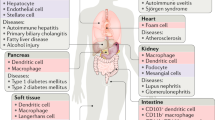
Abstract
Cells can die as a consequence of being phagocytosed by other cells — a form of cell death that has been called phagotrophy, cell cannibalism, programmed cell removal and primary phagocytosis. However, these are all different manifestations of cell death by phagocytosis (termed ‘phagoptosis’ for short). The engulfed cells die as a result of cytotoxic oxidants, peptides and degradative enzymes within acidic phagolysosomes. Cell death by phagocytosis was discovered by Metchnikov in the 1880s, but was neglected until recently. It is now known to contribute to developmental cell death in nematodes, Drosophila and mammals, and is central to innate and adaptive immunity against pathogens. Cell death by phagocytosis mediates physiological turnover of erythrocytes and other leucocytes, making it the most abundant form of cell death in the mammalian body. Immunity against cancer is also partly mediated by macrophage phagocytosis of cancer cells, but cancer cells can also phagocytose host cells and other cancer cells in order to survive. Recent evidence indicates neurodegeneration and other neuropathologies can be mediated by microglial phagocytosis of stressed neurons. Thus, despite cell death by phagocytosis being poorly recognized, it is one of the oldest, commonest and most important forms of cell death.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Uribe-Querol, E. & Rosales, C. Phagocytosis: our current understanding of a universal biological process. Front. Immunol. 11, 1066 (2020).
Kay, M. M. Mechanism of removal of senescent cells by human macrophages in situ. Proc. Natl Acad. Sci. USA 72, 3521–3525 (1975).
Khandelwal, S., van Rooijen, N. & Saxena, R. K. Reduced expression of CD47 during murine red blood cell (RBC) senescence and its role in RBC clearance from the circulation. Transfusion 47, 1725–1732 (2007).
Feng, M. et al. Phagocytosis checkpoints as new targets for cancer immunotherapy. Nat. Rev. Cancer 19, 568–586 (2019).
Jauslin, T. et al. How phagocytic cells kill different bacteria: a quantitative analysis using Dictyostelium discoideum. mBio 12, e03169-20 (2021).
Metchnikoff, E. in The Evolutionary Biology Papers of Elie Metchnikoff (eds Gourko, H. et al.) (Springer, 2000).
Mills, D. B. The origin of phagocytosis in earth history interface focus. Interface Focus 10, 20200019 (2020).
Sebé-Pedrós, A., Degnan, B. & Ruiz-Trillo, I. The origin of Metazoa: a unicellular perspective. Nat. Rev. Genet. 18, 498–512 (2017).
Xu, X., Pan, M. & Jin, T. How phagocytes acquired the capability of hunting and removing pathogens from a human body: lessons learned from chemotaxis and phagocytosis of Dictyostelium discoideum. Front. Cell Dev. Biol. 9, 724940 (2021).
Leander, B. S. Predatory protists. Curr. Biol. 30, R510–R516 (2020).
Jagus, R., Bachvaroff, T. R., Joshi, B. & Place, A. R. Diversity of eukaryotic translational initiation factor eIF4E in protists. Comp. Funct. Genomics 2012, 134839 (2012).
Sherr, E. B. & Sherr, B. F. in Aquatic Microbial Ecology and Biogeochemistry: A Dual Perspective (eds Glibert P., Kana T.) (Springer, 2016).
Serizier, S. B., Peterson, J. S. & McCall, K. Non-autonomous cell death induced by the Draper phagocytosis receptor requires signaling through the JNK and SRC pathways. J. Cell Sci. 135, jcs250134 (2022).
Timmons, A. K. et al. Phagocytosis genes nonautonomously promote developmental cell death in the Drosophila ovary. Proc. Natl Acad. Sci. USA 113, E1246–E1255 (2016).
Hakim-Mishnaevski, K., Flint-Brodsly, N., Shklyar, B., Levy-Adam, F. & Kurant, E. Glial phagocytic receptors promote neuronal loss in adult Drosophila brain. Cell Rep. 29, 1438–1448.e3 (2019).
Zohar-Fux, M. et al. The phagocytic cyst cells in Drosophila testis eliminate germ cell progenitors via phagoptosis. Sci. Adv. 8, eabm4937 (2022).
Reddien, P., Cameron, S. & Horvitz, H. R. Phagocytosis promotes programmed cell death in C. elegans. Nature 412, 198–202 (2001).
Hoeppner, D. J., Hengartner, M. O. & Schnabel, R. Engulfment genes cooperate with ced-3 to promote cell death in Caenorhabditis elegans. Nature 412, 202–206 (2001). Together with Reddien et al. (2001), this study shows that phagoptosis contributes to developmental cell death in C. elegans. Note that Horvitz was awarded the Nobel prize for elucidating apoptosis in C. elegans.
Johnsen, H. L. & Horvitz, H. R. Both the apoptotic suicide pathway and phagocytosis are required for a programmed cell death in Caenorhabditis elegans. BMC Biol. 14, 39 (2016).
Walker, C., Lesser, P. & Unuma, T. Sea urchin gametogenesis — structural, functional and molecular/genomic biology. Dev. Aquac. Fish. Sci. 38, 25–43 (2013).
Galluzzi, L. et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 25, 486–541 (2018).
Brown, G. C., Vilalta, A. & Fricker, M. Phagoptosis — cell death by phagocytosis — plays central roles in physiology, host defense and pathology. Curr. Mol. Med. 15, 842–851 (2015).
Boada-Romero, E., Martinez, J., Heckmann, B. L. & Green, D. R. The clearance of dead cells by efferocytosis. Nat. Rev. Mol. Cell Biol. 21, 398–414 (2020).
Neher, J. J. et al. Inhibition of microglial phagocytosis is sufficient to prevent inflammatory neuronal death. J. Immunol. 186, 4973–4983 (2011). This study shows that microglial phagocytosis of neurons causes neuronal death in inflammatory conditions, and elucidates the signalling involved.
Neniskyte, U., Neher, J. J. & Brown, G. C. Neuronal death induced by nanomolar amyloid β is mediated by primary phagocytosis of neurons by microglia. J. Biol. Chem. 286, 39904–39913 (2011).
Overholtzer, M. et al. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell 131, 966–979 (2007).
Chao, M. P., Majeti, R. & Weissman, I. L. Programmed cell removal: a new obstacle in the road to developing cancer. Nat. Rev. Cancer 12, 58–67 (2011).
Malorni, W., Matarrese, P., Tinari, A., Farrace, M. G. & Piacentini, M. Xeno-cannibalism: a survival “escamotage”. Autophagy 3, 75–77 (2007).
Lozupone, F. & Fais, S. Cancer cell cannibalism: a primeval option to survive. Curr. Mol. Med. 15, 836–841 (2015).
Cano, C. E. et al. Homotypic cell cannibalism, a cell-death process regulated by the nuclear protein 1, opposes to metastasis in pancreatic cancer. EMBO Mol. Med. 13, e14243 (2021).
Lugini, L. et al. Cannibalism of live lymphocytes by human metastatic but not primary melanoma cells. Cancer Res. 66, 3629–3638 (2006).
Marín-Teva, J. L. et al. Microglia promote the death of developing Purkinje cells. Neuron 41, 535–547 (2004).
Wang, S. et al. Internalization of NK cells into tumor cells requires ezrin and leads to programmed cell-in-cell death. Cell Res. 19, 1350–1362 (2009).
Hornik, T. C., Vilalta, A. & Brown, G. C. Activated microglia cause reversible apoptosis of pheochromocytoma cells, inducing their cell death by phagocytosis. J. Cell Sci. 129, 65–79 (2016).
Taban, Q., Mumtaz, P. T., Masoodi, K. Z., Haq, E. & Ahmad, S. M. Scavenger receptors in host defense: from functional aspects to mode of action. Cell Commun. Signal. 20, 2 (2022).
Cockram, T. O., Dundee, J. M., Popescu, A. S. & Brown, G. C. The phagocytic code regulating phagocytosis of mammalian cells. Front. Immunol. 12, 629979 (2021).
Ogden, C. A. et al. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J. Exp. Med. 194, 781–795 (2001).
Martel, C. M. Conceptual bases for prey biorecognition and feeding selectivity in the microplanktonic marine phagotroph Oxyrrhis marina. Microb. Ecol. 57, 589–597 (2009).
Oldenborg, P. A. et al. Role of CD47 as a marker of self on red blood cells. Science 288, 2051–2054 (2000).
Ishidome, T., Yoshida, T. & Hanayama, R. Induction of live cell phagocytosis by a specific combination of inflammatory stimuli. EBioMedicine 22, 89–99 (2017).
Elliott, J. I. et al. Membrane phosphatidylserine distribution as a nonapoptotic signaling mechanism in lymphocytes. Nat. Cell Biol. 7, 808–816 (2005).
Fischer, K. et al. Antigen recognition induces phosphatidylserine exposure on the cell surface of human CD8+ T cells. Blood 108, 4094–4101 (2006).
Balasubramanian, K., Mirnikjoo, B. & Schroit, A. J. Regulated externalization of phosphatidylserine at the cell surface: implications for apoptosis. J. Biol. Chem. 282, 18357–64. (2007).
Jitkaew, S., Witasp, E., Zhang, S., Kagan, V. E. & Fadeel, B. Induction of caspase- and reactive oxygen species-independent phosphatidylserine externalization in primary human neutrophils: role in macrophage recognition and engulfment. J. Leukoc. Biol. 85, 427–437 (2009).
Segawa, K. et al. Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science 344, 1164–1168 (2014).
Gardai, S. J. et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 123, 321–334 (2005). This study shows that translocation of calreticulin to the surface of live cells induces phagocytes to phagocytose such cells, particularly neutrophils.
Feng, M. et al. Programmed cell removal by calreticulin in tissue homeostasis and cancer. Nat. Commun. 9, 3194 (2018).
Fucikova, J., Spisek, R., Kroemer, G. & Galluzzi, L. Calreticulin and cancer. Cell Res. 31, 5–16 (2021).
Klaus, C., Liao, H., Allendorf, D. H., Brown, G. C. & Neumann, H. Sialylation acts as a checkpoint for innate immune responses in the central nervous system. Glia 69, 1619–1636 (2021).
Barkal, A. A. et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature 572, 392–396 (2019).
Bennett, M. R., Gibson, D. F., Schwartz, S. M. & Tait, J. F. Binding and phagocytosis of apoptotic vascular smooth muscle cells is mediated in part by exposure of phosphatidylserine. Circ. Res. 77, 1136–1142 (1995).
He, M. et al. Receptor for advanced glycation end products binds to phosphatidylserine and assists in the clearance of apoptotic cells. EMBO Rep. 12, 358–364 (2011).
Liu, G. et al. High mobility group protein-1 inhibits phagocytosis of apoptotic neutrophils through binding to phosphatidylserine. J. Immunol. 181, 4240–4246 (2008).
Law, A. L. et al. Cleavage of Mer tyrosine kinase (MerTK) from the cell surface contributes to the regulation of retinal phagocytosis. J. Biol. Chem. 290, 4941–4952 (2015).
Park, Y.-J. et al. PAI-1 inhibits neutrophil efferocytosis. Proc. Natl Acad. Sci. USA 105, 11784–11789 (2008).
Nauta, A. J. et al. Biochemical and functional characterization of the interaction between pentraxin 3 and C1q. Eur. J. Immunol. 33, 465–473 (2003).
Nakamura, K. et al. Targeting an adenosine-mediated “Don’t eat me signal” augments anti-lymphoma immunity by anti-CD20 monoclonal antibody. Leukemia 34, 2708–2721 (2020).
Koizumi, S. et al. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature 446, 1091–1095 (2007).
Segawa, K., Suzuki, J. & Nagata, S. Constitutive exposure of phosphatidylserine on viable cells. Proc. Natl Acad. Sci. USA 108, 19246–19251 (2011).
Riazanski, V., Sui, Z. & Nelson, D. J. Kinetic separation of oxidative and non-oxidative metabolism in single phagosomes from alveolar macrophages: impact on bacterial killing. iScience 23, 101759 (2020).
Kim, S. E., Zhang, J., Jiang, E. & Overholtzer, M. Amino acids and mechanistic target of rapamycin regulate the fate of live engulfed cells. FASEB J. 35, e21909 (2021).
Cwiklik, L. & Jungwirth, P. Massive oxidation of phospholipid membranes leads to pore creation and bilayer disintegration. Chem. Phys. Lett. 486, 99–103 (2010).
Winterbourn, C. C. & Kettle, A. J. Redox reactions and microbial killing in the neutrophil phagosome. Antioxid. Redox Signal. 18, 642–660 (2013).
Dingjan, I. et al. Lipid peroxidation causes endosomal antigen release for cross-presentation. Sci. Rep. 6, 22064 (2016).
Canton, J. et al. The receptor DNGR-1 signals for phagosomal rupture to promote cross-presentation of dead-cell-associated antigens. Nat. Immunol. 22, 140–153 (2021).
Mayadas, T. N., Cullere, X. & Lowell, C. A. The multifaceted functions of neutrophils. Annu. Rev. Pathol. 9, 181–218 (2014).
Bagaitkar, J. et al. NADPH oxidase activation regulates apoptotic neutrophil clearance by murine macrophages. Blood 131, 2367–2378 (2018).
Muñoz-Espín, D. et al. Programmed cell senescence during mammalian embryonic development. Cell 155, 1104–1118 (2013).
Cunningham, C. L., Martinez-Cerdeno, V. & Noctor, S. C. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J. Neurosci. 33, 4216–4233 (2013).
Luo, C., Koyama, R. & Ikegaya, Y. Microglia engulf viable newborn cells in the epileptic dentate gyrus. Glia 64, 1508–1517 (2016).
Nelson, L. H., Warden, S. & Lenz, K. M. Sex differences in microglial phagocytosis in the neonatal hippocampus. Brain Behav. Immun. 64, 11–22 (2017).
Anderson, S. R. et al. Complement targets newborn retinal ganglion cells for phagocytic elimination by microglia. J. Neurosci. 39, 2025–2040 (2019).
Nemes-Baran, A. D., White, D. R. & DeSilva, T. M. Fractalkine-dependent microglial pruning of viable oligodendrocyte progenitor cells regulates myelination. Cell Rep. 32, 108047 (2020).
Irfan, M., Evonuk, K. S. & DeSilva, T. M. Microglia phagocytose oligodendrocyte progenitor cells and synapses during early postnatal development: implications for white versus gray matter maturation. FEBS J. 289, 2110–2127 (2022).
Jaiswal, S. et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 138, 271–285 (2009). This study shows that stem cells and leukaemic cells overexpress CD47 to prevent themselves being phagocytosed alive. The Weissman laboratory subsequently showed that anti-CD47 antibodies can induce phagocytosis of cancer and other pathogenic cells, as reviewed by Majeti et al. (2022).
Kuriyama, T. et al. Engulfment of hematopoietic stem cells caused by down-regulation of CD47 is critical in the pathogenesis of hemophagocytic lymphohistiocytosis. Blood 120, 4058–4067 (2012).
Li, J. et al. Overexpression of CD47 is associated with brain overgrowth and 16p11.2 deletion syndrome. Proc. Natl Acad. Sci. USA 118, e2005483118 (2021).
Tarique, I. et al. In vivo cellular evidence of autophagic associated spermiophagy within the principal cells during sperm storage in epididymis of the turtle. Aging 12, 8987–8999 (2020).
Murakami, M., Sugita, A. & Hamasaki, M. Scanning electron microscopic observations of the vas deferens in man and monkey with special reference to spermiophagy in its ampullary region. Scan Electron. Microsc. 3, 1333–1339 (1982).
He, C. et al. The semenogelin I-derived peptide SgI-52 in seminal plasma participates in sperm selection and clearance by macrophages. Peptides 153, 170799 (2022).
Batra, V. et al. A higher abundance of O-linked glycans confers a selective advantage to high fertile buffalo spermatozoa for immune-evasion from neutrophils. Front. Immunol. 11, 1928 (2020).
Föller, M. & Lang, F. Ion transport in eryptosis, the suicidal death of erythrocytes. Front. Cell Dev. Biol. 8, 597 (2020).
Klei, T. R., Meinderts, S. M., van den Berg, T. K. & van Bruggen, R. From the cradle to the grave: the role of macrophages in erythropoiesis and erythrophagocytosis. Front. Immunol. 8, 73 (2017).
Burger, P., Hilarius-Stokman, P., de Korte, D., van den Berg, T. K. & van Bruggen, R. CD47 functions as a molecular switch for erythrocyte phagocytosis. Blood 119, 5512–5521 (2012).
Cao, H. et al. Red blood cell mannoses as phagocytic ligands mediating both sickle cell anaemia and malaria resistance. Nat. Commun. 12, 1792 (2021).
Casanova-Acebes, M. et al. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell 153, 1025–1035 (2013).
Lagasse, E. & Weissman, I. L. bcl-2 inhibits apoptosis of neutrophils but not their engulfment by macrophages. J. Exp. Med. 179, 1047–1052 (1994).
Lovewell, R. R., Patankar, Y. R. & Berwin, B. Mechanisms of phagocytosis and host clearance of Pseudomonas aeruginosa. Am. J. Physiol. Lung Cell Mol. Physiol. 306, L591–L603 (2014).
Alfaro, C. et al. Dendritic cells take up and present antigens from viable and apoptotic polymorphonuclear leukocytes. PLoS ONE 6, e29300 (2011). This study shows that dendritic cells avidly phagocytose live neutrophils, and present antigens from pathogens that the neutrophils have phagocytosed.
Savina, A. & Amigorena, S. Phagocytosis and antigen presentation in dendritic cells. Immunol. Rev. 219, 143–156 (2007).
Feldman, M. B., Vyas, J. M. & Mansour, M. K. It takes a village: phagocytes play a central role in fungal immunity. Semin. Cell Dev. Biol. 89, 16–23 (2019).
Albacker, L. A. et al. TIM-4, a receptor for phosphatidylserine, controls adaptive immunity by regulating the removal of antigen-specific T cells. J. Immunol. 185, 6839–6849 (2010).
Kurd, N. S. et al. A role for phagocytosis in inducing cell death during thymocyte negative selection. eLife 8, e48097 (2019). This study shows that thymocyte death during negative selection is mediated by phagoptosis.
Birkle, T. & Brown, G. C. I’m infected, eat me! Innate immunity mediated by live, infected cells signaling to be phagocytosed. Infect. Immun. 89, e00476-20 (2021).
Lemke, G. How macrophages deal with death. Nat. Rev. Immunol. 19, 539–549 (2019).
Tufail, Y. et al. Phosphatidylserine exposure controls viral innate immune responses by microglia. Neuron 93, 574–586.e8 (2017).
Torrez Dulgeroff, L. B. et al. CD47 blockade reduces the pathologic features of experimental cerebral malaria and promotes survival of hosts with Plasmodium infection. Proc. Natl Acad. Sci. USA 118, e1907653118 (2021).
Gül, N. et al. Macrophages eliminate circulating tumor cells after monoclonal antibody therapy. J. Clin. Invest. 124, 812–823 (2014).
Munn, D. H. & Cheung, N. K. Antibody-independent phagocytosis of tumor cells by human monocyte-derived macrophages cultured in recombinant macrophage colony-stimulating factor. Cancer Immunol. Immunother. 41, 46–52 (1995).
Xie, R. et al. Phagocytosis by macrophages and endothelial cells inhibits procoagulant and fibrinolytic activity of acute promyelocytic leukemia cells. Blood 119, 2325–2334 (2012).
Chao, M. P. et al. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci. Transl. Med. 2, 63ra94 (2010).
Métayer, L. E., Vilalta, A., Burke, G. A. A. & Brown, G. C. Anti-CD47 antibodies induce phagocytosis of live, malignant B cells by macrophages via the Fc domain, resulting in cell death by cell death by phagocytosis. Oncotarget 8, 60892–60903 (2017).
Majeti, R. et al. Clonal expansion of stem/progenitor cells in cancer, fibrotic diseases, and atherosclerosis, and CD47 protection of pathogenic cells. Annu. Rev. Med. 73, 307–320 (2022). This paper reviews the Weissman laboratory observation that anti-CD47 antibodies can induce phagocytosis of cancer and other pathogenic cells.
Fricker, M., Tolkovsky, A. M., Borutaite, V., Coleman, M. & Brown, G. C. Neuronal cell death. Physiol. Rev. 98, 813–880 (2018).
Butler, C. A. et al. Microglial phagocytosis of neurons in neurodegeneration, and its regulation. J. Neurochem. 158, 621–639 (2021).
Brown, G. C. Neuronal loss after stroke due to microglial phagocytosis of stressed neurons. Int. J. Mol. Sci. 22, 13442 (2021).
Tolkovsky, A. M. & Spillantini, M. G. Tau aggregation and its relation to selected forms of neuronal cell death. Essays Biochem. 65, 847–857 (2021).
Yanuck, S. F. Microglial phagocytosis of neurons: diminishing neuronal loss in traumatic, infectious, inflammatory, and autoimmune CNS disorders. Front. Psychiatry 10, 712 (2019).
Neher, J. J., Neniskyte, U., Hornik, T. & Brown, G. C. Inhibition of UDP/P2Y6 purinergic signaling prevents phagocytosis of viable neurons by activated microglia in vitro and in vivo. Glia 62, 1463–1475 (2014).
Neniskyte, U. & Brown, G. C. Lactadherin/MFG-E8 is essential for microglia-mediated neuronal loss and cell death by phagocytosis induced by amyloid β. J. Neurochem. 126, 312–317 (2013).
Neniskyte, U., Fricker, M. & Brown, G. C. Amyloid β induces microglia to phagocytose neurons via activation of protein kinase Cs and NADPH oxidase. Int. J. Biochem. Cell Biol. 81, 346–355 (2016).
Brelstaff, J., Tolkovsky, A. M., Ghetti, B., Goedert, M. & Spillantini, M. G. Living neurons with Tau filaments aberrantly expose phosphatidylserine and are phagocytosed by microglia. Cell Rep. 24, 1939–1948.e4 (2018).
Pampuscenko, K. et al. Extracellular tau induces microglial phagocytosis of living neurons in cell cultures. J. Neurochem. 154, 316–329 (2020).
Puigdellívol, M. et al. The microglial P2Y6 receptor mediates neuronal loss and memory deficits in neurodegeneration. Cell Rep. 37, 110148 (2021). This study shows that microglial phagocytosis of neurons contributes to neurodegeneration induced by amyloid-β and tau, so that blocking phagocytosis prevents neurodegeneration.
Milde, S. et al. Inflammatory neuronal loss in the substantia nigra induced by systemic lipopolysaccharide is prevented by knockout of the P2Y6 receptor in mice. J. Neuroinflammation 18, 225 (2021).
Dundee, J. M., Puigdellívol, M., Butler, R., Cockram, T. O. J. & Brown, G. C. P2Y6 receptor-dependent microglial phagocytosis of synapses mediates synaptic and memory loss in aging. Aging Cell 22, e13761 (2023).
Romero-Molina, C., Garretti, F., Andrews, S. J., Marcora, E. & Goate, A. M. Microglial efferocytosis: diving into the Alzheimer’s disease gene pool. Neuron 110, 3513–3533 (2022).
Popescu, A. S. et al. Alzheimer’s disease-associated R47H TREM2 increases, but wild-type TREM2 decreases, microglial phagocytosis of synaptosomes and neuronal loss. Glia 71, 974–990 (2023).
Linnartz-Gerlach, B. et al. TREM2 triggers microglial density and age-related neuronal loss. Glia 67, 539–550 (2019).
Zhao, L. et al. Microglial phagocytosis of living photoreceptors contributes to inherited retinal degeneration. EMBO Mol. Med. 7, 1179–1197 (2015).
Hollingsworth, T. J., Wang, X., White, W. A., Simpson, R. N. & Jablonski, M. M. Chronic proinflammatory signaling accelerates the rate of degeneration in a spontaneous polygenic model of inherited retinal dystrophy. Front. Pharmacol. 13, 839424 (2022).
Bourseguin, J. et al. Persistent DNA damage associated with ATM kinase deficiency promotes microglial dysfunction. Nucleic Acids Res. 50, 2700–2718 (2022).
Vasek, M. J. et al. A complement–microglial axis drives synapse loss during virus-induced memory impairment. Nature 534, 538–543 (2016).
Hu, D. D. et al. Glucocorticoids prevent enterovirus 71 capsid protein VP1 induced calreticulin surface exposure by alleviating neuronal ER stress. Neurotox. Res. 31, 204–217 (2017).
Rodríguez, A. M. et al. Brucella abortus-activated microglia induce neuronal death through primary phagocytosis. Glia 65, 1137–1151 (2017).
Sierra-Martín, A. et al. LPS-stimulated microglial cells promote ganglion cell death in organotypic cultures of quail embryo retina. Front. Cell Neurosci. 17, 1120400 (2023).
Neher, J. J. et al. Phagocytosis executes delayed neuronal death after focal brain ischemia. Proc. Natl Acad. Sci. USA 110, E4098–E4107 (2013).
Milde, S. & Brown, G. C. Knockout of the P2Y6 receptor prevents peri-infarct neuronal loss after transient, focal ischemia in mouse brain. Int. J. Mol. Sci. 23, 2304 (2022).
Katayama, T. et al. Accumulating microglia phagocytose injured neurons in hippocampal slice cultures: involvement of p38 MAP kinase. PLoS ONE 7, e40813 (2012).
Li, L. et al. Resolvin D1 reprograms energy metabolism to promote microglia to phagocytize neutrophils after ischemic stroke. Cell Rep. 42, 112617 (2023).
Pickett, L. A., VanRyzin, J. W., Marquardt, A. E. & McCarthy, M. M. Microglia phagocytosis mediates the volume and function of the rat sexually dimorphic nucleus of the preoptic area. Proc. Natl Acad. Sci. USA 120, e2212646120 (2023).
Fourgeaud, L. et al. TAM receptors regulate multiple features of microglial physiology. Nature 532, 240–244 (2016).
Huang, Y. & Lemke, G. Early death in a mouse model of Alzheimer’s disease exacerbated by microglial loss of TAM receptor signaling. Proc. Natl Acad. Sci. USA 119, e2204306119 (2022).
Sterling, N. A., Park, J. Y., Park, R., Cho, S. H. & Kim, S. An entosis-like process induces mitotic disruption in Pals1 microcephaly pathogenesis. Nat. Commun. 14, 82 (2023).
Schloesser, D. et al. Senescent cells suppress macrophage-mediated corpse removal via upregulation of the CD47–QPCT/L axis. J. Cell Biol. 222, e202207097 (2023).
Gao, L., He, Z. & Wu, Y. Advances in anti-metabolic disease treatments targeting CD47. Curr. Pharm. Des. 28, 3720–3728 (2022).
Kojima, Y. et al. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature 536, 86–90 (2016).
Kang, T. W. et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 479, 547–551 (2011).
Tonnessen-Murray, C. A. et al. Chemotherapy-induced senescent cancer cells engulf other cells to enhance their survival. J. Cell Biol. 218, 3827–3844 (2019).
Thomas, A. L., Lehn, M. A., Janssen, E. M., Hildeman, D. A. & Chougnet, C. A. Naturally-aged microglia exhibit phagocytic dysfunction accompanied by gene expression changes reflective of underlying neurologic disease. Sci. Rep. 12, 19471 (2022).
Tomaiuolo, R. et al. Activity of mannose-binding lectin in centenarians. Aging Cell 11, 394–400 (2012).
Kanaan, D., Shklyar, B., Porat-Kuperstein, L. & Toledano, H. Live imaging of phagoptosis in ex vivo Drosophila testis. Bio Protoc. 13, e4637 (2023).
Acknowledgements
The author thanks the many different researchers who have contributed to this field. The author thanks A. Tolkovsky, V. Borutaite, J. Neher, M. Fricker and U. Neniskyte, who have contributed to his understanding of cell death by phagocytosis.
Author information
Authors and Affiliations
Contributions
The author is the sole contributor to this article.
Corresponding author
Ethics declarations
Competing interest
The author declares that there are no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks G. Lemke and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Cell cannibalism
-
Cells phagocytosing other cells, which may be dead or alive.
- Desialylation
-
The removal of terminal sialic acid residues from glycoproteins or glycolipids.
- Don’t-eat-me signals
-
Molecules on a cell that inhibit a phagocyte eating that cell.
- Eat-me signal
-
A molecule on a cell that induces a phagocyte to eat that cell.
- Efferocytosis
-
Phagocytosis of a cell dying by apoptosis.
- Eryptosis
-
A mechanism of cell death of erythrocytes.
- Entotic cell death
-
The death of a cell that has invaded into another cell by entosis.
- Find-me signals
-
Molecules released from a cell that encourage a phagocyte to chemotactically migrate to the cell.
- Haemophagocytosis
-
The phagocytosis of blood cells.
- NADPH oxidase
-
A membrane-bound enzyme that uses cytosolic NADPH to reduce oxygen to superoxide that is released into phagosomes to kill engulfed cells.
- Nurse cells
-
Specialized cells that support the growth and stability of neighbouring cells.
- Opsonins
-
Normally extracellular molecules that when bound to a cell induce a phagocyte to eat that cell.
- Phagocytic receptors
-
Receptors that directly bind eat-me signals or opsonins and then induce phagocytosis.
- Programmed cell removal
-
The phagocytic removal of cells that may be dead, dying or alive.
- Primary phagocytosis
-
The same as cell death by phagocytosis.
- Scavenger receptors
-
A diverse set of receptors that mediate phagocytosis or endocytosis.
- Secondary phagocytosis
-
Phagocytosis of a dead or dying cell.
- Sialic acid-binding immunoglobulin-type lectin (SIGLEC) receptors
-
A family of receptors binding sialic acid residues.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brown, G.C. Cell death by phagocytosis. Nat Rev Immunol 24, 91–102 (2024). https://doi.org/10.1038/s41577-023-00921-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-023-00921-6
This article is cited by
-
Role of imbalanced gut microbiota in promoting CRC metastasis: from theory to clinical application
Cell Communication and Signaling (2024)
-
Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases
Signal Transduction and Targeted Therapy (2024)