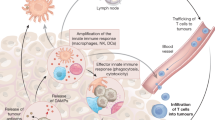
Abstract
There have been major advances in the immunotherapy of cancer in recent years, including the development of T cell engagers — antibodies engineered to redirect T cells to recognize and kill cancer cells — for the treatment of haematological malignancies. However, the field still faces several challenges to develop agents that are consistently effective in a majority of patients and cancer types, such as optimizing drug dose, overcoming treatment resistance and improving efficacy in solid tumours. A new generation of T cell-targeted molecules was developed to tackle these issues that are potentially more effective and safer. In addition, agents designed to engage the antitumour activities of other immune cells, including natural killer cells and myeloid cells, are showing promise and have the potential to treat a broader range of cancers.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Shimabukuro-Vornhagen, A. et al. Cytokine release syndrome. J. Immunother. Cancer 6, 56 (2018).
Ma, L., Feng, Y. & Zhou, Z. A close look at current γδ T-cell immunotherapy. Front. Immunol. 14, 1140623 (2023).
Park, J. H. & Lee, H. K. Function of γδ T cells in tumor immunology and their application to cancer therapy. Exp. Mol. Med. 53, 318–327 (2021).
Demaria, O. et al. Harnessing innate immunity in cancer therapy. Nature 574, 45–56 (2019).
Wolf, N. K., Kissiov, D. U. & Raulet, D. H. Roles of natural killer cells in immunity to cancer, and applications to immunotherapy. Nat. Rev. Immunol. 23, 90–105 (2023).
Barry, S. T., Gabrilovich, D. I., Sansom, O. J., Campbell, A. D. & Morton, J. P. Therapeutic targeting of tumour myeloid cells. Nat. Rev. Cancer 23, 216–237 (2023).
Chaib, M., Chauhan, S. C. & Makowski, L. Friend or foe? Recent strategies to target myeloid cells in cancer. Front. Cell Dev. Biol. 8, 351 (2020).
Goswami, S., Anandhan, S., Raychaudhuri, D. & Sharma, P. Myeloid cell-targeted therapies for solid tumours. Nat. Rev. Immunol. 23, 106–120 (2023).
Stork, R., Campigna, E., Robert, B., Muller, D. & Kontermann, R. E. Biodistribution of a bispecific single-chain diabody and its half-life extended derivatives. J. Biol. Chem. 284, 25612–25619 (2009).
Muller, D. et al. Improved pharmacokinetics of recombinant bispecific antibody molecules by fusion to human serum albumin. J. Biol. Chem. 282, 12650–12660 (2007).
Bacac, M. et al. A novel carcinoembryonic antigen T-cell bispecific antibody (CEA TCB) for the treatment of solid tumors. Clin. Cancer Res. 22, 3286–3297 (2016).
Brinkmann, U. & Kontermann, R. E. The making of bispecific antibodies. MAbs 9, 182–212 (2017).
Ellerman, D. Bispecific T-cell engagers: towards understanding variables influencing the in vitro potency and tumor selectivity and their modulation to enhance their efficacy and safety. Methods 154, 102–117 (2019).
Husain, B. & Ellerman, D. Expanding the boundaries of biotherapeutics with bispecific antibodies. BioDrugs 32, 441–464 (2018).
McAleese, F. & Eser, M. RECRUIT-TandAbs: harnessing the immune system to kill cancer cells. Future Oncol. 8, 687–695 (2012).
Runcie, K., Budman, D. R., John, V. & Seetharamu, N. Bi-specific and tri-specific antibodies — the next big thing in solid tumor therapeutics. Mol. Med. 24, 50 (2018).
Shiraiwa, H. et al. Engineering a bispecific antibody with a common light chain: identification and optimization of an anti-CD3ε and anti-GPC3 bispecific antibody, ERY974. Methods 154, 10–20 (2019).
Spiess, C., Zhai, Q. & Carter, P. J. Alternative molecular formats and therapeutic applications for bispecific antibodies. Mol. Immunol. 67, 95–106 (2015). This paper presents the different molecular formats of bispecific antibodies and their therapeutic applications.
Hong, D. S. et al. Autologous T cell therapy for MAGE-A4+ solid cancers in HLA-A*02+ patients: a phase 1 trial. Nat. Med. 29, 104–114 (2023).
Jungmichel, S. et al. Enhanced anti-tumor responses with a novel dual pMHC T-cell engager bispecific antibody. Cancer Res. Suppl. 82, 2891 (2022).
Roskopf, C. C. et al. Dual-targeting triplebody 33-3-19 mediates selective lysis of biphenotypic CD19+ CD33+ leukemia cells. Oncotarget 7, 22579–22589 (2016).
Tapia-Galisteo, A. et al. Trispecific T-cell engagers for dual tumor-targeting of colorectal cancer. Oncoimmunology 11, 2034355 (2022).
Moore, G. L. et al. Tuning T cell affinity improves efficacy and safety of anti-CD38 × anti-CD3 bispecific antibodies in monkeys — a potential therapy for multiple myeloma. Blood 126, 1798 (2015).
Leong, S. R. et al. An anti-CD3/anti-CLL-1 bispecific antibody for the treatment of acute myeloid leukemia. Blood 129, 609–618 (2017).
Alderson, R. et al. Combinatorial anti-tumor activity in animal models of a novel CD123 × CD3 bispecific Dart® molecule (MGD024) with cytarabine, venetoclax or azacitidine supports combination therapy in acute myeloid leukemia. Blood 138, 1165 (2021).
Rangaswamy, U. et al. A novel T-cell bispecific antibody platform for efficient T-cell mediated killing of tumor cells with minimal cytokine release. J. Clin. Oncol. 36, 209 (2018).
Trinklein, N. D. et al. Efficient tumor killing and minimal cytokine release with novel T-cell agonist bispecific antibodies. MAbs 11, 639–652 (2019).
Goebeler, M. E. & Bargou, R. C. T cell-engaging therapies — BiTEs and beyond. Nat. Rev. Clin. Oncol. 17, 418–434 (2020).
Singh, A., Dees, S. & Grewal, I. S. Overcoming the challenges associated with CD3+ T-cell redirection in cancer. Br. J. Cancer 124, 1037–1048 (2021).
Weigelin, B. et al. Focusing and sustaining the antitumor CTL effector killer response by agonist anti-CD137 mAb. Proc. Natl Acad. Sci. USA 112, 7551–7556 (2015).
Williams, J. B. et al. The EGR2 targets LAG-3 and 4-1BB describe and regulate dysfunctional antigen-specific CD8+ T cells in the tumor microenvironment. J. Exp. Med. 214, 381–400 (2017).
Piha-Paul, S. et al. A phase I dose escalation study of PRS-343, a HER2/4-1BB bispecific molecule, in patients with HER2-positive malignancies. J. Immunother. Cancer Suppl. 8, A1.2–A2 (2020).
Melero, I. et al. A first-in-human study of the fibroblast activation protein-targeted, 4-1BB agonist RO7122290 in patients with advanced solid tumors. Sci. Transl. Med. 15, eabp9229 (2023).
Claus, C. et al. Tumor targeted 4-1BB agonists for combination with T cell bispecific antibodies as off-the-shelf therapy. Sci. Transl. Med. 11, eaav5989 (2019).
Melero, I. et al. First-in-human (FIH) phase I study of RO7122290 (RO), a novel FAP-targeted 4-1BB agonist, administered as single agent and in combination with atezolizumab (ATZ) to patients with advanced solid. Ann. Oncol. Suppl. 31, S707 (2020).
de Bono, J. S. et al. A phase 1 open-label, dose escalation and expansion trial to investigate the safety, pharmacokinetics and pharmacodynamics of CB307, a trispecific Humabody® T-cell enhancer, in patients with PSMA+ advanced and/or metastatic solid tumors (POTENTIA). Cancer Res. https://doi.org/10.1158/1538-7445.AM2022-CT207 (2022).
Hangiu, O. et al. Tumor targeted 4-1BB agonist antibody–albumin fusions with high affinity to FcRn induce anti-tumor immunity without toxicity. iScience 25, 104958 (2022).
Mandrup, O. A. et al. Programmable half-life and anti-tumour effects of bispecific T-cell engager–albumin fusions with tuned FcRn affinity. Commun. Biol. 4, 310 (2021).
Suntharalingam, G. et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 355, 1018–1028 (2006).
Wu, L. et al. Trispecific antibodies enhance the therapeutic efficacy of tumor-directed T cells through T cell receptor co-stimulation. Nat. Cancer 1, 86–98 (2020). This paper reports the generation of a trispecific antibody that interacts with CD38, CD3 and CD28 to enhance both T cell activation and tumour targeting.
Liang, J. et al. A CLDN18.2-targeting bispecific T cell co-stimulatory activator for cancer immunotherapy. Cancer Manag. Res. 13, 6977–6987 (2021).
Suh, H., Pillai, K. & Morris, D. L. Mucins in pancreatic cancer: biological role, implications in carcinogenesis and applications in diagnosis and therapy. Am. J. Cancer Res. 7, 1372–1383 (2017).
Skokos, D. et al. A class of costimulatory CD28-bispecific antibodies that enhance the antitumor activity of CD3-bispecific antibodies. Sci. Transl. Med. 12, eaaw7888 (2020). This paper reports the generation of bispecific antibodies that mimic signal 2 to T cells by bridging a tumour-specific antigen to the costimulatory receptor CD28 on T cells.
Wei, J. et al. CD22-targeted CD28 bispecific antibody enhances antitumor efficacy of odronextamab in refractory diffuse large B cell lymphoma models. Sci. Transl. Med. 14, eabn1082 (2022).
Jin, X. et al. Production of soluble matriptase by human cancer cell lines and cell surface activation of its zymogen by trypsin. J. Cell Biochem. 95, 632–647 (2005).
LeBeau, A. M. et al. Imaging a functional tumorigenic biomarker in the transformed epithelium. Proc. Natl Acad. Sci. USA 110, 93–98 (2013).
Liu, C., Sun, C., Huang, H., Janda, K. & Edgington, T. Overexpression of legumain in tumors is significant for invasion/metastasis and a candidate enzymatic target for prodrug therapy. Cancer Res. 63, 2957–2964 (2003).
Liu, G., Shuman, M. A. & Cohen, R. L. Co-expression of urokinase, urokinase receptor and PAI-1 is necessary for optimum invasiveness of cultured lung cancer cells. Int. J. Cancer 60, 501–506 (1995).
Uhland, K. Matriptase and its putative role in cancer. Cell Mol. Life Sci. 63, 2968–2978 (2006).
Ulisse, S., Baldini, E., Sorrenti, S. & D’Armiento, M. The urokinase plasminogen activator system: a target for anti-cancer therapy. Curr. Cancer Drug Targets 9, 32–71 (2009).
Desnoyers, L. R. et al. Tumor-specific activation of an EGFR-targeting probody enhances therapeutic index. Sci. Transl. Med. 5, 207ra144 (2013).
Autio, K. A., Boni, V., Humphrey, R. W. & Naing, A. Probody therapeutics: an emerging class of therapies designed to enhance on-target effects with reduced off-tumor toxicity for use in immuno-oncology. Clin. Cancer Res. 26, 984–989 (2020).
Boustany, L. M. et al. EGFR-CD3 bispecific probody therapeutic induces tumor regressions and increases maximum tolerated dose >60-fold in preclinical studies. Mol. Cancer Ther. 17, A164 (2018).
Boustany, L. M. et al. A Probody T cell-engaging bispecific antibody targeting EGFR and CD3 inhibits colon cancer growth with limited toxicity. Cancer Res. 82, 4288–4298 (2022).
Kamata-Sakurai, M. et al. Antibody to CD137 activated by extracellular adenosine triphosphate is tumor selective and broadly effective in vivo without systemic immune activation. Cancer Discov. 11, 158–175 (2021).
Rohani, N. et al. Acidification of tumor at stromal boundaries drives transcriptome alterations associated with aggressive phenotypes. Cancer Res. 79, 1952–1966 (2019).
Chang, H. W. et al. Generating tumor-selective conditionally active biologic anti-CTLA4 antibodies via protein-associated chemical switches. Proc. Natl Acad. Sci. USA 118, e2020606118 (2021).
La Gruta, N. L., Gras, S., Daley, S. R., Thomas, P. G. & Rossjohn, J. Understanding the drivers of MHC restriction of T cell receptors. Nat. Rev. Immunol. 18, 467–478 (2018).
Saura-Esteller, J. et al. Gamma delta T-cell based cancer immunotherapy: past-present-future. Front. Immunol. 13, 915837 (2022).
Donia, M., Ellebaek, E., Andersen, M. H., Straten, P. T. & Svane, I. M. Analysis of Vδ1 T cells in clinical grade melanoma-infiltrating lymphocytes. Oncoimmunology 1, 1297–1304 (2012).
Gentles, A. J. et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 21, 938–945 (2015).
Lu, H. et al. High abundance of intratumoral γδ T cells favors a better prognosis in head and neck squamous cell carcinoma: a bioinformatic analysis. Front. Immunol. 11, 573920 (2020).
Meraviglia, S. et al. Distinctive features of tumor-infiltrating γδ T lymphocytes in human colorectal cancer. Oncoimmunology 6, e1347742 (2017).
Tosolini, M. et al. Assessment of tumor-infiltrating TCRVγ9Vδ2 γδ lymphocyte abundance by deconvolution of human cancers microarrays. Oncoimmunology 6, e1284723 (2017).
Wang, J. et al. Tumor-infiltrating γδT cells predict prognosis and adjuvant chemotherapeutic benefit in patients with gastric cancer. Oncoimmunology 6, e1353858 (2017).
Zhao, N. et al. Intratumoral γδ T-cell infiltrates, chemokine (C-C motif) ligand 4/chemokine (C-C motif) ligand 5 protein expression and survival in patients with hepatocellular carcinoma. Hepatology 73, 1045–1060 (2021).
Benyamine, A. et al. BTN3A molecules considerably improve Vγ9Vδ2T cells-based immunotherapy in acute myeloid leukemia. Oncoimmunology 5, e1146843 (2016).
Tokuyama, H. et al. Vγ9Vδ2T cell cytotoxicity against tumor cells is enhanced by monoclonal antibody drugs — rituximab and trastuzumab. Int. J. Cancer 122, 2526–2534 (2008).
de Silva, S. et al. Antigen-specific targeting of tissue-resident gamma delta T cells with recombinant butyrophilin heterodimeric fusion proteins. Cancer Res. 81, 1736 (2021).
Oberg, H. H. et al. Bispecific antibodies enhance tumor-infiltrating T cell cytotoxicity against autologous HER-2-expressing high-grade ovarian tumors. J. Leukoc. Biol. 107, 1081–1095 (2020).
Oberg, H. H. et al. γδ T cell activation by bispecific antibodies. Cell Immunol. 296, 41–49 (2015).
Oberg, H. H. et al. Novel bispecific antibodies increase γδ T-cell cytotoxicity against pancreatic cancer cells. Cancer Res. 74, 1349–1360 (2014).
Ganesan, R. et al. Selective recruitment of γδ T cells by a bispecific antibody for the treatment of acute myeloid leukemia. Leukemia 35, 2274–2284 (2021).
de Weerdt, I. et al. A bispecific single-domain antibody boosts autologous Vγ9Vδ2-T cell responses toward CD1d in chronic lymphocytic leukemia. Clin. Cancer Res. 27, 1744–1755 (2021).
Hoeres, T. et al. Improving immunotherapy against B-cell malignancies using γδ T-cell-specific stimulation and therapeutic monoclonal antibodies. J. Immunother. 42, 331–344 (2019).
Schiller, C. B. et al. CD19-specific triplebody SPM-1 engages NK and γδ T cells for rapid and efficient lysis of malignant B-lymphoid cells. Oncotarget 7, 83392–83408 (2016).
Seidel, U. J. et al. γδ T cell-mediated antibody-dependent cellular cytotoxicity with CD19 antibodies assessed by an impedance-based label-free real-time cytotoxicity assay. Front. Immunol. 5, 618 (2014).
de Bruin, R. C. G. et al. A bispecific nanobody approach to leverage the potent and widely applicable tumor cytolytic capacity of Vγ9Vδ2-T cells. Oncoimmunology 7, e1375641 (2017).
de Weerdt, I. et al. A bispecific antibody antagonizes prosurvival CD40 signaling and promotes Vγ9Vδ2 T cell-mediated antitumor responses in human B-cell malignancies. Cancer Immunol. Res. 9, 50–61 (2021).
King, L. A. et al. A bispecific γδ T-cell engager targeting EGFR activates a potent Vγ9Vδ2 T cell-mediated immune response against EGFR-expressing tumors. Cancer Immunol. Res. 11, 1237–1252 (2023).
Lameris, R. et al. Generation and characterization of CD1d-specific single-domain antibodies with distinct functional features. Immunology 149, 111–121 (2016).
Ames, E. et al. NK cells preferentially target tumor cells with a cancer stem cell phenotype. J. Immunol. 195, 4010–4019 (2015).
Castriconi, R. et al. NK cells recognize and kill human glioblastoma cells with stem cell-like properties. J. Immunol. 182, 3530–3539 (2009).
Tallerico, R. et al. Human NK cells selective targeting of colon cancer-initiating cells: a role for natural cytotoxicity receptors and MHC class I molecules. J. Immunol. 190, 2381–2390 (2013).
Ilander, M. et al. Increased proportion of mature NK cells is associated with successful imatinib discontinuation in chronic myeloid leukemia. Leukemia 31, 1108–1116 (2017).
Street, S. E. et al. Innate immune surveillance of spontaneous B cell lymphomas by natural killer cells and γδ T cells. J. Exp. Med. 199, 879–884 (2004).
Habif, G., Crinier, A., Andre, P., Vivier, E. & Narni-Mancinelli, E. Targeting natural killer cells in solid tumors. Cell Mol. Immunol. 16, 415–422 (2019).
Devillier, R. et al. Phase I trial of prophylactic donor-derived IL-2-activated NK cell infusion after allogeneic hematopoietic stem cell transplantation from a matched sibling donor. Cancers 13, 2673 (2021).
Li, Y., Hermanson, D. L., Moriarity, B. S. & Kaufman, D. S. Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell 23, 181–192.e5 (2018).
Marshall, M. J. E., Stopforth, R. J. & Cragg, M. S. Therapeutic antibodies: what have we learnt from targeting CD20 and where are we going? Front. Immunol. 8, 1245 (2017).
Molina, A. A decade of rituximab: improving survival outcomes in non-Hodgkin’s lymphoma. Annu. Rev. Med. 59, 237–250 (2008).
Kaplon, H., Muralidharan, M., Schneider, Z. & Reichert, J. M. Antibodies to watch in 2020. MAbs 12, 1703531 (2020).
Shields, R. L. et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcγ RIII and antibody-dependent cellular toxicity. J. Biol. Chem. 277, 26733–26740 (2002).
Suzuki, E. et al. A nonfucosylated anti-HER2 antibody augments antibody-dependent cellular cytotoxicity in breast cancer patients. Clin. Cancer Res. 13, 1875–1882 (2007).
Wang, X., Mathieu, M. & Brezski, R. J. IgG Fc engineering to modulate antibody effector functions. Protein Cell 9, 63–73 (2018).
van der Horst, H. J., Nijhof, I. S., Mutis, T. & Chamuleau, M. E. D. Fc-engineered antibodies with enhanced Fc-effector function for the treatment of B-cell malignancies. Cancers 12, 3041 (2020).
Demaria, O., Gauthier, L., Debroas, G. & Vivier, E. Natural killer cell engagers in cancer immunotherapy: next generation of immuno-oncology treatments. Eur. J. Immunol. 51, 1934–1942 (2021).
Myers, J. A. & Miller, J. S. Exploring the NK cell platform for cancer immunotherapy. Nat. Rev. Clin. Oncol. 18, 85–100 (2021).
Rothe, A. et al. A phase 1 study of the bispecific anti-CD30/CD16A antibody construct AFM13 in patients with relapsed or refractory Hodgkin lymphoma. Blood 125, 4024–4031 (2015).
Bartlett, N. L. et al. A phase 1b study of AFM13 in combination with pembrolizumab in patients with relapsed or refractory Hodgkin lymphoma. Blood 136, 2401–2409 (2020). This clinical trial evaluates the combination of the CD30–CD16A-bispecific antibody AFM13 with pembrolizumab (anti-PD1) and reports an objective response rate of 88% at the highest treatment dose, with an 83% overall response rate for the overall population.
Gauthier, L. et al. Multifunctional natural killer cell engagers targeting NKp46 trigger protective tumor immunity. Cell 177, 1701–1713.e16 (2019). This paper reports the generation of trifunctional NK cell engagers targeting two activating receptors, NKp46 and CD16, on NK cells and a tumour antigen on cancer cells.
Vallera, D. A. et al. IL15 trispecific killer engagers (TriKE) make natural killer cells specific to CD33+ targets while also inducing persistence, in vivo expansion, and enhanced function. Clin. Cancer Res. 22, 3440–3450 (2016). Compared with the bispecific antibody, the 16-15-33 TriKE molecule induced higher cytotoxicity, degranulation and cytokine production by NK cells against CD33-positive targets in vitro and induced persistence and survival of human NK cells in preclinical mouse models.
Schmohl, J. U. et al. Engineering of anti-CD133 trispecific molecule capable of inducing NK expansion and driving antibody-dependent cell-mediated cytotoxicity. Cancer Res. Treat. 49, 1140–1152 (2017). This work describes the production of a bispecific NK cell engager targeting CD16 and tumour antigen coupled to IL-15 to promote NK cell expansion, function and survival.
Vallera, D. A. et al. NK-cell-mediated targeting of various solid tumors using a B7-H3 tri-specific killer engager in vitro and in vivo. Cancers 12, 2659 (2020).
Gavira-O’Neill, C. E., Dong, J. X. & Trimmer, J. S. Development, screening, and validation of camelid-derived nanobodies for neuroscience research. Curr. Protoc. Neurosci. 94, e107 (2020).
Miller, J. et al. Second-generation CD19 targeting trispecific killer engager drives robust NK cell function against B cell malignancies. HemaSpere 6, 517–518 (2022).
Chan, W. K. et al. A CS1-NKG2D bispecific antibody collectively activates cytolytic immune cells against multiple myeloma. Cancer Immunol. Res. 6, 776–787 (2018).
von Strandmann, E. P. et al. A novel bispecific protein (ULBP2-BB4) targeting the NKG2D receptor on natural killer (NK) cells and CD138 activates NK cells and has potent antitumor activity against human multiple myeloma in vitro and in vivo. Blood 107, 1955–1962 (2006).
Wang, Y. et al. BCMA-targeting bispecific antibody that simultaneously stimulates NKG2D-enhanced efficacy against multiple myeloma. J. Immunother. 43, 175–188 (2020).
Wensveen, F. M., Jelencic, V. & Polic, B. NKG2D: a master regulator of immune cell responsiveness. Front. Immunol. 9, 441 (2018).
Chitadze, G., Bhat, J., Lettau, M., Janssen, O. & Kabelitz, D. Generation of soluble NKG2D ligands: proteolytic cleavage, exosome secretion and functional implications. Scand. J. Immunol. 78, 120–129 (2013).
Rezvaya, A. et al. NKp46 engaging Bicycle® NK-TICA™ drives targeted tumor cytotoxicity. J. Immunother. Cancer 10, 1207 (2022).
Colomar-Carando, N. et al. Exploiting natural killer cell engagers to control pediatric B-cell precursor acute lymphoblastic leukemia. Cancer Immunol. Res. 10, 291–302 (2022).
Gauthier, L. et al. Control of acute myeloid leukemia by a trifunctional NKp46-CD16a-NK cell engager targeting CD123. Nat. Biotechnol. 41, 1296–1306 (2023). This paper reports the efficiency of the ANKET molecule targeting CD123 in AML without dose-limiting toxicity.
Uy, G. L. et al. Preliminary results of a phase 1 study of flotetuzumab, a CD123 × CD3 bispecific Dart® protein, in patients with relapsed/refractory acute myeloid leukemia and myelodysplastic syndrome. Blood 130, 637 (2017).
Stein, A. S. et al. A first-in-human study of CD123 NK cell engager SAR443579 in relapsed or refractory acute myeloid leukemia, B-cell acute lymphoblastic leukemia, or high-risk myelodysplasia. J. Clin. Oncol. 41, 7005 (2023).
Demaria, O. et al. Antitumor immunity induced by antibody-based natural killer cell engager therapeutics armed with not-alpha IL-2 variant. Cell Rep. Med. 3, 100783 (2022). This paper reports the design of tetraspecific molecules engaging the NK cell-activating receptors NKp46 and CD16A, the β-chain of the IL-2 receptor and a tumour-associated antigen.
Lin, L. et al. Novel multifunctional tetravalent CD38 NKp46 FLEX-NK engagers actively target and kill multiple myeloma cells. Hemasphere 6, 736–737 (2022).
Arulanandam, A. et al. Glypican-3 (GPC3) and NKp46 directed FLEX-NK engager antibody (CYT-303) recruits natural killer (NK) cells to tumors in a preclinical hepatocellular carcinoma (HCC) mouse model. Ann. Oncol. https://doi.org/10.1016/j.annonc.2022.07.882 (2022).
Pekar, L. et al. Affinity maturation of B7-H6 translates into enhanced NK cell-mediated tumor cell lysis and improved proinflammatory cytokine release of bispecific immunoligands via NKp30 engagement. J. Immunol. 206, 225–236 (2021).
Xiao, X. et al. Bispecific NK-cell engager targeting BCMA elicits stronger antitumor effects and produces less proinflammatory cytokines than T-cell engager. Front. Immunol. 14, 1113303 (2023). This work reports the generation and testing of two IgG-like bispecific antibodies: one attracts T cells and tumour cells (BCMA × CD3) and the other targets NK cells and tumour cells (BCMA × CD16). The results suggest that the NK cell antibodies are more effective and produce fewer pro-inflammatory cytokines.
Dysthe, M. & Parihar, R. Myeloid-derived suppressor cells in the tumor microenvironment. Adv. Exp. Med. Biol. 1224, 117–140 (2020).
Kim, J. & Bae, J. S. Tumor-associated macrophages and neutrophils in tumor microenvironment. Mediators Inflamm. 2016, 6058147 (2016).
Wellenstein, M. D. & de Visser, K. E. Cancer-cell-intrinsic mechanisms shaping the tumor immune landscape. Immunity 48, 399–416 (2018).
Overdijk, M. B. et al. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs 7, 311–321 (2015). This paper reports that antibody-mediated phagocytosis is a clinically relevant mechanism of action for the anti-CD38 mAb daratumumab.
VanDerMeid, K. R. et al. Cellular cytotoxicity of next-generation CD20 monoclonal antibodies. Cancer Immunol. Res. 6, 1150–1160 (2018).
Shi, Y. et al. Trastuzumab triggers phagocytic killing of high HER2 cancer cells in vitro and in vivo by interaction with Fcγ receptors on macrophages. J. Immunol. 194, 4379–4386 (2015).
Nimmerjahn, F. & Ravetch, J. V. Fcγ receptors as regulators of immune responses. Nat. Rev. Immunol. 8, 34–47 (2008).
Hogarth, P. M. & Pietersz, G. A. Fc receptor-targeted therapies for the treatment of inflammation, cancer and beyond. Nat. Rev. Drug Discov. 11, 311–331 (2012).
Braster, R., O’Toole, T. & van Egmond, M. Myeloid cells as effector cells for monoclonal antibody therapy of cancer. Methods 65, 28–37 (2014).
Futosi, K., Fodor, S. & Mocsai, A. Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int. Immunopharmacol. 17, 638–650 (2013).
Valerius, T. et al. Involvement of the high-affinity receptor for IgG (FcγRI; CD64) in enhanced tumor cell cytotoxicity of neutrophils during granulocyte colony-stimulating factor therapy. Blood 82, 931–939 (1993).
Sundarapandiyan, K. et al. Bispecific antibody-mediated destruction of Hodgkin’s lymphoma cells. J. Immunol. Methods 248, 113–123 (2001).
Watanabe, M. et al. Antibody dependent cellular phagocytosis (ADCP) and antibody dependent cellular cytotoxicity (ADCC) of breast cancer cells mediated by bispecific antibody, MDX-210. Breast Cancer Res. Treat. 53, 199–207 (1999).
Sewnath, C. A., Behrens, L. M. & van Egmond, M. Targeting myeloid cells with bispecific antibodies as novel immunotherapies of cancer. Expert Opin. Biol. Ther. 22, 983–995 (2022).
Aleyd, E., Heineke, M. H. & van Egmond, M. The era of the immunoglobulin A Fc receptor FcαRI; its function and potential as target in disease. Immunol. Rev. 268, 123–138 (2015).
Dechant, M. et al. Effector mechanisms of recombinant IgA antibodies against epidermal growth factor receptor. J. Immunol. 179, 2936–2943 (2007).
van Tetering, G., Evers, M., Chan, C., Stip, M. & Leusen, J. Fc engineering strategies to advance IgA antibodies as therapeutic agents. Antibodies 9, 70 (2020).
Xu, L. et al. Targeting CD89 on tumor-associated macrophages overcomes resistance to immune checkpoint blockade. J. Immunother. Cancer 10, e005447 (2022).
Li, B. et al. CD89-mediated recruitment of macrophages via a bispecific antibody enhances anti-tumor efficacy. Oncoimmunology 7, e1380142 (2017).
Kelton, W. et al. IgGA: a “cross-isotype” engineered human Fc antibody domain that displays both IgG-like and IgA-like effector functions. Chem. Biol. 21, 1603–1609 (2014).
Borrok, M. J. et al. Enhancement of antibody-dependent cell-mediated cytotoxicity by endowing IgG with FcαRI (CD89) binding. MAbs 7, 743–751 (2015).
Li, B. et al. Simultaneous exposure to FcγR and FcαR on monocytes and macrophages enhances antitumor activity in vivo. Oncotarget 8, 39356–39366 (2017).
Heemskerk, N. et al. Augmented antibody-based anticancer therapeutics boost neutrophil cytotoxicity. J. Clin. Invest. 131, e134680 (2021).
Long, K. B. et al. IFNγ and CCL2 cooperate to redirect tumor-infiltrating monocytes to degrade fibrosis and enhance chemotherapy efficacy in pancreatic carcinoma. Cancer Discov. 6, 400–413 (2016).
Beatty, G. L. et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 331, 1612–1616 (2011). This paper reports the mechanism of action of an agonist CD40-specific antibody that requires macrophages but not T cells.
Dahlen, E., Veitonmaki, N. & Norlen, P. Bispecific antibodies in cancer immunotherapy. Ther. Adv. Vaccines Immunother. 6, 3–17 (2018).
Ye, S. et al. A bispecific molecule targeting CD40 and tumor antigen mesothelin enhances tumor-specific immunity. Cancer Immunol. Res. 7, 1864–1875 (2019).
Hagerbrand, K. et al. Bispecific antibodies targeting CD40 and tumor-associated antigens promote cross-priming of T cells resulting in an antitumor response superior to monospecific antibodies. J. Immunother. Cancer 10, e005018 (2022).
Pastan, I. & Hassan, R. Discovery of mesothelin and exploiting it as a target for immunotherapy. Cancer Res. 74, 2907–2912 (2014).
Luke, J. J. et al. Phase I study of ABBV-428, a mesothelin-CD40 bispecific, in patients with advanced solid tumors. J. Immunother. Cancer 9, e002015 (2021).
Rigamonti, N. et al. A multispecific anti-CD40 DARPin construct induces tumor-selective CD40 activation and tumor regression. Cancer Immunol. Res. 10, 626–640 (2022).
Urban-Wojciuk, Z. et al. The role of TLRs in anti-cancer immunity and tumor rejection. Front. Immunol. 10, 2388 (2019).
Sagiv-Barfi, I. et al. Eradication of spontaneous malignancy by local immunotherapy. Sci. Transl. Med. 10, eaan4488 (2018).
Carmi, Y. et al. Allogeneic IgG combined with dendritic cell stimuli induce antitumour T-cell immunity. Nature 521, 99–104 (2015).
Allen, B. M. et al. Systemic dysfunction and plasticity of the immune macroenvironment in cancer models. Nat. Med. 26, 1125–1134 (2020).
Milhem, M. M. et al. Intratumoral Toll-like receptor (TLR9) agonist, CMP-001, in combination with pembrolizumab can reverse resistance to PD-1 inhibition in a phase 1b trial in subjects with advanced melanoma. Cancer Res. Suppl. 78, CT144 (2018).
Ackerman, S. E. et al. Immune-stimulating antibody conjugates elicit robust myeloid activation and durable antitumor immunity. Nat. Cancer 2, 18–33 (2021). This paper reports the generation of a novel class of immune-stimulating antibody conjugates comprising a TLR7–TLR8 dual agonist conjugated to tumour-targeting antibodies.
Janku, F. et al. Preclinical characterization and phase I study of an anti-HER2-TLR7 immune-stimulator antibody conjugate in patients with HER2+ malignancies. Cancer Immunol. Res. 10, 1441–1461 (2022).
Blum, L. K. et al. The CEA-targeted ISAC, BDC-2034, shows preclinical efficacy associated with innate immune activation, phagocytosis, and myeloid reprogramming. Cancer Res. 82, 2911 (2022).
Appleman, V. et al. Preclinical activity of C-C chemokine receptor 2 (CCR2)-targeted immune stimulating antibody conjugate (ISAC), motivating clinical testing of TAK-500. J. Immunother. Cancer 10, 3503 (2022).
Duvall, J. R. et al. XMT-2056, a HER2-targeted Immunosynthen STING-agonist antibody-drug conjugate, binds a novel epitope of HER2 and shows increased anti-tumor activity in combination with trastuzumab and pertuzumab. Cancer Res. Suppl. 82, 3503 (2022).
Wu, Y. T. et al. Tumor-targeted delivery of a STING agonist improvescancer immunotherapy. Proc. Natl Acad. Sci. USA 119, e2214278119 (2022).
Barnett, L. et al. DC-SIGN antibody conjugates comprising STING agonists. Patent WO/2020/092617 (2020).
Ye, Y. et al. JAB-X1800: a potent immunostimulatory antibody-drug conjugate (iADC) targeting CD73. Cancer Res. Suppl. 83, 2923 (2023).
Duell, J. et al. Frequency of regulatory T cells determines the outcome of the T-cell-engaging antibody blinatumomab in patients with B-precursor ALL. Leukemia 31, 2181–2190 (2017).
Dutta, A. et al. Altered T-bet dominance in IFN-gamma-decoupled CD4+ T cells with attenuated cytokine storm and preserved memory in influenza. J. Immunol. 190, 4205–4214 (2013).
Penaloza-MacMaster, P. et al. Vaccine-elicited CD4 T cells induce immunopathology after chronic LCMV infection. Science 347, 278–282 (2015).
Leclercq, G. et al. JAK and mTOR inhibitors prevent cytokine release while retaining T cell bispecific antibody in vivo efficacy. J. Immunother. Cancer 10, e003766 (2022).
Kerbauy, L. N. et al. Combining AFM13, a bispecific CD30/CD16 antibody, with cytokine-activated blood and cord blood-derived NK cells facilitates CAR-like responses against CD30+ malignancies. Clin. Cancer Res. 27, 3744–3756 (2021). This paper reports the combination of the CD30–CD16-bispecific antibody AFM13 and pre-activation of blood-derived and cord blood-derived NK cells with IL-12, IL-15 and IL-18.
Nieto, Y. et al. Innate cell engager AFM13 combined with preactivated and expanded cord blood-derived NK cells for patients with double refractory CD30+ lymphoma. Blood Suppl. 140, 415–416 (2022).
Malik, H., Buelow, B., Rangaswamy, U., Balasubramani, A. & Schooten, W. V. TNB-486, a novel fully human bispecific CD19 × CD3 antibody that kills CD19-positive tumor cells with minimal cytokine secretion. Blood 134, 4070 (2019).
Izhak, L. et al. Potent antitumor activity of duvortuxizumab, a CD19 × CD3 DART® molecule, in lymphoma models. Cancer Res. Suppl. 13, 3636 (2017).
Topp, M. et al. Safety of AFM11 in the treatment of patients with B-cell malignancies: findings from two phase 1 studies. Trials 24, 4 (2023).
Kantarjian, H. et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N. Engl. J. Med. 376, 836–847 (2017).
Stein, A. S. et al. Blinatumomab for acute lymphoblastic leukemia relapse after allogeneic hematopoietic stem cell transplantation. Biol. Blood Marrow Transpl. 25, 1498–1504 (2019).
Frey, N. V. & Porter, D. L. Cytokine release syndrome with novel therapeutics for acute lymphoblastic leukemia. Hematol. Am. Soc. Hematol. Educ. Program 2016, 567–572 (2016).
Dimitriou, F. et al. Cytokine release syndrome during sequential treatment with immune checkpoint inhibitors and kinase inhibitors for metastatic melanoma. J. Immunother. 42, 29–32 (2019).
Coyle, L. et al. Open-label, phase 2 study of blinatumomab as second salvage therapy in adults with relapsed/refractory aggressive B-cell non-Hodgkin lymphoma. Leuk. Lymphoma 61, 2103–2112 (2020).
Gokbuget, N. et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood 131, 1522–1531 (2018).
Martinelli, G. et al. Complete hematologic and molecular response in adult patients with relapsed/refractory philadelphia chromosome-positive B-precursor acute lymphoblastic leukemia following treatment with blinatumomab: results from a phase II, single-arm, multicenter study. J. Clin. Oncol. 35, 1795–1802 (2017).
Topp, M. S. et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 16, 57–66 (2015).
Topp, M. S. et al. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J. Clin. Oncol. 32, 4134–4140 (2014).
Viardot, A. et al. Phase 2 study of the bispecific T-cell engager (BiTE) antibody blinatumomab in relapsed/refractory diffuse large B-cell lymphoma. Blood 127, 1410–1416 (2016).
Kohnke, T., Krupka, C., Tischer, J., Knosel, T. & Subklewe, M. Increase of PD-L1 expressing B-precursor ALL cells in a patient resistant to the CD19/CD3-bispecific T cell engager antibody blinatumomab. J. Hematol. Oncol. 8, 111 (2015).
Krupka, C. et al. Blockade of the PD-1/PD-L1 axis augments lysis of AML cells by the CD33/CD3 BiTE antibody construct AMG 330: reversing a T-cell-induced immune escape mechanism. Leukemia 30, 484–491 (2016).
Feucht, J. et al. T-cell responses against CD19+ pediatric acute lymphoblastic leukemia mediated by bispecific T-cell engager (BiTE) are regulated contrarily by PD-L1 and CD80/CD86 on leukemic blasts. Oncotarget 7, 76902–76919 (2016).
Yu, H. et al. Repeated loss of target surface antigen after immunotherapy in primary mediastinal large B cell lymphoma. Am. J. Hematol. 92, E11–E13 (2017).
Bukhari, A. et al. Rapid relapse of large B-cell lymphoma after CD19 directed CAR-T-cell therapy due to CD-19 antigen loss. Am. J. Hematol. 94, E273–E275 (2019).
Zhao, Y. Q. et al. Tumor-intrinsic and -extrinsic determinants of response to blinatumomab in adults with B-ALL. Blood 137, 471–484 (2021).
Nathan, P. et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N. Engl. J. Med. 385, 1196–1206 (2021). A phase III clinical trial evaluating tebentafusp, a bispecific (gp100 × CD3) ImmTAC molecule, in patients with metastatic uveal melanoma and showing longer overall survival than with the control therapy.
Nagele, V. et al. Changes in clinical laboratory parameters and pharmacodynamic markers in response to blinatumomab treatment of patients with relapsed/refractory ALL. Exp. Hematol. Oncol. 6, 14 (2017).
Carvajal, R. D. et al. Phase I study of safety, tolerability, and efficacy of tebentafusp using a step-up dosing regimen and expansion in patients with metastatic uveal melanoma. J. Clin. Oncol. 40, 1939–1948 (2022).
Budde, L. E. et al. Single-agent mosunetuzumab shows durable complete responses in patients with relapsed or refractory B-cell lymphomas: phase I dose-escalation study. J. Clin. Oncol. 40, 481–491 (2022).
Engelberts, P. J. et al. DuoBody-CD3×CD20 induces potent T-cell-mediated killing of malignant B cells in preclinical models and provides opportunities for subcutaneous dosing. EBioMedicine 52, 102625 (2020).
Martínez Sánchez, P. et al. Safety and efficacy of subcutaneous (SC) blinatumomab for the treatment of adults with relapsed or refractory B cell precursor acute lymphoblastic leukemia (R/R B-ALL). Blood 138, 2303 (2021).
Rossi, G. et al. A phase 1b study of blinatumomab including subcutaneous administration in relapsed/refractory (R/R) indolent non Hodgkin’s lymphoma (NHL). Blood 138, 2436 (2021).
Acknowledgements
The Vivier laboratory at the Centre d’Immunologie de Marseille-Luminy and Assistance-Publique-Hôpitaux de Marseille is supported by funding from the European Research Council under the European Union’s Horizon 2020 research and innovation program, the Agence Nationale de la Recherche, including the PIONEER Project, MSDAvenir, Innate Pharma and institutional grants awarded to the Centre d’Immunologie de Marseille-Luminy and Marseille Immunopôle.
Author information
Authors and Affiliations
Contributions
E.N.-M. and E.V. wrote the manuscript with the help of the other authors. A.F. designed the figures. All authors contributed to the revision of the manuscript.
Corresponding author
Ethics declarations
Competing interests
A.F., O.D., L.G. and E.V. are employees of Innate Pharma. E.N.-M. declares no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks M. Peipp and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fenis, A., Demaria, O., Gauthier, L. et al. New immune cell engagers for cancer immunotherapy. Nat Rev Immunol (2024). https://doi.org/10.1038/s41577-023-00982-7
Accepted:
Published:
DOI: https://doi.org/10.1038/s41577-023-00982-7