagno, C. D. Engineering an artificial amoeba propelled by nanoparticle-triggered actin polymerization. Nanotechnology 20, 085101 (2009).
Sato, Y., Hiratsuka, Y., Kawamata, I., Murata, S. & Nomura, S.-i.M. Micrometer-sized molecular robot changes its shape in response to signal molecules. Sci. Robot. 2, eaal3735 (2017).
Terentjev, E. M. & Weitz, D. A. The Oxford Handbook of Soft Condensed Matter. (Oxford Univ. Press, 2015).
Marchetti, M. C. et al. Hydrodynamics of soft active matter. Rev. Mod. Phys. 85, 1143 (2013).
Needleman, D. & Dogic, Z. Active matter at the interface between materials science and cell biology. Nat. Rev. Mater. 2, 17048 (2017).
Ramaswamy, S. The mechanics and statistics of active matter. Annu. Rev. Condens. Matter Phys. 1, 323–345 (2010).
Illien, P., Golestanian, R. & Sen, A. ‘Fuelled’ motion: phoretic motility and collective behaviour of active colloids. Chem. Soc. Rev. 46, 5508–5518 (2017).
Anderson, J. L. Colloid transport by interfacial forces. Annu. Rev. Fluid Mechan. 21, 61–99 (1989).
Moran, J. L. & Posner, J. D. Phoretic self-propulsion. Annu. Rev. Fluid Mechan. 49, 511–540 (2017).
Bechinger, C. et al. Active particles in complex and crowded environments. Rev. Mod. Phys. 88, 045006 (2016).
Jiang, H.-R., Yoshinaga, N. & Sano, M. Active motion of a Janus particle by self-thermophoresis in a defocused laser beam. Phys. Rev. Lett. 105, 268302 (2010).
Ning, H., Buitenhuis, J., Dhont, J. K. G. & Wiegand, S. Thermal diffusion behavior of hard-sphere suspensions. J. Chem. Phys. 125, 204911 (2006).
Paxton, W. F., Sundararajan, S., Mallouk, T. E. & Sen, A. Chemical locomotion. Angew. Chem. Int. Ed. 45, 5420–5429 (2006).
Sánchez, S., Soler, L. & Katuri, J. Chemically powered micro- and nanomotors. Angew. Chem. Int. Ed. 54, 1414–1444 (2015).
Kapral, R. Perspective: Nanomotors without moving parts that propel themselves in solution. J. Chem. Phys. 138, 020901 (2013).
Paxton, W. F. et al. Catalytic nanomotors: autonomous movement of striped nanorods. J. Am. Chem. Soc. 126, 13424–13431 (2004).
Howse, J. R. et al. Self-motile colloidal particles: from directed propulsion to random walk. Phys. Rev. Lett. 99, 048102 (2007).
Solovev, A. A., Mei, Y., Bermúdez Ureña, E., Huang, G. & Schmidt, O. G. Catalytic microtubular jet engines self-propelled by accumulated gas bubbles. Small 5, 1688–1692 (2009).
Golestanian, R., Liverpool, T. B. & Ajdari, A. Propulsion of a molecular machine by asymmetric distribution of reaction products. Phys. Rev. Lett. 94, 220801 (2005).
Popescu, M. N., Uspal, W. E. & Dietrich, S. Self-diffusiophoresis of chemically active colloids. Eur. Phys. J. Special Top. 225, 2189–2206 (2016).
Uspal, W. E., Popescu, M. N., Dietrich, S. & Tasinkevych, M. Guiding catalytically active particles with chemically patterned surfaces. Phys. Rev. Lett. 117, 048002 (2016).
Brown, A. & Poon, W. Ionic effects in self-propelled Pt-coated Janus swimmers. Soft Matter 10, 4016–4027 (2014).
Paxton, W. F., Sen, A. & Mallouk, T. E. Motility of catalytic nanoparticles through self-generated forces. Chem. Eur. J. 11, 6462–6470 (2005).
Chen, K. et al. “Z”-shaped rotational Au/Pt micro-nanorobot. Micromachines 8, 183 (2017).
Wang, Y. et al. Bipolar electrochemical mechanism for the propulsion of catalytic nanomotors in hydrogen peroxide solutions. Langmuir 22, 10451–10456 (2006).
Wang, S. & Wu, N. Selecting the swimming mechanisms of colloidal particles: bubble propulsion versus self-diffusiophoresis. Langmuir 30, 3477–3486 (2014).
Fomin, V. M. et al. Propulsion mechanism of catalytic microjet engines. IEEE Trans. Robot. 30, 40–48 (2014).
Abdelmohsen, L. K. E. A., Peng, F., Tu, Y. & Wilson, D. A. Micro- and nano-motors for biomedical applications. J. Mater. Chem. B 2, 2395–2408 (2014).
Abdelmohsen, L. K. E. A. et al. Dynamic loading and unloading of proteins in polymeric stomatocytes: formation of an enzyme-loaded supramolecular nanomotor. ACS Nano 10, 2652–2660 (2016).
Gao, W. et al. Artificial micromotors in the mouse’s stomach: a step toward in vivo use of synthetic motors. ACS Nano 9, 117–123 (2015).
Dong, R., Zhang, Q., Gao, W., Pei, A. & Ren, B. Highly efficient light-driven TiO2–Au Janus micromotors. ACS Nano 10, 839–844 (2016).
Dong, R. et al. Visible-light-driven BiOI-based Janus micromotor in pure water. J. Am. Chem. Soc. 139, 1722–1725 (2017).
Pohl, O. & Stark, H. Dynamic clustering and chemotactic collapse of self-phoretic active particles. Phys. Rev. Lett. 112, 238303 (2014).
Berg, H. C. The rotary motor of bacterial flagella. Annu. Rev. Biochem. 72, 19–54 (2003).
Dusenbery, D. B. Living at Micro Scale: The Unexpected Physics of Being Small. (Harvard Univ. Press, 2009).
Palacci, J. et al. Artificial rheotaxis. Sci. Adv. 1, e1400214 (2015).
Dai, B. et al. Programmable artificial phototactic microswimmer. Nat. Nanotechnol 11, 1087–1092 (2016).
Lozano, C., ten Hagen, B., Löwen, H. & Bechinger, C. Phototaxis of synthetic microswimmers in optical landscapes. Nat. Commun. 7, 12828 (2016).
Zhuang, J. & Sitti, M. Chemotaxis of bio-hybrid multiple bacteria-driven microswimmers. Sci. Rep. 6, 32135 (2016).
Felfoul, O. et al. Magneto-aerotactic bacteria deliver drug-containing nanoliposomes to tumour hypoxic regions. Nat. Nanotechnol. 11, 941–947 (2016).
Wang, W., Duan, W., Sen, A. & Mallouk, T. E. Catalytically powered dynamic assembly of rod-shaped nanomotors and passive tracer particles. Proc. Natl Acad. Sci. USA 110, 17744–17749 (2013).
Nourhani, A., Brown, D., Pletzer, N. & Gibbs, J. G. Engineering contactless particle–particle interactions in active microswimmers. Adv. Mater. 29, 1703910 (2017).
Buttinoni, I. et al. Dynamical clustering and phase separation in suspensions of self-propelled colloidal particles. Phys. Rev. Lett. 110, 238301 (2013).
Palacci, J., Sacanna, S., Steinberg, A. P., Pine, D. J. & Chaikin, P. M. Living crystals of light-activated colloidal surfers. Science 339, 936–940 (2013).
Singh, D. P., Choudhury, U., Fischer, P. & Mark, A. G. Non-equilibrium assembly of light-activated colloidal mixtures. Adv. Mater. 29, 1701328 (2017).
Bricard, A., Caussin, J.-B., Desreumaux, N., Dauchot, O. & Bartolo, D. Emergence of macroscopic directed motion in populations of motile colloids. Nature 503, 95–98 (2013).
Yan, J. et al. Reconfiguring active particles by electrostatic imbalance. Nat. Mater. 15, 1095 (2016).
Brooks, R. A. Intelligence without representation. Artif. Intell. 47, 139–159 (1991).
Braitenberg, V. Vehicles: Experiments in Synthetic Psychology (MIT Press, 1986).
Brooks, R. A. Cambrian Intelligence: The Early History of the New AI (MIT Press, 1999).
Brooks, R. A. & Connell, J. H. in Proceedings of SPIE https://doi.org/10.1117/12.937785 (1987).
Murphy, R. Introduction to AI Robotics (MIT Press, 2000).
Acknowledgements
The authors acknowledge helpful discussions with D. Singh and M. Popescu.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the preparation of this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Palagi, S., Fischer, P. Bioinspired microrobots. Nat Rev Mater 3, 113–124 (2018). https://doi.org/10.1038/s41578-018-0016-9
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41578-018-0016-9
This article is cited by
-
Spatially selective delivery of living magnetic microrobots through torque-focusing
Nature Communications (2024)
-
Materials consideration for the design, fabrication and operation of microscale robots
Nature Reviews Materials (2024)
-
Light-controlled soft bio-microrobot
Light: Science & Applications (2024)
-
Nanoscale anisotropy for biomedical applications
Nature Reviews Bioengineering (2024)
-
Functionally antagonistic polyelectrolyte for electro-ionic soft actuator
Nature Communications (2024)