Abstract
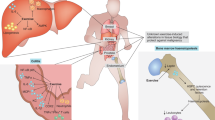
Regular physical activity is associated with lower cancer incidence and mortality, as well as with a lower rate of tumour recurrence. The epidemiological evidence is supported by preclinical studies in animal models showing that regular exercise delays the progression of cancer, including highly aggressive malignancies. Although the mechanisms underlying the antitumorigenic effects of exercise remain to be defined, an improvement in cancer immunosurveillance is likely important, with different immune cell subtypes stimulated by exercise to infiltrate tumours. There is also evidence that immune cells from blood collected after an exercise bout could be used as adoptive cell therapy for cancer. In this Perspective, we address the importance of muscular activity for maintaining a healthy immune system and discuss the effects of a single bout of exercise (that is, ‘acute’ exercise) and those of ‘regular’ exercise (that is, repeated bouts) on anticancer immunity, including tumour infiltrates. We also address the postulated mechanisms and the clinical implications of this emerging area of research.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
01 February 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41577-024-00999-6
References
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
GBD 2019 Cancer Risk Factors Collaborators. The global burden of cancer attributable to risk factors, 2010–19: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 400, 563–591 (2022).
Guthold, R., Stevens, G. A., Riley, L. M. & Bull, F. C. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1.9 million participants. Lancet Glob. Health 6, e1077–e1086 (2018).
Bull, F. C. et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 54, 1451–1462 (2020).
Garcia-Hermoso, A. et al. Adherence to aerobic and muscle-strengthening activities guidelines: a systematic review and meta-analysis of 3.3 million participants across 32 countries. Br. J. Sports Med. 57, 225–229 (2023).
Moore, S. C. et al. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern. Med. 176, 816–825 (2016).
Matthews, C. E. et al. Amount and intensity of leisure-time physical activity and lower cancer risk. J. Clin. Oncol. 38, 686–697 (2020).
Ahmadi, M. N. et al. Vigorous physical activity, incident heart disease, and cancer: how little is enough? Eur. Heart J. 43, 4801–4814 (2022).
Morishita, S. et al. Effect of exercise on mortality and recurrence in patients with cancer: a systematic review and meta-analysis. Integr. Cancer Ther. 19, 1534735420917462 (2020).
Friedenreich, C. M., Stone, C. R., Cheung, W. Y. & Hayes, S. C. Physical activity and mortality in cancer survivors: a systematic review and meta-analysis. JNCI Cancer Spectr. 4, pkz080 (2019).
Arem, H. et al. Leisure time physical activity and mortality: a detailed pooled analysis of the dose-response relationship. JAMA Intern. Med. 175, 959–967 (2015).
Larrabee, R. C. Leucocytosis after violent exercise. J. Med. Res. 7, 76–82 (1902).
Nieman, D. C. & Wentz, L. M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 8, 201–217 (2019).
Simpson, R. J., Bigley, A. B., Agha, N., Hanley, P. J. & Bollard, C. M. Mobilizing immune cells with exercise for cancer immunotherapy. Exerc. Sport Sci. Rev. 45, 163–172 (2017).
Simpson, R. J. et al. Human cytomegalovirus infection and the immune response to exercise. Exerc. Immunol. Rev. 22, 8–27 (2016).
Simpson, R. J., Kunz, H., Agha, N. & Graff, R. Exercise and the regulation of immune functions. Prog. Mol. Biol. Transl. Sci. 135, 355–380 (2015).
Anane, L. H. et al. Mobilization of gammadelta T lymphocytes in response to psychological stress, exercise, and beta-agonist infusion. Brain Behav. Immun. 23, 823–829 (2009).
Steppich, B. et al. Selective mobilization of CD14(+)CD16(+) monocytes by exercise. Am. J. Physiol. Cell Physiol. 279, C578–C586 (2000).
Peake, J. et al. Changes in neutrophil surface receptor expression, degranulation, and respiratory burst activity after moderate- and high-intensity exercise. J. Appl. Physiol. 97, 612–618 (2004).
Fiuza-Luces, C., Valenzuela, P. L., Castillo-García, A. & Lucia, A. Exercise benefits meet cancer immunosurveillance: implications for immunotherapy. Trends Cancer 7, 91–93 (2021).
Zuazo, M. et al. Functional systemic CD4 immunity is required for clinical responses to PD-L1/PD-1 blockade therapy. EMBO Mol. Med. 11, e10293 (2019).
Spitzer, M. H. et al. Systemic immunity is required for effective cancer immunotherapy. Cell 168, 487–502 (2017).
Gustafson, M. P. et al. Immune monitoring using the predictive power of immune profiles. J. Immunother. Cancer 1, 7 (2013).
Chow, L. S. et al. Exerkines in health, resilience and disease. Nat. Rev. Endocrinol. 18, 273–289 (2022).
Gustafson, M. P. et al. Exercise and the immune system: taking steps to improve responses to cancer immunotherapy. J. Immunother. Cancer 9, e001872 (2021).
Fischer, C. P. Interleukin-6 in acute exercise and training: what is the biological relevance? Exerc. Immunol. Rev. 12, 6–33 (2006).
Steensberg, A., Fischer, C. P., Keller, C., Moller, K. & Pedersen, B. K. IL-6 enhances plasma IL-1RA, IL-10, and cortisol in humans. Am. J. Physiol. Endocrinol. Metab. 285, E433–E437 (2003).
Starkie, R., Ostrowski, S. R., Jauffred, S., Febbraio, M. & Pedersen, B. K. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-α production in humans. FASEB J. 17, 884–886 (2003).
Pedersen, B. K. & Febbraio, M. A. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol. Rev. 88, 1379–1406 (2008).
Bay, M. L. et al. Human immune cell mobilization during exercise: effect of IL-6 receptor blockade. Exp. Physiol. 105, 2086–2098 (2020).
Pedersen, L. et al. Voluntary running suppresses tumor growth through epinephrine- and IL-6-dependent NK cell mobilization and redistribution. Cell Metab. 23, 554–562 (2016). This elegant, mechanistic study shows the involvement of two exerkines, epinephrine and IL-6, on NK-cell mobilization into several tumour types.
Quinn, L. S., Haugk, K. L. & Grabstein, K. H. Interleukin-15: a novel anabolic cytokine for skeletal muscle. Endocrinology 136, 3669–3672 (1995).
Haugen, F. et al. IL-7 is expressed and secreted by human skeletal muscle cells. Am. J. Physiol. Cell Physiol. 298, C807–C816 (2010).
Nielsen, A. R. et al. Expression of interleukin-15 in human skeletal muscle effect of exercise and muscle fibre type composition. J. Physiol. 584, 305–312 (2007).
Tamura, Y. et al. Upregulation of circulating IL-15 by treadmill running in healthy individuals: is IL-15 an endocrine mediator of the beneficial effects of endurance exercise? Endocr. J. 58, 211–225 (2011).
Capitini, C. M., Chisti, A. A. & Mackall, C. L. Modulating T-cell homeostasis with IL-7: preclinical and clinical studies. J. Intern. Med. 266, 141–153 (2009).
Fry, T. J. & Mackall, C. L. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J. Immunol. 174, 6571–6576 (2005).
Goldrath, A. W. et al. Cytokine requirements for acute and basal homeostatic proliferation of naïve and memory CD8+ T cells. J. Exp. Med. 195, 1515–1522 (2002).
Wu, J. et al. Skeletal muscle antagonizes antiviral CD8+ T cell exhaustion. Sci. Adv. 6, eaba3458 (2020). This study provides a mechanistic link between two seemingly isolated events that are prevalent in the context of cancer — loss of muscle mass and T cell exhaustion — and shows the importance of skeletal muscle preservation to protect the proliferative potential of these cells.
Wallace, D. L. et al. Prolonged exposure of naïve CD8+ T cells to interleukin-7 or interleukin-15 stimulates proliferation without differentiation or loss of telomere length. Immunology 119, 243–253 (2006).
Cieri, N. et al. Il-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood 121, 573–584 (2013).
Campbell, K. L. et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med. Sci. Sports Exerc. 51, 2375–2390 (2019).
Yao, J. et al. Human double negative T cells target lung cancer via ligand-dependent mechanisms that can be enhanced by IL-15. J. Immunother. Cancer 7, 17 (2019).
Van Acker, H. H. et al. Interleukin-15 enhances the proliferation, stimulatory phenotype, and antitumor effector functions of human gamma delta T cells. J. Hematol. Oncol. 9, 101 (2016).
Xu, Y. et al. Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15. Blood 123, 3750–3759 (2014).
Kochenderfer, J. N. et al. Lymphoma remissions caused by anti-CD19 chimeric antigen receptor T cells are associated with high serum interleukin-15 levels. J. Clin. Oncol. 35, 1803–1813 (2017).
Kurz, E. et al. Exercise-induced engagement of the IL-15/IL-15Rα axis promotes anti-tumor immunity in pancreatic cancer. Cancer Cell 40, 720–737.e5 (2022). This mechanistic preclinical and clinical study highlights the therapeutic potential of regular exercise against one of the deadliest malignancies, pancreatic ductal adenocarcinoma, through IL-15-mediated activation of CD8+ T cells.
Nelke, C., Dziewas, R., Minnerup, J., Meuth, S. G. & Ruck, T. Skeletal muscle as potential central link between sarcopenia and immune senescence. eBioMedicine 49, 381–388 (2019).
López-Otín, C., Pietrocola, F., Roiz-Valle, D., Galluzzi, L. & Kroemer, G. Meta-hallmarks of aging and cancer. Cell Metab. 35, 12–35 (2023).
Izquierdo, M., Morley, J. E. & Lucia, A. Exercise in people over 85. BMJ 368, m402 (2020).
Fiuza-Luces, C. et al. Exercise benefits in cardiovascular disease: beyond attenuation of traditional risk factors. Nat. Rev. Cardiol. 15, 731–743 (2018).
Furman, D. et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 25, 1822–1832 (2019).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. Hallmarks of aging: an expanding universe. Cell 186, 243–278 (2023).
Michels, N., van Aart, C., Morisse, J., Mullee, A. & Huybrechts, I. Chronic inflammation towards cancer incidence: a systematic review and meta-analysis of epidemiological studies. Crit. Rev. Oncol. Hematol. 157, 103177 (2021).
Ridker, P. M. et al. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 390, 1833–1842 (2017).
Fane, M. & Weeraratna, A. T. How the ageing microenvironment influences tumour progression. Nat. Rev. Cancer 20, 89–106 (2020).
Simpson, R. J. et al. Exercise and adrenergic regulation of immunity. Brain Behav. Immun. 97, 303–318 (2021).
Campbell, J. P. & Turner, J. E. Debunking the myth of exercise-induced immune suppression: redefining the impact of exercise on immunological health across the lifespan. Front. Immunol. 9, 648 (2018).
Campbell, J. P. et al. Acute exercise mobilises CD8+ T lymphocytes exhibiting an effector-memory phenotype. Brain Behav. Immun. 23, 767–775 (2009).
Bigley, A. B. et al. Acute exercise preferentially redeploys NK-cells with a highly differentiated phenotype and augments cytotoxicity against lymphoma and multiple myeloma target cells. Brain Behav. Immun. 39, 160–171 (2014). This is a pioneering study showing that acute exercise may serve as a simple strategy to enrich the blood compartment of highly cytotoxic NK-cell subsets that can be harvested for clinical use (adoptive transfer immunotherapy).
Batatinha, H. et al. Human lymphocytes mobilized with exercise have an anti-tumor transcriptomic profile and exert enhanced graft-versus-leukemia effects in xenogeneic mice. Front. Immunol. 14, 1067369 (2023).
Zúñiga, T. M. et al. Acute exercise mobilizes NKT-like cells with a cytotoxic transcriptomic profile but does not augment the potency of cytokine-induced killer (CIK) cells. Front. Immunol. 13, 938106 (2022).
Turner, J. E. et al. Exercise-induced B cell mobilisation: preliminary evidence for an influx of immature cells into the bloodstream. Physiol. Behav. 164, 376–382 (2016).
Shephard, R. J. Adhesion molecules, catecholamines and leucocyte redistribution during and following exercise. Sports Med. 33, 261–284 (2003).
Graff, R. M. et al. β2-Adrenergic receptor signaling mediates the preferential mobilization of differentiated subsets of CD8+ T-cells, NK-cells and non-classical monocytes in response to acute exercise in humans. Brain Behav. Immun. 74, 143–153 (2018).
Dimitrov, S., Lange, T. & Born, J. Selective mobilization of cytotoxic leukocytes by epinephrine. J. Immunol. 184, 503–511 (2010).
Rehman, J. et al. Dynamic exercise leads to an increase in circulating ICAM-1: further evidence for adrenergic modulation of cell adhesion. Brain Behav. Immun. 11, 343–351 (1997).
Goossens, G. H. et al. Short-term beta-adrenergic regulation of leptin, adiponectin and interleukin-6 secretion in vivo in lean and obese subjects. Diabetes Obes. Metab. 10, 1029–1038 (2008).
Kruger, K., Lechtermann, A., Fobker, M., Volker, K. & Mooren, F. C. Exercise-induced redistribution of T lymphocytes is regulated by adrenergic mechanisms. Brain Behav. Immun. 22, 324–338 (2008). This is a study in rodents that elegantly shows (using fluorescent cell tracking) a redistribution of T cells from the spleen to target organs (the lung, bone marrow and gut (Peyer’s patches)) in the 24 hours after an acute exercise bout.
Baker, F. L. et al. Systemic β-adrenergic receptor activation augments the ex vivo expansion and anti-tumor activity of Vγ9Vδ2 T-cells. Front. Immunol. 10, 3082 (2020).
Kruger, K. & Mooren, F. C. T cell homing and exercise. Exerc. Immunol. Rev. 13, 37–54 (2007).
Kruger, K. et al. Apoptosis of T-cell subsets after acute high-intensity interval exercise. Med. Sci. Sports Exerc. 48, 2021–2029 (2016).
Simpson, R. J. Aging, persistent viral infections, and immunosenescence: can exercise “make space”? Exerc. Sport Sci. Rev. 39, 23–33 (2011).
Schenk, A. et al. Distinct distribution patterns of exercise-induced natural killer cell mobilization into the circulation and tumor tissue of patients with prostate cancer. Am. J. Physiol. Cell Physiol. 323, C879–C884 (2022).
Schauer, T., Djurhuus, S. S., Simonsen, C., Brasso, K. & Christensen, J. F. The effects of acute exercise and inflammation on immune function in early-stage prostate cancer. Brain Behav. Immun. Health 25, 100508 (2022).
Djurhuus, S. S. et al. Effects of acute exercise training on tumor outcomes in men with localized prostate cancer: a randomized controlled trial. Physiol. Rep. 10, e15408 (2022).
Djurhuus, S. S. et al. Exercise training to increase tumour natural killer-cell infiltration in men with localised prostate cancer: a randomised controlled trial. BJU Int. 131, 116–124 (2023). This paper is a clinical trial showing that regular, intense exercise can increase NK cell infiltration in prostate tumours.
Thienger, P. & Rubin, M. A. Prostate cancer hijacks the microenvironment. Nat. Cell Biol. 23, 3–5 (2021).
Martori, C. et al. Macrophages as a therapeutic target in metastatic prostate cancer: a way to overcome immunotherapy resistance? Cancers 14, 440 (2022).
Clifford, B. K., Kaakoush, N. O., Tedla, N., Goldstein, D. & Simar, D. The effect of exercise intensity on the inflammatory profile of cancer survivors: a randomized crossover study. Eur. J. Clin. Invest. 53, e13984 (2023).
Valenzuela, P. L. et al. Exercise training and natural killer cells in cancer survivors: current evidence and research gaps based on a systematic review and meta-analysis. Sports Med. Open 8, 36 (2022).
Coletta, A. M. et al. The impact of high-intensity interval exercise training on NK-cell function and circulating myokines for breast cancer prevention among women at high risk for breast cancer. Breast Cancer Res. Treat. 187, 407–416 (2021).
Llavero, F. et al. Exercise training effects on natural killer cells: a preliminary proteomics and systems biology approach. Exerc. Immunol. Rev. 27, 125–141 (2021).
MacDonald, G. et al. A pilot study of high-intensity interval training in older adults with treatment naïve chronic lymphocytic leukemia. Sci. Rep. 11, 3137 (2021).
Toffoli, E. C. et al. Effects of physical exercise on natural killer cell activity during (neo)adjuvant chemotherapy: a randomized pilot study. Physiol. Rep. 9, e14919 (2021).
Mace, E. M. Phosphoinositide-3-kinase signaling in human natural killer cells: new insights from primary immunodeficiency. Front. Immunol. 9, 445 (2018).
Takahashi, N. et al. Tumor marker nucleoporin 88 kDa regulates nucleocytoplasmic transport of NF-kappaB. Biochem. Biophys. Res. Commun. 374, 424–430 (2008).
Spielmann, G. et al. Aerobic fitness is associated with lower proportions of senescent blood T-cells in man. Brain Behav. Immun. 25, 1521–1529 (2011).
Himbert, C. et al. Differences in the gut microbiome by physical activity and BMI among colorectal cancer patients. Am. J. Cancer Res. 12, 4789–4801 (2022).
O’Sullivan, O. et al. Exercise and the microbiota. Gut Microbes 6, 131–136 (2015).
Clarke, S. F. et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 63, 1913–1920 (2014).
Lozupone, C. A., Stombaugh, J. I., Gordon, J. I., Jansson, J. K. & Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 489, 220–230 (2012).
Wirbel, J. et al. Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for colorectal cancer. Nat. Med. 25, 679–689 (2019).
Routy, B. et al. The gut microbiota influences anticancer immunosurveillance and general health. Nat. Rev. Clin. Oncol. 15, 382–396 (2018).
Park, E. M. et al. Targeting the gut and tumor microbiota in cancer. Nat. Med. 28, 690–703 (2022).
Gopalakrishnan, V. et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359, 97–103 (2018).
Wang, B. et al. Synergetic inhibition of daidzein and regular exercise on breast cancer in bearing-4T1 mice by regulating NK cells and apoptosis pathway. Life Sci. 245, 117387 (2020).
Dufresne, S. et al. Exercise training improves radiotherapy efficiency in a murine model of prostate cancer. FASEB J. 34, 4984–4996 (2020).
Laskowski, T. J., Biederstädt, A. & Rezvani, K. Natural killer cells in antitumour adoptive cell immunotherapy. Nat. Rev. Cancer 22, 557–575 (2022).
Di Vito, C. et al. NK cells to cure cancer. Semin. Immunol. 41, 101272 (2019).
Huntington, N. D., Cursons, J. & Rautela, J. The cancer–natural killer cell immunity cycle. Nat. Rev. Cancer 20, 437–454 (2020).
López-Soto, A., Gonzalez, S., Smyth, M. J. & Galluzzi, L. Control of metastasis by NK cells. Cancer Cell 32, 135–154 (2017).
Li, B., Jiang, Y., Li, G., Fisher, G. A. & Li, R. Natural killer cell and stroma abundance are independently prognostic and predict gastric cancer chemotherapy benefit. JCI Insight 5, e136570 (2020).
Lee, H. et al. Integrated molecular and immunophenotypic analysis of NK cells in anti-PD-1 treated metastatic melanoma patients. Oncoimmunology 8, e1537581 (2019).
Cursons, J. et al. A gene signature predicting natural killer cell infiltration and improved survival in melanoma patients. Cancer Immunol. Res. 7, 1162–1174 (2019).
Buss, L. A. et al. Effects of exercise and anti-PD-1 on the tumour microenvironment. Immunol. Lett. 239, 60–71 (2021).
Garritson, J. et al. Physical activity delays accumulation of immunosuppressive myeloid-derived suppressor cells. PLoS ONE 15, e0234548 (2020).
Wennerberg, E. et al. Exercise reduces immune suppression and breast cancer progression in a preclinical model. Oncotarget 11, 452–461 (2020).
Mengos, A. E., Gastineau, D. A. & Gustafson, M. P. The CD14+HLA-DRlo/neg monocyte: an immunosuppressive phenotype that restrains responses to cancer immunotherapy. Front. Immunol. 10, 1147 (2019).
Kim, I. S. et al. Immuno-subtyping of breast cancer reveals distinct myeloid cell profiles and immunotherapy resistance mechanisms. Nat. Cell Biol. 21, 1113–1126 (2019).
Weber, R. et al. Myeloid-derived suppressor cells hinder the anti-cancer activity of immune checkpoint inhibitors. Front. Immunol. 9, 1310 (2018).
Martín-Ruiz, A. et al. Benefits of exercise and immunotherapy in a murine model of human non-small-cell lung carcinoma. Exerc. Immunol. Rev. 26, 100–115 (2020).
Bay, M. L. et al. Voluntary wheel running can lead to modulation of immune checkpoint molecule expression. Acta Oncol. 59, 1447–1454 (2020).
Denton, N. L., Chen, C. Y., Scott, T. R. & Cripe, T. P. Tumor-associated macrophages in oncolytic virotherapy: friend or foe? Biomedicines 4, 13 (2016).
Rolny, C. et al. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell 19, 31–44 (2011).
Ruffell, B., Affara, N. I. & Coussens, L. M. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 33, 119–126 (2012).
Goh, J. et al. Exercise training in transgenic mice is associated with attenuation of early breast cancer growth in a dose-dependent manner. PLoS ONE 8, e80123 (2013).
McClellan, J. L. et al. Exercise effects on polyp burden and immune markers in the ApcMin/+ mouse model of intestinal tumorigenesis. Int. J. Oncol. 45, 861–868 (2014).
Ge, Z., Wu, S., Qi, Z. & Ding, S. Exercise modulates polarization of TAMs and expression of related immune checkpoints in mice with lung cancer. J. Cancer 13, 3297–3307 (2022).
Lamkin, D. M. et al. Physical activity modulates mononuclear phagocytes in mammary tissue and inhibits tumor growth in mice. PeerJ 9, e10725 (2021).
Castanedo-Rincón, C. et al. Combined exercise intervention in a mouse model of high-risk neuroblastoma: effects on physical, immune, tumor and clinical outcomes. Exerc. Immunol. Rev. 29, 86–110 (2023).
Singh, G. & Singh, S. M. Role of host’s antitumor immunity in exercise-dependent regression of murine T-cell lymphoma. Comp. Immunol. Microbiol. Infect. Dis. 28, 231–248 (2005).
Broz, M. L. et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell 26, 638–652 (2014).
Ruffell, B. et al. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell 26, 623–637 (2014).
Layer, J. P. et al. Amplification of N-Myc is associated with a T-cell-poor microenvironment in metastatic neuroblastoma restraining interferon pathway activity and chemokine expression. Oncoimmunology 6, e1320626 (2017).
Zafari, R., Razi, S. & Rezaei, N. The role of dendritic cells in neuroblastoma: implications for immunotherapy. Immunobiology 227, 152293 (2022).
Martín-Ruiz, A. et al. Effects of anti-PD-1 immunotherapy on tumor regression: insights from a patient-derived xenograft model. Sci. Rep. 10, 7078 (2020).
Hagar, A. et al. Endurance training slows breast tumor growth in mice by suppressing Treg cells recruitment to tumors. BMC Cancer 9, 536 (2019).
Gomes-Santos, I. L. et al. Exercise training improves tumor control by increasing CD8+ T-cell infiltration via CXCR3 signaling and sensitizes breast cancer to immune checkpoint blockade. Cancer Immunol. Res. 9, 765–778 (2021).
Rundqvist, H. et al. Cytotoxic T-cells mediate exercise-induced reductions in tumor growth. eLife 9, e59996 (2020). Together with Gomes-Santos et al., this paper provides preclinical support for an increase in the anticancer effector function of CD8+ T cells with regular exercise.
Feng, Q. et al. Lactate increases stemness of CD8+ T cells to augment anti-tumor immunity. Nat. Commun. 13, 4981 (2022).
Kaymak, I. et al. Carbon source availability drives nutrient utilization in CD8+ T cells. Cell Metab. 34, 1298–1311.e6 (2022).
Barbieri, L. et al. Lactate exposure shapes the metabolic and transcriptomic profile of CD8+ T cells. Front. Immunol. 14, 1101433 (2023).
Balachandran, V. P., Beatty, G. L. & Dougan, S. K. Broadening the impact of immunotherapy to pancreatic cancer: challenges and opportunities. Gastroenterology 156, 2056–2072 (2019).
Bear, A. S., Vonderheide, R. H. & O’Hara, M. H. Challenges and opportunities for pancreatic cancer immunotherapy. Cancer Cell 38, 788–802 (2020).
Gupta, P. et al. Comparison of three exercise interventions with and without gemcitabine treatment on pancreatic tumor growth in mice: no impact on tumor infiltrating lymphocytes. Front. Physiol. 13, 1039988 (2022).
Laumont, C. M. & Nelson, B. H. B cells in the tumor microenvironment: multi-faceted organizers, regulators, and effectors of anti-tumor immunity. Cancer Cell 41, 466–489 (2023).
Wieland, A. et al. Defining HPV-specific B cell responses in patients with head and neck cancer. Nature 597, 274–278 (2021).
Cabrita, R. et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 577, 561–565 (2020).
Petitprez, F. et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 577, 556–560 (2020).
Zitvogel, L., Tesniere, A. & Kroemer, G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat. Rev. Immunol. 10, 715–727 (2006).
Rodríguez-Cañamero, S. et al. Impact of physical exercise in advanced-stage cancer patients: systematic review and meta-analysis. Cancer Med. 11, 3714–3727 (2022).
Schmitz, K. H. et al. Exercise is medicine in oncology: engaging clinicians to help patients move through cancer. CA Cancer J. Clin. 69, 468–484 (2019).
Siversten, I. & Dahlstrom, A. W. Relation of muscular activity to carcinoma: a preliminary report. J. Cancer Res. 6, 365–378 (1921).
Rusch, H. P. & Kline, B. E. The effect of exercise on the growth of a mouse tumor. Cancer Res. 4, 116–118 (1944).
Newton, G. Tumor susceptibility in rats: role of infantile manipulation and later exercise. Psychol. Rep. 16, 127–132 (1965).
Deuster, P. A., Morrison, S. D. & Ahrens, R. A. Endurance exercise modifies cachexia of tumor growth in rats. Med. Sci. Sports Exerc. 17, 385–392 (1985).
MacNeil, B. & Hoffman-Goetz, L. Exercise training and tumour metastasis in mice: influence of time of exercise onset. Anticancer Res. 13, 2085–2088 (1993).
Virchow, R. Cellular pathology. As based upon physiological and pathological histology. Lecture XVI — atheromatous affection of arteries. 1858. Nutr. Rev. 47, 23–25 (1989).
Starnes, C. O. Coley’s toxins. Nature 360, 23 (1992).
Starnes, C. O. Coley’s toxins in perspective. Nature 357, 11–12 (1992).
MacNeil, B. & Hoffman-Goetz, L. Effect of exercise on natural cytotoxicity and pulmonary tumor metastases in mice. Med. Sci. Sports Exerc. 25, 922–928 (1993). This preclinical study investigates on whether regular exercise improves anticancer function (as assessed with splenic NK cell cytotoxic activity).
Hutt, D. Feasibility of leukapheresis for CAR T-cell production in heavily pre-treated pediatric patients. Transfus. Apher. Sci. 59, 102769 (2020).
Korell, F. et al. Current challenges in providing good leukapheresis products for manufacturing of CAR-T cells for patients with relapsed/refractory NHL or ALL. Cells 9, 1225 (2020).
Tuazon, S. A. et al. Factors affecting lymphocyte collection efficiency for the manufacture of chimeric antigen receptor T cells in adults with B-cell malignancies. Transfusion 59, 1773–1780 (2019).
Allen, E. S. et al. Autologous lymphapheresis for the production of chimeric antigen receptor T cells. Transfusion 57, 1133–1141 (2017).
Hont, A. B. et al. The generation and application of antigen-specific T cell therapies for cancer and viral-associated disease. Mol. Ther. 30, 2130–2152 (2022).
LaVoy, E. C. et al. A single bout of dynamic exercise enhances the expansion of MAGE-A4 and PRAME-specific cytotoxic T-cells from healthy adults. Exerc. Immunol. Rev. 21, 144–153 (2015).
Acknowledgements
The authors are grateful to K. McCreath for helpful comments on the text. Research by A.L. and C.F.-L. in exercise and cancer is funded by the Wereld Kanker Onderzoek Fonds (WKOF), as part of the World Cancer Research Fund International grant programme (grant number IIG_FULL_2021_007), and the Spanish Ministry of Science and Innovation (Fondo de Investigaciones Sanitarias (FIS)) and Fondos FEDER (grant numbers PI18/00139 and PI20/00645), and European Union’s Horizon 2020 research and innovation programme under grant agreement number 945153. Research by C.F.-L. is funded by a Miguel Servet postdoctoral contract granted by Instituto de Salud Carlos III (CP18/00034). Research by P.L.V. is funded by a Sara Borrell postdoctoral contract granted by Instituto de Salud Carlos III (CD21/00138).
Author information
Authors and Affiliations
Contributions
A.L. wrote the first manuscript draft with the help of C.F.-L. All authors researched data for the article, contributed to the discussion of content, and also reviewed and edited the article in depth before submission. B.G.G. and C.F.-L. made the figures.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Peer review
Peer review information
Nature Reviews Immunology thanks Bente Klarlund Pedersen, Karsten Krueger and John P. Campbell for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Exercise (or ‘exercise training’)
-
A form of structured leisure-time physical activity with the purpose of improving or maintaining health — training for a 10-km running race, or resistance training (for example, weight lifting) to increase muscle mass. Although physical activity and exercise are often used interchangeably, the bulk of observational epidemiological evidence is based on physical activity data, whereas exercise is frequently used in intervention trials and preclinical studies.
- Physical activity
-
Any bodily movement produced by skeletal muscles that requires energy expenditure and includes the domains of occupational, domestic, transportation and leisure time (such as walking to work or walking the dog).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fiuza-Luces, C., Valenzuela, P.L., Gálvez, B.G. et al. The effect of physical exercise on anticancer immunity. Nat Rev Immunol 24, 282–293 (2024). https://doi.org/10.1038/s41577-023-00943-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-023-00943-0