Abstract
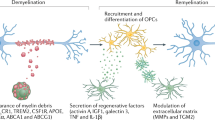
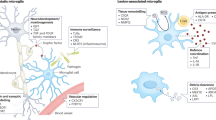
Microglia are resident macrophages of the central nervous system that have key functions in its development, homeostasis and response to damage and infection. Although microglia have been increasingly implicated in contributing to the pathology that underpins neurological dysfunction and disease, they also have crucial roles in neurological homeostasis and regeneration. This includes regulation of the maintenance and regeneration of myelin, the membrane that surrounds neuronal axons, which is required for axonal health and function. Myelin is damaged with normal ageing and in several neurodegenerative diseases, such as multiple sclerosis and Alzheimer disease. Given the lack of approved therapies targeting myelin maintenance or regeneration, it is imperative to understand the mechanisms by which microglia support and restore myelin health to identify potential therapeutic approaches. However, the mechanisms by which microglia regulate myelin loss or integrity are still being uncovered. In this Review, we discuss recent work that reveals the changes in white matter with ageing and neurodegenerative disease, how this relates to microglia dynamics during myelin damage and regeneration, and factors that influence the regenerative functions of microglia.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Borst, K., Dumas, A. A. & Prinz, M. Microglia: immune and non-immune functions. Immunity 54, 2194–2208 (2021).
Prinz, M., Jung, S. & Priller, J. Microglia biology: one century of evolving concepts. Cell 179, 292–311 (2019).
Ginhoux, F. et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845 (2010).
Askew, K. et al. Coupled proliferation and apoptosis maintain the rapid turnover of microglia in the adult brain. Cell Rep. 18, 391–405 (2017).
Tay, T. L. et al. Microglia across the lifespan: from origin to function in brain development, plasticity and cognition. J. Physiol. 595, 1929–1945 (2017).
Réu, P. et al. The lifespan and turnover of microglia in the human brain. Cell Rep. 20, 779–784 (2017).
Füger, P. et al. Microglia turnover with aging and in an Alzheimer’s model via long-term in vivo single-cell imaging. Nat. Neurosci. 20, 1371–1376 (2017).
Bryois, J. et al. Cell-type-specific cis-eQTLs in eight human brain cell types identify novel risk genes for psychiatric and neurological disorders. Nat. Neurosci. 25, 1104–1112 (2022).
Schwartzentruber, J. et al. Genome-wide meta-analysis, fine-mapping and integrative prioritization implicate new Alzheimer’s disease risk genes. Nat. Genet. 53, 392–402 (2021).
International Multiple Sclerosis Genetics Consortium. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science 365, eaav7188 (2019).
Fünfschilling, U. et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485, 517–521 (2012).
Saab, A. S. et al. Oligodendroglial NMDA receptors regulate glucose import and axonal energy metabolism. Neuron 91, 119–132 (2016).
Nemes-Baran, A. D., White, D. R. & DeSilva, T. M. Fractalkine-dependent microglial pruning of viable oligodendrocyte progenitor cells regulates myelination. Cell Rep. 32, 108047 (2020).
Hughes, A. N. & Appel, B. Microglia phagocytose myelin sheaths to modify developmental myelination. Nat. Neurosci. 23, 1055–1066 (2020).
Djannatian, M. et al. Myelination generates aberrant ultrastructure that is resolved by microglia. J. Cell Biol. 222, e202204010 (2023).
Franklin, R. J. M. & ffrench-Constant, C. Regenerating CNS myelin — from mechanisms to experimental medicines. Nat. Rev. Neurosci. 18, 753–769 (2017).
Lloyd, A. F. & Miron, V. E. The pro-remyelination properties of microglia in the central nervous system. Nat. Rev. Neurol. 15, 447–458 (2019).
Yong, V. W. Microglia in multiple sclerosis: protectors turn destroyers. Neuron 110, 3534–3548 (2022).
Xie, F. et al. Gene profiling in the dynamic regulation of the lifespan of the myelin sheath structure in the optic nerve of rats. Mol. Med. Rep. 10, 217–222 (2014).
Sugiyama, I. et al. Ultrastructural analysis of the paranodal junction of myelinated fibers in 31-month-old-rats. J. Neurosci. Res. 70, 309–317 (2002).
Peters, A. The effects of normal aging on myelinated nerve fibers in monkey central nervous system. Front. Neuroanat. 3, 11 (2009).
Peters, A., Sethares, C. & Killiany, R. J. Effects of age on the thickness of myelin sheaths in monkey primary visual cortex. J. Comp. Neurol. 435, 241–248 (2001).
Masuda, T. et al. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 566, 388–392 (2019). Seminal study demonstrating microglial heterogeneity across the lifespan in mouse brain regions and in acute lesion biopsy samples from patients with MS.
Kuhlmann, T. et al. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 133, 13–24 (2017).
Hansen, D. V., Hanson, J. E. & Sheng, M. Microglia in Alzheimer’s disease. J. Cell Biol. 217, 459–472 (2017).
Hoy, A. R. et al. Microstructural white matter alterations in preclinical Alzheimer’s disease detected using free water elimination diffusion tensor imaging. PLoS ONE 12, e0173982 (2017).
Kenigsbuch, M. et al. A shared disease-associated oligodendrocyte signature among multiple CNS pathologies. Nat. Neurosci. 25, 876–886 (2022). Critical study that brings together previous scRNA-seq studies to demonstrate a common oligodendrocyte subpopulation that appears in mouse models of CNS pathology.
Lee, S.-H. et al. TREM2-independent oligodendrocyte, astrocyte, and T cell responses to tau and amyloid pathology in mouse models of Alzheimer disease. Cell Rep. 37, 110158 (2021).
Mathys, H. et al. Single-cell transcriptomic analysis of Alzheimer’s disease. Nature 570, 332–337 (2019). snRNA-seq of AD demonstrating alterations in oligodendrocyte lineage cell transcriptomes and changes in other cell types in pathways associated with myelination.
Sadick, J. S. et al. Astrocytes and oligodendrocytes undergo subtype-specific transcriptional changes in Alzheimer’s disease. Neuron 110, 1788–1805.e10 (2022).
Poon, K. W. C. et al. Lipid biochemical changes detected in normal appearing white matter of chronic multiple sclerosis by spectral coherent Raman imaging. Chem. Sci. 9, 1586–1595 (2018).
Bosch, A.van den et al. Neurofilament light chain levels in multiple sclerosis correlate with lesions containing foamy macrophages and with acute axonal damage. Neurol. Neuroimmunol. Neuroinflamm. 9, e1154 (2022).
Traka, M., Podojil, J. R., McCarthy, D. P., Miller, S. D. & Popko, B. Oligodendrocyte death results in immune-mediated CNS demyelination. Nat. Neurosci. 19, 65–74 (2016).
Caprariello, A. V. et al. Biochemically altered myelin triggers autoimmune demyelination. Proc. Natl Acad. Sci. USA 115, 5528–5533 (2018).
Bando, Y. et al. Abnormal morphology of myelin and axon pathology in murine models of multiple sclerosis. Neurochem. Int. 81, 16–27 (2015).
Recks, M. S. et al. Early axonal damage and progressive myelin pathology define the kinetics of CNS histopathology in a mouse model of multiple sclerosis. Clin. Immunol. 149, 32–45 (2013).
Schäffner, E. et al. Myelin insulation as a risk factor for axonal degeneration in autoimmune demyelinating disease. Preprint at bioRxiv https://doi.org/10.1101/2021.11.11.468223 (2021).
Chapman, T. W., Olveda, G. E., Bame, X., Pereira, E., & Hill, R. A. Oligodendrocyte death initiates synchronous remyelination to restore cortical myelin patterns in mice. Nat. Neurosci. 26, 555–569 (2023).
Rodriguez, M. & Scheithauer, B. Ultrastructure of multiple sclerosis. Ultrastruct. Pathol. 18, 3–13 (1994).
Romanelli, E. et al. Myelinosome formation represents an early stage of oligodendrocyte damage in multiple sclerosis and its animal model. Nat. Commun. 7, 13275 (2016).
Aber, E. R. et al. Oligodendroglial macroautophagy is essential for myelin sheath turnover to prevent neurodegeneration and death. Cell Rep. 41, 111480 (2022).
Hu, X. et al. Sustained ErbB activation causes demyelination and hypomyelination by driving necroptosis of mature oligodendrocytes and apoptosis of oligodendrocyte precursor cells. J. Neurosci. 41, 9872–9890 (2021).
Chen, W.-T. et al. Spatial transcriptomics and in situ sequencing to study Alzheimer’s disease. Cell 182, 976–991.e19 (2020). Spatial transcriptomics surrounding amyloid-β plaques in a mouse model of AD indicates altered gene expression relating to oligodendrocytes and myelin.
Ferreira, S. et al. Amyloidosis is associated with thicker myelin and increased oligodendrogenesis in the adult mouse brain. J. Neurosci. Res. 98, 1905–1932 (2020).
Desai, M. K. et al. Early oligodendrocyte/myelin pathology in Alzheimer’s disease mice constitutes a novel therapeutic target. Am. J. Pathol. 177, 1422–1435 (2010).
Wheeler, D., Bandaru, V. V. R., Calabresi, P. A., Nath, A. & Haughey, N. J. A defect of sphingolipid metabolism modifies the properties of normal appearing white matter in multiple sclerosis. Brain 131, 3092–3102 (2008).
van der Poel, M. et al. Transcriptional profiling of human microglia reveals grey–white matter heterogeneity and multiple sclerosis-associated changes. Nat. Commun. 10, 1139 (2019).
Fitzner, D. et al. Cell-type- and brain-region-resolved mouse brain lipidome. Cell Rep. 32, 108132 (2020).
Han, X. Multi-dimensional mass spectrometry-based shotgun lipidomics and the altered lipids at the mild cognitive impairment stage of Alzheimer’s disease. Biochim. Biophys. Acta 1801, 774–783 (2010).
Jorissen, W. et al. Relapsing-remitting multiple sclerosis patients display an altered lipoprotein profile with dysfunctional HDL. Sci. Rep. 7, 43410 (2017).
Wang, F. et al. Myelin degeneration and diminished myelin renewal contribute to age-related deficits in memory. Nat. Neurosci. 23, 481–486 (2020). Study demonstrating reduced production of myelin with ageing in the mouse CNS and that encouraging myelination can improve cognitive deficits.
Chen, J.-F. et al. Enhancing myelin renewal reverses cognitive dysfunction in a murine model of Alzheimer’s disease. Neuron 109, 2292–2307.e5 (2021). Study demonstrating increased demyelination and remyelination in a mouse model of AD. However, remyelination cannot overcome the robust demyelination associated with cognitive dysfunction.
Bacmeister, C. M. et al. Motor learning drives dynamic patterns of intermittent myelination on learning-activated axons. Nat. Neurosci. 25, 1300–1313 (2022).
Steadman, P. E. et al. Disruption of oligodendrogenesis impairs memory consolidation in adult mice. Neuron 105, 150–164.e6 (2020).
McKenzie, I. A. et al. Motor skill learning requires active central myelination. Science 346, 318–322 (2014).
Neumann, B. et al. Metformin restores CNS remyelination capacity by rejuvenating aged stem cells. Cell Stem Cell 25, 473–485.e8 (2019).
Sim, F. J., Zhao, C., Penderis, J. & Franklin, R. J. M. The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J. Neurosci. 22, 2451–2459 (2002).
Heß, K. et al. Lesion stage-dependent causes for impaired remyelination in MS. Acta Neuropathol. 140, 359–375 (2020).
Lubetzki, C., Zalc, B., Williams, A., Stadelmann, C. & Stankoff, B. Remyelination in multiple sclerosis: from basic science to clinical translation. Lancet Neurol. 19, 678–688 (2020).
Starost, L. et al. Extrinsic immune cell-derived, but not intrinsic oligodendroglial factors contribute to oligodendroglial differentiation block in multiple sclerosis. Acta Neuropathol. 140, 715–736 (2020).
Mozafari, S. et al. Multiple sclerosis iPS-derived oligodendroglia conserve their properties to functionally interact with axons and glia in vivo. Sci. Adv. 6, eabc6983 (2020).
Neumann, B. et al. Myc determines the functional age state of oligodendrocyte progenitor cells. Nat. Aging 1, 826–837 (2021).
Segel, M. et al. Niche stiffness underlies the ageing of central nervous system progenitor cells. Nature 573, 130–134 (2019).
Behrendt, G. et al. Dynamic changes in myelin aberrations and oligodendrocyte generation in chronic amyloidosis in mice and men. Glia 61, 273–286 (2013).
Desai, M. K., Guercio, B. J., Narrow, W. C. & Bowers, W. J. An Alzheimer’s disease-relevant presenilin-1 mutation augments amyloid-beta-induced oligodendrocyte dysfunction. Glia 59, 627–640 (2011).
Yeung, M. S. Y. et al. Dynamics of oligodendrocyte generation in multiple sclerosis. Nature 566, 538–542 (2019).
Neely, S. A. et al. New oligodendrocytes exhibit more abundant and accurate myelin regeneration than those that survive demyelination. Nat. Neurosci. 25, 415–420 (2022).
Bacmeister, C. M. et al. Motor learning promotes remyelination via new and surviving oligodendrocytes. Nat. Neurosci. 23, 819–831 (2020).
Mezydlo, A. et al. Remyelination by surviving oligodendrocytes is inefficient in the inflamed mammalian cortex. Neuron 111, 1748–1759.e8 (2023).
Pernin, F. et al. Diverse injury responses of human oligodendrocyte to mediators implicated in multiple sclerosis. Brain 145, 4320–4333 (2022).
Seeker, L. A. et al. Brain matters: unveiling the distinct contributions of region, age, and sex to glia diversity and CNS function. Acta Neuropathol. Commun. 11, 84 (2023).
Luo, J. X. X. et al. Human oligodendrocyte myelination potential; relation to age and differentiation. Ann. Neurol. 91, 178–191 (2022).
Crawford, A. H., Tripathi, R. B., Richardson, W. D. & Franklin, R. J. M. Developmental origin of oligodendrocyte lineage cells determines response to demyelination and susceptibility to age-associated functional decline. Cell Rep. 15, 761–773 (2016).
Bechler, M. E., Byrne, L. & Ffrench-Constant, C. CNS myelin sheath lengths are an intrinsic property of oligodendrocytes. Curr. Biol. 25, 2411–2416 (2015).
Marisca, R. et al. Functionally distinct subgroups of oligodendrocyte precursor cells integrate neural activity and execute myelin formation. Nat. Neurosci. 23, 363–374 (2020).
Zeisel, A. et al. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science 347, 1138–1142 (2015).
Marques, S. et al. Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science 352, 1326–1329 (2016). Study demonstrating oligodendrocyte transcriptional heterogeneity by scRNA-seq in the mouse brain.
Jäkel, S. et al. Altered human oligodendrocyte heterogeneity in multiple sclerosis. Nature 566, 543–547 (2019). Study demonstrating oligodendrocyte transcriptional heterogeneity shown by snRNA-seq in the human brain, in both controls and patients with MS.
Schirmer, L. et al. Neuronal vulnerability and multilineage diversity in multiple sclerosis. Nature 573, 75–82 (2019).
Perlman, K. et al. Developmental trajectory of oligodendrocyte progenitor cells in the human brain revealed by single cell RNA sequencing. Glia 68, 1291–1303 (2020).
Yaqubi, M. et al. Regional and age-related diversity of human mature oligodendrocytes. Glia 70, 1938–1949 (2022).
Falcão, A. M. et al. Disease-specific oligodendrocyte lineage cells arise in multiple sclerosis. Nat. Med. 24, 1837–1844 (2018).
Zhou, Y. et al. Human and mouse single-nucleus transcriptomics reveal TREM2-dependent and TREM2-independent cellular responses in Alzheimer’s disease. Nat. Med. 26, 131–142 (2020).
Kaya, T. et al. CD8+ T cells induce interferon-responsive oligodendrocytes and microglia in white matter aging. Nat. Neurosci. 25, 1446–1457 (2022).
Pandey, S. et al. Disease-associated oligodendrocyte responses across neurodegenerative diseases. Cell Rep. 40, 111189 (2022).
Absinta, M. et al. A lymphocyte–microglia–astrocyte axis in chronic active multiple sclerosis. Nature 597, 709–714 (2021).
Hilscher, M. M. et al. Spatial and temporal heterogeneity in the lineage progression of fine oligodendrocyte subtypes. BMC Biol. 20, 122 (2022).
Bramow, S. et al. Demyelination versus remyelination in progressive multiple sclerosis. Brain 133, 2983–2998 (2010).
de la Fuente, A. G. et al. Changes in the oligodendrocyte progenitor cell proteome with ageing. Mol. Cell. Proteom. 19, 1281–1302 (2020).
Meijer, M. et al. Epigenomic priming of immune genes implicates oligodendroglia in multiple sclerosis susceptibility. Neuron 110, 1193–1210.e13 (2022).
Kirby, L. et al. Oligodendrocyte precursor cells present antigen and are cytotoxic targets in inflammatory demyelination. Nat. Commun. 10, 3887 (2019).
Grubman, A. et al. A single-cell atlas of entorhinal cortex from individuals with Alzheimer’s disease reveals cell-type-specific gene expression regulation. Nat. Neurosci. 22, 2087–2097 (2019).
Del-Aguila, J. L. et al. A single-nuclei RNA sequencing study of mendelian and sporadic ad in the human brain. Alzheimer’s Res. Ther. 11, 71 (2019).
Lau, S.-F., Cao, H., Fu, A. K. Y. & Ip, N. Y. Single-nucleus transcriptome analysis reveals dysregulation of angiogenic endothelial cells and neuroprotective glia in Alzheimer’s disease. Proc. Natl Acad. Sci. USA 117, 25800–25809 (2020).
Gerrits, E. et al. Distinct amyloid-β and tau-associated microglia profiles in Alzheimer’s disease. Acta Neuropathol. 141, 681–696 (2021).
Leng, K. et al. Molecular characterization of selectively vulnerable neurons in Alzheimer’s disease. Nat. Neurosci. 24, 276–287 (2021).
Morabito, S. et al. Single-nucleus chromatin accessibility and transcriptomic characterization of Alzheimer’s disease. Nat. Genet. 53, 1143–1155 (2021).
Valihrach, L. et al. Recent advances in deciphering oligodendrocyte heterogeneity with single-cell transcriptomics. Front. Cell. Neurosci. 16, 1025012 (2022).
Park, H. et al. Single-cell RNA-sequencing identifies disease-associated oligodendrocytes in male APP NL-G-F and 5XFAD mice. Nat. Commun. 14, 802 (2023).
Lloyd, A. F. et al. Central nervous system regeneration is driven by microglia necroptosis and repopulation. Nat. Neurosci. 22, 1046–1052 (2019). Study demonstrating that a pro-remyelination microglial state appears by repopulation, following spontaneous death of pro-inflammatory microglia after demyelination.
Shen, K. et al. Multiple sclerosis risk gene Mertk is required for microglial activation and subsequent remyelination. Cell Rep. 34, 108835 (2021).
Dulken, B. W. et al. Single-cell analysis reveals T cell infiltration in old neurogenic niches. Nature 571, 205–210 (2019).
Lewis, N. D., Hill, J. D., Juchem, K. W., Stefanopoulos, D. E. & Modis, L. K. RNA sequencing of microglia and monocyte-derived macrophages from mice with experimental autoimmune encephalomyelitis illustrates a changing phenotype with disease course. J. Neuroimmunol. 277, 26–38 (2014).
Yamasaki, R. et al. Differential roles of microglia and monocytes in the inflamed central nervous system. J. Exp. Med. 211, 1533–1549 (2014).
Hammond, T. R. et al. Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity 50, 253–271.e6 (2019). Study demonstrating transcriptional heterogeneity of microglia by scRNA-seq of mouse brain across the lifespan and after demyelination.
Plemel, J. R. et al. Microglia response following acute demyelination is heterogeneous and limits infiltrating macrophage dispersion. Sci. Adv. 6, eaay6324 (2020). Important study demonstrating the interaction between microglia and monocytes in CNS remyelination, with microglia limiting monocyte entry into lesions.
Grabert, K. et al. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nat. Neurosci. 19, 504–516 (2016). Study demonstrating transcriptional heterogeneity of microglia across brain regions by microarray and how microglia in distinct regions age at different rates.
Safaiyan, S. et al. White matter aging drives microglial diversity. Neuron 109, 1100–1117.e10 (2021). Study demonstrating that microglial transcriptional heterogeneity is regulated by phagocytosis of myelin debris in the ageing white matter.
van Horssen, J. et al. Clusters of activated microglia in normal-appearing white matter show signs of innate immune activation. J. Neuroinflamm. 9, 156 (2012).
Keren-Shaul, H. et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169, 1276–1290.e17 (2017).
Olah, M. et al. Single cell RNA sequencing of human microglia uncovers a subset associated with Alzheimer’s disease. Nat. Commun. 11, 6129 (2020).
Zia, S. et al. Single-cell microglial transcriptomics during demyelination defines a microglial state required for lytic carcass clearance. Mol. Neurodegener. 17, 82 (2022).
Clark, I. C. et al. Barcoded viral tracing of single-cell interactions in central nervous system inflammation. Science 372, eabf1230 (2021).
Haimon, Z. et al. Re-evaluating microglia expression profiles using RiboTag and cell isolation strategies. Nat. Immunol. 19, 636–644 (2018).
Böttcher, C. et al. Single-cell mass cytometry reveals complex myeloid cell composition in active lesions of progressive multiple sclerosis. Acta Neuropathol. Commun. 8, 136 (2020).
Ramaglia, V. et al. Multiplexed imaging of immune cells in staged multiple sclerosis lesions by mass cytometry. eLife 8, e48051 (2019).
Mendiola, A. S. et al. Transcriptional profiling and therapeutic targeting of oxidative stress in neuroinflammation. Nat. Immunol. 21, 513–524 (2020).
Gibson, E. M. et al. Methotrexate chemotherapy induces persistent tri-glial dysregulation that underlies chemotherapy-related cognitive impairment. Cell 176, 43–55.e13 (2019).
Doroshenko, E. R. et al. Peroxisome proliferator-activated receptor-δ deficiency in microglia results in exacerbated axonal injury and tissue loss in experimental autoimmune encephalomyelitis. Front. Immunol. 12, 570425 (2021).
Berglund, R. et al. Microglial autophagy–associated phagocytosis is essential for recovery from neuroinflammation. Sci. Immunol. 5, eabb5077 (2020).
Alam, M. M. et al. Deficiency of microglial autophagy increases the density of oligodendrocytes and susceptibility to severe forms of seizures. eNeuro 8, ENEURO.0183-20.2021 (2021).
Mei, F. et al. Accelerated remyelination during inflammatory demyelination prevents axonal loss and improves functional recovery. Elife 5, e18246 (2016).
Nugent, A. A. et al. TREM2 regulates microglial cholesterol metabolism upon chronic phagocytic challenge. Neuron 105, 837–854.e9 (2020).
Gouna, G. et al. TREM2-dependent lipid droplet biogenesis in phagocytes is required for remyelination. J. Exp. Med. 218, e20210227 (2021).
McNamara, N. B. et al. Microglia regulate central nervous system myelin growth and integrity. Nature 613, 120–129 (2023). Study revealing that microglia are not required for developmental myelination but instead for limiting myelin growth and demyelination.
Rojo, R. et al. Deletion of a Csf1r enhancer selectively impacts CSF1R expression and development of tissue macrophage populations. Nat. Commun. 10, 3215 (2019).
Kiani Shabestari, S. et al. Absence of microglia promotes diverse pathologies and early lethality in Alzheimer’s disease mice. Cell Rep. 39, 110961 (2022).
Munro, D. A. D. et al. CNS macrophages differentially rely on an intronic Csf1r enhancer for their development. Development 147, dev194449 (2020).
Miron, V. E. & Priller, J. Investigating microglia in health and disease: challenges and opportunities. Trends Immunol. 41, 785–793 (2020).
Boche, D. & Gordon, M. N. Diversity of transcriptomic microglial phenotypes in aging and Alzheimer’s disease. Alzheimers Dement. 18, 360–376 (2022).
Bosch-Queralt, M. et al. Diet-dependent regulation of TGFβ impairs reparative innate immune responses after demyelination. Nat. Metab. 3, 211–227 (2021).
Berghoff, S. A. et al. Microglia facilitate repair of demyelinated lesions via post-squalene sterol synthesis. Nat. Neurosci. 24, 47–60 (2021).
Dong, Y. et al. Oxidized phosphatidylcholines found in multiple sclerosis lesions mediate neurodegeneration and are neutralized by microglia. Nat. Neurosci. 24, 489–503 (2021). Study demonstrating the protective nature of microglia via phagocytosis of toxic lipids following demyelination.
Luan, W. et al. Microglia impede oligodendrocyte generation in aged brain. J. Inflamm. Res. 14, 6813–6831 (2021).
Shobin, E. et al. Microglia activation and phagocytosis: relationship with aging and cognitive impairment in the rhesus monkey. GeroScience 39, 199–220 (2017).
Safaiyan, S. et al. Age-related myelin degradation burdens the clearance function of microglia during aging. Nat. Neurosci. 19, 995–998 (2016).
Rawji, K. S. et al. Niacin-mediated rejuvenation of macrophage/microglia enhances remyelination of the aging central nervous system. Acta Neuropathol. 139, 893–909 (2020). Study identifying a therapeutic strategy to rejuvenate microglial function and enhance remyelination in ageing via stimulation of phagocytic potential.
Pluvinage, J. V. et al. CD22 blockade restores homeostatic microglial phagocytosis in ageing brains. Nature 568, 187–192 (2019).
Cuollo, L., Antonangeli, F., Santoni, A. & Soriani, A. The senescence-associated secretory phenotype (SASP) in the challenging future of cancer therapy and age-related diseases. Biology 9, 485 (2020).
van Deursen, J. M. The role of senescent cells in ageing. Nature 509, 439–446 (2014).
Thériault, P. & Rivest, S. Microglia: senescence impairs clearance of myelin debris. Curr. Biol. 26, R772–R775 (2016).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Roy, A. L. et al. A blueprint for characterizing senescence. Cell 183, 1143–1146 (2020).
González-Gualda, E., Baker, A. G., Fruk, L. & Muñoz-Espín, D. A guide to assessing cellular senescence in vitro and in vivo. FEBS J. 288, 56–80 (2021).
Tuttle, C. S. L. et al. Cellular senescence and chronological age in various human tissues: a systematic review and meta-analysis. Aging Cell 19, e13083 (2020).
Yousefzadeh, M. J. et al. Tissue specificity of senescent cell accumulation during physiologic and accelerated aging of mice. Aging Cell 19, e13094 (2020).
Martínez-Cué, C. & Rueda, N. Cellular senescence in neurodegenerative diseases. Front. Cell. Neurosci. 14, 16 (2020).
Nicaise, A. M. et al. Cellular senescence in progenitor cells contributes to diminished remyelination potential in progressive multiple sclerosis. Proc. Natl Acad. Sci. USA 116, 9030–9039 (2019).
Streit, W. J. Microglia and Alzheimer’s disease pathogenesis. J. Neurosci. Res. 77, 1–8 (2004).
Angelova, D. M. & Brown, D. R. Microglia and the aging brain: are senescent microglia the key to neurodegeneration? J. Neurochem. 151, 676–688 (2019).
Streit, W. J., Xue, Q.-S., Tischer, J. & Bechmann, I. Microglial pathology. Acta Neuropathol. Commun. 2, 142 (2014).
Streit, W. J., Khoshbouei, H. & Bechmann, I. Dystrophic microglia in late-onset Alzheimer’s disease. Glia 68, 845–854 (2020).
Streit, W. J., Sammons, N. W., Kuhns, A. J. & Sparks, D. L. Dystrophic microglia in the aging human brain. Glia 45, 208–212 (2004).
Streit, W. J., Braak, H., Xue, Q.-S. & Bechmann, I. Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer’s disease. Acta Neuropathol. 118, 475–485 (2009).
Shahidehpour, R. K. et al. Dystrophic microglia are associated with neurodegenerative disease and not healthy aging in the human brain. Neurobiol. Aging 99, 19–27 (2021).
Neumann, P., Lenz, D. E., Streit, W. J. & Bechmann, I. Is microglial dystrophy a form of cellular senescence? An analysis of senescence markers in the aged human brain. Glia 71, 377–390 (2023).
Gorgoulis, V. et al. Cellular senescence: defining a path forward. Cell 179, 813–827 (2019).
Zhou, D., Borsa, M. & Simon, A. K. Hallmarks and detection techniques of cellular senescence and cellular ageing in immune cells. Aging Cell 20, e13316 (2021).
Hu, Y. et al. Replicative senescence dictates the emergence of disease-associated microglia and contributes to Aβ pathology. Cell Rep. 35, 109228 (2021).
Bussian, T. J. et al. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature 562, 578–582 (2018).
Xie, Y.-Y. et al. Clemastine ameliorates myelin deficits via preventing senescence of oligodendrocytes precursor cells in Alzheimer’s disease model mouse. Front. Cell Dev. Biol. 9, 733945 (2021).
Zhang, P. et al. Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat. Neurosci. 22, 719–728 (2019).
Ritschka, B. et al. The senescence-associated secretory phenotype induces cellular plasticity and tissue regeneration. Genes Dev. 31, 172–183 (2017).
Linnerbauer, M., Wheeler, M. A. & Quintana, F. J. Astrocyte crosstalk in CNS inflammation. Neuron 108, 608–622 (2020).
Rothhammer, V. et al. Microglial control of astrocytes in response to microbial metabolites. Nature 557, 724–728 (2018).
Skripuletz, T. et al. Astrocytes regulate myelin clearance through recruitment of microglia during cuprizone-induced demyelination. Brain 136, 147–167 (2013).
Sen, M. K., Mahns, D. A., Coorssen, J. R. & Shortland, P. J. The roles of microglia and astrocytes in phagocytosis and myelination: Insights from the cuprizone model of multiple sclerosis. Glia 70, 1215–1250 (2022).
Bellver-Landete, V. et al. Microglia are an essential component of the neuroprotective scar that forms after spinal cord injury. Nat. Commun. 10, 518 (2019).
Brennan, F. H. et al. Microglia coordinate cellular interactions during spinal cord repair in mice. Nat. Commun. 13, 4096 (2022).
Greenhalgh, A. D. et al. Peripherally derived macrophages modulate microglial function to reduce inflammation after CNS injury. PLoS Biol. 16, e2005264 (2018).
De Schepper, S. et al. Perivascular cells induce microglial phagocytic states and synaptic engulfment via SPP1 in mouse models of Alzheimer’s disease. Nat. Neurosci. 26, 406–415 (2023).
Perry, V. H. & Holmes, C. Microglial priming in neurodegenerative disease. Nat. Rev. Neurol. 10, 217–224 (2014).
Xie, J. et al. Low-grade peripheral inflammation affects brain pathology in the AppNL-G-Fmouse model of Alzheimer’s disease. Acta Neuropathol. Commun. 9, 163 (2021).
Tejera, D. et al. Systemic inflammation impairs microglial Aβ clearance through NLRP3 inflammasome. EMBO J. 38, e101064 (2019).
García-Domínguez, I. et al. Peripheral inflammation enhances microglia response and nigral dopaminergic cell death in an in vivo MPTP model of Parkinson’s disease. Front. Cell Neurosci. 12, 398 (2018).
Kho, Z. Y. & Lal, S. K. The human gut microbiome – a potential controller of wellness and disease. Front. Microbiol. 9, 1835 (2018).
Chen, J. et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci. Rep. 6, 28484 (2016).
Vogt, N. M. et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 7, 13537 (2017).
Zapała, B. et al. Differences in the composition of gut microbiota between patients with Parkinson’s disease and healthy controls: a cohort study. J. Clin. Med. 10, 5698 (2021).
Zhai, C.-D., Zheng, J.-J., An, B.-C., Huang, H.-F. & Tan, Z.-C. Intestinal microbiota composition in patients with amyotrophic lateral sclerosis: establishment of bacterial and archaeal communities analyses. Chin. Med. J. 132, 1815–1822 (2019).
Onisiforou, A. & Spyrou, G. M. Immunomodulatory effects of microbiota-derived metabolites at the crossroad of neurodegenerative diseases and viral infection: network-based bioinformatics insights. Front. Immunol. 13, 843128 (2022).
Erny, D. et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 18, 965–977 (2015). First study demonstrating the impact of the gut microbiome on microglial responses.
McMurran, C. E. et al. The microbiota regulates murine inflammatory responses to toxin-induced CNS demyelination but has minimal impact on remyelination. Proc. Natl Acad. Sci. USA 116, 25311–25321 (2019). First study assessing the impact of manipulating the gut microbiome on microglial responses and remyelination efficiency.
Chen, T., Noto, D., Hoshino, Y., Mizuno, M. & Miyake, S. Butyrate suppresses demyelination and enhances remyelination. J. Neuroinflamm. 16, 165 (2019).
Wuerch, E., Lozinski, B. & Yong, V. W. MedXercise: a promising strategy to promote remyelination. Curr. Opin. Pharmacol. 61, 120–126 (2021).
Jensen, S. K. et al. Multimodal enhancement of remyelination by exercise with a pivotal role for oligodendroglial PGC1α. Cell Rep. 24, 3167–3179 (2018). Critical study demonstrating the positive impact of exercise on oligodendrocyte lineage cell responses and remyelination in mice.
Lozinski, B. M. & Yong, V. W. Exercise and the brain in multiple sclerosis. Mult. Scler. 28, 1167–1172 (2022).
Zaychik, Y. et al. High-Intensity exercise training protects the brain against autoimmune neuroinflammation: regulation of microglial redox and pro-inflammatory functions. Front. Cell. Neurosci. 15, 640724 (2021).
Lozinski, B. M. et al. Exercise rapidly alters proteomes in mice following spinal cord demyelination. Sci. Rep. 11, 7239 (2021).
Garraud, O., Hozzein, W. N. & Badr, G. Wound healing: time to look for intelligent, ‘natural’ immunological approaches? BMC Immunol. 18, 23 (2017).
Brickman, A. M. et al. Regional white matter hyperintensity volume, not hippocampal atrophy, predicts incident alzheimer disease in the community. Arch. Neurol. 69, 1621–1627 (2012).
Brickman, A. M. et al. Reconsidering harbingers of dementia: progression of parietal lobe white matter hyperintensities predicts Alzheimer’s disease incidence. Neurobiol. Aging 36, 27–32 (2015).
Tosto, G. et al. The effect of white matter hyperintensities on neurodegeneration in mild cognitive impairment. Alzheimers Dement. 11, 1510–1519 (2015).
McAleese, K. E. et al. Cortical tau load is associated with white matter hyperintensities. Acta Neuropathol. Commun. 3, 60 (2015).
Braak, H. & Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82, 239–259 (1991).
Bartzokis, G., Lu, P. H. & Mintz, J. Human brain myelination and amyloid beta deposition in Alzheimer’s disease. Alzheimers Dement. 3, 122–125 (2007).
Braak, H. & Braak, E. Development of Alzheimer-related neurofibrillary changes in the neocortex inversely recapitulates cortical myelogenesis. Acta Neuropathol. 92, 197–201 (1996).
Radde, R. et al. Aβ42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep. 7, 940–946 (2006).
Jankowsky, J. L. & Zheng, H. Practical considerations for choosing a mouse model of Alzheimer’s disease. Mol. Neurodegener. 12, 1–22 (2017).
Saito, T. et al. Single App knock-in mouse models of Alzheimer’s disease. Nat. Neurosci. 17, 661–663 (2014).
Deczkowska, A. et al. Disease-associated microglia: a universal immune sensor of neurodegeneration. Cell 173, 1073–1081 (2018).
Li, Q. et al. Developmental heterogeneity of microglia and brain myeloid cells revealed by deep single-cell RNA aequencing. Neuron 101, 207–223.e10 (2019).
Chen, Y. & Colonna, M. Microglia in Alzheimer’s disease at single-cell level. Are there common patterns in humans and mice? J. Exp. Med. 218, e20202717 (2021).
Lee, K.-A., Flores, R. R., Jang, I. H., Saathoff, A. & Robbins, P. D. Immune senescence, immunosenescence and aging. Front. Aging 3, 900028 (2022).
Rodier, F. & Campisi, J. Four faces of cellular senescence. J. Cell Biol. 192, 547–556 (2011).
Walford, R. L. The immunologic theory of aging. Immunol. Rev. 2, 171–171 (1969).
Huang, W., Hickson, L. J., Eirin, A., Kirkland, J. L. & Lerman, L. O. Cellular senescence: the good, the bad and the unknown. Nat. Rev. Nephrol. 18, 611–627 (2022).
Acknowledgements
S.A.K. discloses support for this work from the Wellcome Trust Translational Neuroscience PhD Programme (108890/Z/15/Z) at The University of Edinburgh. V.E.M. discloses support for this work from the John David Eaton Chair in Multiple Sclerosis Research (St Michael’s Hospital Foundation and Barlo MS Centre) and a Medical Research Council Senior Non-Clinical Fellowship.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the Review.
Corresponding author
Ethics declarations
Competing interests
V.E.M. currently receives research funds from Astex Pharmaceuticals relating to some of the topics covered in this Review. S.A.K. declares no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks V. Wee Yong and the other anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- 3×Tg mouse model of Alzheimer disease
-
Mice that harbour the human APP transgene containing the Swedish mutation, a PSEN1 knock-in with the M146V mutation and the MAPT transgene containing the P301L mutation. Unlike some of the other commonly used Alzheimer disease mouse models, this model progressively develops both amyloid-β plaques and neurofibrillary tangles, with extracellular amyloid-β deposited at 6 months of age and hyperphosphorylated tau aggregates observed at 12–15 months of age. These mice exhibit synaptic dysfunction before the detection of plaques and tangles, and cognitive impairment is seen at 4 months of age.
- App NL-G-F mouse model of Alzheimer disease
-
Mice that possess a single amyloid-β precursor protein gene (APP) knock-in, containing a humanized amyloid-β domain with three mutations, namely the Swedish ‘NL’, Arctic ‘G’ and Iberian ‘F’ mutations. Although APP is expressed at wild-type levels, the NL, G and F mutations boost total amyloid-β production, encourage amyloid-β aggregation and increase the amyloid-β42:amyloid-β40 ratio, respectively. These mice exhibit amyloid-β plaque accumulation with deposition beginning at 2 months of age. They also display microgliosis, astrocytosis and synapse loss, in addition to cognitive impairment at 6 months of age. Neurofibrillary tangles are absent from this model.
- APP/PS1 mouse model of Alzheimer disease
-
Mice that express two human transgenes for APP and PSEN1 (presenilin-1, also known as PS1), which contain the Swedish and L166P mutations, respectively. Both are under the control of the Thy1 promoter, and the mice demonstrate overexpression of APP with levels approximately threefold higher than those endogenously expressed. Amyloid-β deposition begins at 6 weeks of age, with microgliosis and astrocytosis, and dendritic spine loss is also observed in the mice. Cognitive impairment is seen at 7 months of age, and neurofibrillary tangles are absent from this model.
- Border-associated macrophages
-
Also known as central nervous system (CNS)-associated macrophages, these macrophages reside in the border regions of the CNS including the perivascular spaces, the choroid plexus and the meninges.
- Dietary cuprizone-induced demyelination
-
In this model, mice are fed with the copper chelator cuprizone, leading to oligodendrocyte death and subsequent demyelination followed by spontaneous remyelination, which is initiated during the late demyelination phase and continues robustly over the subsequent 3–6 weeks after withdrawal of cuprizone.
- Lysophosphatidylcholine (LPC)-induced demyelination
-
A widely used model to study remyelination, involving injection of LPC to induce focal demyelination in either the corpus callosum or spinal cord, which is typically complete by 3 days after injection and is followed by robust remyelination without ongoing demyelination over the subsequent 2–4 weeks.
- Monocytes
-
Monocytes originate from haematopoietic stem and progenitor cells in the bone marrow, from which they emigrate to circulate in the blood before entering tissues and differentiating into either macrophages or monocyte-derived dendritic cells.
- PDGF–APP Sw.Ind mouse model of Alzheimer disease
-
Mice that express the human APP transgene containing the Swedish and Indiana (V717F) mutations, under the control of the PDGFB promoter. These mice overexpress APP, with amyloid-β seen at 6 weeks of age and plaques evident at 5–7 months of age. This model also demonstrates synapse loss, astrogliosis and microgliosis, in addition to cognitive deficits by 4 months of age. Neurofibrillary tangles are absent from this model.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kent, S.A., Miron, V.E. Microglia regulation of central nervous system myelin health and regeneration. Nat Rev Immunol 24, 49–63 (2024). https://doi.org/10.1038/s41577-023-00907-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-023-00907-4
This article is cited by
-
Preclinical translational platform of neuroinflammatory disease biology relevant to neurodegenerative disease
Journal of Neuroinflammation (2024)
-
Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases
Signal Transduction and Targeted Therapy (2024)
-
Neural circuits regulating visceral pain
Communications Biology (2024)
-
Commentary on: Bai, X., Zhao, N., Koupourtidou, C., Fang, L.-P., Schwarz, V., Caudal, L.C., Zhao, R., Hirrlinger, J., Walz, W., Bian, S., Huang, W., Ninkovic, J., Kirchhoff, F., Scheller, A. “In the mouse cortex, oligodendrocytes regain a plastic capacity, transforming into astrocytes after acute injury”
Pflügers Archiv - European Journal of Physiology (2023)