Abstract
Breakthrough infections with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in fully vaccinated individuals are receiving intense scrutiny because of their importance in determining how long restrictions to control virus transmission will need to remain in place in highly vaccinated populations as well as in determining the need for additional vaccine doses or changes to the vaccine formulations and/or dosing intervals. Measurement of breakthrough infections is challenging outside of randomized, placebo-controlled, double-blind field trials. However, laboratory and observational studies are necessary to understand the impact of waning immunity, viral variants and other determinants of changing vaccine effectiveness against various levels of coronavirus disease 2019 (COVID-19) severity. Here, we describe the approaches being used to measure vaccine effectiveness and provide a synthesis of the burgeoning literature on the determinants of vaccine effectiveness and breakthrough rates. We argue that, rather than trying to tease apart the contributions of factors such as age, viral variants and time since vaccination, the rates of breakthrough infection are best seen as a consequence of the level of immunity at any moment in an individual, the variant to which that individual is exposed and the severity of disease being considered. We also address key open questions concerning the transition to endemicity, the potential need for altered vaccine formulations to track viral variants, the need to identify immune correlates of protection, and the public health challenges of using various tools to counter breakthrough infections, including boosters in an era of global vaccine shortages.
Similar content being viewed by others
Introduction
No vaccine is perfectly effective, even those against yellow fever, which seem to be very close1. For a virus like severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), sterilizing immunity is difficult to achieve, even with vaccines, and protection is expected to decline with time since vaccination2. Therefore, the key questions for scientists studying breakthrough infections — a term used to describe infections in fully vaccinated people — surround their timing, frequency, causes, severity and levels of infectiousness.
The answers to these questions matter for several reasons. First, identifying the frequency, severity and causes of breakthrough infections may inform the choice of public health responses: watchful waiting may be appropriate if breakthroughs are comparatively rare or mild and unlikely to markedly increase transmission rates. By contrast, if breakthrough infections are common, severe or highly transmissible, then there may be a need for additional vaccine doses, changes in vaccine formulations or non-pharmaceutical interventions (or a combination of these approaches) to reduce the incidence of infection. Identifying the range of clinical outcomes seen in breakthrough infections and determining how severe they can be as well as which clinical and demographic individual characteristics are associated with a severe outcome, will indicate how information about vaccination history can be used in prognostic scores to identify who should receive priority for additional vaccinations or treatments.
In this Perspective, we first describe the approaches used to measure breakthrough infections and then consider the causes and impact of these breakthrough infections. Finally, we discuss some of the critical questions that remain to be addressed concerning breakthrough infections.
Measuring breakthrough infections
When a population reaches a high enough level of vaccine coverage, most infections will occur in vaccinated people, simply because most people are vaccinated3. Therefore, to interpret the occurrence of breakthrough infections, it is important to compare the incidence rate of breakthrough infections to the rate of (non-breakthrough) infections in unvaccinated people who, apart from their vaccination status, are similar to the vaccinated. This comparison provides an estimate of vaccine effectiveness. We define vaccine effectiveness (generically to include efficacy as measured in trials) as the proportional reduction caused by vaccination in the probability that a single exposure will give rise to an infection4.
Measuring vaccine effectiveness is challenging for several reasons. Given the substantial burden of infection before vaccines became available, some individuals who are unvaccinated will nonetheless have some immunity as a result of prior infection, complicating comparisons of immunity between these groups (although there are approaches to account for this complexity)5,6. Additionally, SARS-CoV-2 infection has a spectrum of disease severity from asymptomatic to fatal, and vaccine effectiveness against each outcome may be different7. Initial phase III randomized, double-blind, placebo-controlled trials (RCTs) mainly used PCR-confirmed symptomatic disease as a primary end point8,9,10, although the Janssen vaccine trial used moderate-to-severe/critical coronavirus disease 2019 (COVID-19) as its primary end point.11 To measure all infections (whether symptomatic or not), some reported post-trial serological measurements (detecting antibodies to SARS-CoV-2 antigens not contained in the vaccine)11 as a secondary end point, while others estimated the reduction in prevalence of infection among participants tested irrespective of symptoms or asymptomatic participants8,10. Effectiveness against more severe outcomes, such as hospitalization or development of severe, critical or fatal COVID-19, has also been standard in phase III trials, although not all trials have had the power to make precise estimates of efficacy against the rarer, more severe end points9,10,11,12 or in subgroups of the population defined by age or comorbidities13.
The advantage of randomized trials is that, when well-designed and adequately sized, they ensure comparability of the vaccinated and unvaccinated people by assigning vaccination at random and blinding participants and researchers to the vaccine status of each individual. These features promote confidence that any differences observed in infection rates are due to the biological effect of the vaccine rather than due to other differences between those who did and did not receive it. Counteracting this advantage are several important limitations in what RCTs can measure.
For example, a critical public health question at the time of writing is to what extent protection from vaccines declines as time passes or as new viral variants circulate, thus increasing the rate of breakthrough infections given a particular level of exposure. Long-term measurement of vaccine efficacy in phase III RCTs has been limited because randomized efficacy trials of vaccines offered vaccination to placebo recipients soon after the vaccines became authorized for emergency use14. Nonetheless, such data were available for a period up to 6 months post first dose15,16). Moreover, unpublished data from the open-label phase III clinical trials of the Pfizer17 and Moderna18 mRNA vaccines compares breakthrough infections during a period in July and August 2021 among individuals randomized to vaccination at the start of the trial versus those originally randomized to placebo who received the vaccine later, following unblinding. In each case, breakthroughs were more frequent in the earlier-vaccinated individuals, providing randomized evidence for waning vaccine efficacy.
Another limitation of RCT data is that RCTs have been able to precisely estimate protection against only one viral variant, rather than to compare protection between variants. The timing of phase III trials was such that, in each country, one variant was dominant during the trial period. Efficacy in each country was thus assessed mainly against one variant19,20 and therefore higher rates of breakthrough infections in a country with a certain variant (in particular the Beta (B.1.351) variant in South Africa)20 could not conclusively be attributed to the variant as other factors also differed between the countries. Likewise, each major RCT, sponsored by the manufacturer of one vaccine, has compared that vaccine against placebo, preventing a head-to-head comparison of more than one vaccine in an RCT setting21. Observational studies have addressed, fully or partially, each of these limitations of RCTs by comparing rates in unvaccinated people to those in vaccinated people to assess effectiveness during periods of predominance of the Delta (B.1.617.2) variant22,23,24, comparing effectiveness between Delta and prior variants25,26,27, comparing different vaccine products22,26,28,29,30,31, or following vaccinated individuals post-vaccination to assess waning23,24,27. Observational studies can also achieve higher sample sizes, thereby making precise estimates of vaccine effectiveness in small subgroups of the population, for example, in distinct 10-year age groups32,33, in patients with solid organ tumours34 or in pregnant women35.
In settings with well-followed cohorts, such as integrated health-care organizations or cohorts of health-care workers, it has been possible to emulate randomized efficacy trials with cohort studies27,28,33,35,36. In some such studies, vaccinated and unvaccinated persons are matched on a number of potential confounders in order to make them as similar as possible apart from their vaccine status33,35. The availability of ‘gold-standard’ evidence from a randomized trial that the effect of Pfizer-BioNTech COVID-19 vaccination begins 10–14 days after the first dose9 provided a negative control outcome37 whereby investigators could assess how well vaccinated and unvaccinated individuals had been matched by showing that no difference in the rate of breakthrough infections occurred in this early post-vaccine period33,35,36. This approach found consistently high effectiveness in the early months after the second dose across various disease outcomes and across multiple subgroups in the population, with some small exceptions.
A second prospective, observational approach to estimate vaccine effectiveness compares incidence rates of infection (and more severe outcomes) each week among vaccinated versus unvaccinated individuals, stratified by age group and sex. Such a study in Israel32 found similar results to those in trials and in observational cohort studies.
Retrospective case–control studies, in which COVID-19 cases are detected and then vaccine status ascertained, have been a more common approach to evaluate COVID-19 vaccine effectiveness, in part because this approach requires far less infrastructure than prospective designs. The World Health Organization recommends38 this approach, and specifically the test-negative design39 in which laboratory-confirmed COVID-19 cases are compared against individuals that are tested for SARS-CoV-2 infection due to similar symptoms but are negative on the test. This approach has been widely used in the past for evaluation of effectiveness of influenza vaccines and numerous other vaccines40. It is susceptible to several sources of bias common to other observational studies and some that are specific to this design37,39, yet a number of approaches exist to mitigate these biases, making it a preferred option in many settings22,24,38.
Experience with COVID-19 has stimulated a number of new approaches to estimating, eliminating or compensating for biases in estimates of vaccine effectiveness23,33,41 as well as the pioneering of new study designs, such as contact tracing-based vaccine effectiveness studies42,43, to estimate the reduced risk of COVID-19 infection given a close contact with an infected person44. Cohorts of closely monitored health-care workers have been especially informative in studying vaccine effectiveness, breakthrough infections and the causes of each of these36,45,46,47.
In vitro measurements of antibody levels or activity can shed light on the comparative risk of breakthrough infections. Neutralization assays provide quantifiable data on the ability of SARS-CoV-2-specific antibodies in a given sample to prevent the virus from infecting cells. While the gold standard neutralization assay uses live virus, the requirement for a BSL3 facility and the long incubation time prompted the development of SARS-CoV-2 pseudotyped viral particles, which express, upon infection, only a reporter protein and thus need a shorter incubation time and can be used under BSL2 conditions. As SARS-CoV-2 pseudotyped particles contain only the spike protein, they are not suitable for research on functions and processes related to other viral proteins and neutralizing antibodies identified by this approach should be validated using live virus neutralization. However, overall, they are considered a useful virological tool for the study of SARS-CoV-2. When such in vitro measurements are combined with population-level48,49,50 or individual-level45,51 observations of the level of protection a vaccine offers, they can identify immune correlates of protection52,53. Neutralizing activity and, to a lesser degree, the quantity of anti-spike IgG, have been suggested as partial correlates of protection. In vitro measurements of these parameters have shown that they decline with time since vaccination54,55 and that there is reduced activity against some viral variants (see below), providing an independent line of evidence on increased risk of breakthrough infections with time and variants. These can be particularly important for deciding whether a new vaccine formulation is needed to counter breakthrough infections with viral variants. For example, data showing that a third dose of the Pfizer-BioNTech vaccine in the original formulation induced high neutralizing titres against the Delta variant17 was a consideration in recommending a third dose rather than reformulating the vaccine to track variant evolution. The same has been shown for the Moderna vaccine56.
Evidence about breakthrough infections should be interpreted in the context of the type of study in which they are measured. The strength of evidence depends on the rigour, size and quality of individual studies and becomes greatest when multiple approaches to measurement in different settings reach similar conclusions.
Causes of breakthrough infections
Vaccines against viruses work by generating immune responses that inhibit the infection process (mainly serum antibodies that bind and/or neutralize virus particles and, for mucosally applied vaccines, also mucosal secretory IgA) and by creating immune memory in the form of antigen-specific memory B cells and T cells that are primed to produce a rapid anamnestic response when the infection reintroduces the vaccine antigen into the body. These mechanisms can prevent initial proliferation of the virus or, failing that, rapidly control it, reducing the amount of virus to which the host is ultimately exposed and the duration of the exposure. While the amount of circulating antibody present following vaccination (or any antigenic stimulus) increases rapidly, on a timescale of days to weeks, it also declines rapidly from its peak on a timescale of weeks to months47, and then more slowly over a time scale of decades57. The first phase reflects antibody secreted by short-lived plasmablast populations, which expand right after antigen exposure as a first line of defense58,59. They typically die within 1–2 weeks after antigen exposure and the antibody they secreted declines based on the specific antibody half-life (approximately 21 days for IgG). The second, usually very slow, phase of decline likely reflects the kinetics of long-lived plasma cells, which migrate to the bone marrow and from there secrete antibody into the blood, often maintaining stable titres for many years60,61. Importantly, although peripherally injected vaccines can induce low levels of IgG and monomeric IgA antibodies at the mucosal surfaces of the upper respiratory tract (which are the main entry portal for respiratory viruses) they do not induce secretory IgA efficiently62. The small proportions of IgG and IgA that land on the mucosal surfaces of the upper respiratory tract after intramuscular vaccination disappear relatively quickly as serum antibodies wane.
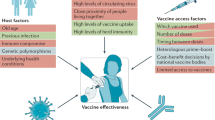
Whether a breakthrough infection occurs when a vaccinated host is exposed to an infectious person depends on whether the immune response present in that person at the moment of exposure is sufficient to abort or rapidly control the infection (Fig. 1). Given the kinetics of immune responses, it is not surprising that the amount of protection offered by a vaccine against infection might decline over time, allowing more breakthrough infections as the immune response wanes over months (as observed for influenza virus vaccines63) and/or as immune memory wanes over years (as observed for mumps vaccines64). Likewise, protection might increase after a breakthrough infection or after a subsequent vaccine dose, which enhances the person’s immune response. It is also unsurprising that older individuals, whose neutralizing antibody responses to COVID-19 vaccines are typically lower65, appear to be at greater risk of breakthrough infections at any given time following vaccination23,25.
The figure illustrates how the interplay between the age of the vaccinated individual, immune competent or compromised state, the variant of SARS-CoV-2, and time since vaccination determine the susceptibility to breakthrough infection. The blue lines chart the levels of immune response that develop following a two-dose primary vaccine regimen, which peaks and wanes (first rapidly and the more slowly) and is then boosted by a third dose (booster) 6–7 months after the second. At the time of writing, insufficient data exist to define the kinetics of immunity following the third dose. The outcome of an exposure (protection or breakthrough) depends on the relative magnitude of (1) the current level of immunity of an individual and the level required to prevent infection (long dashed lines) or severe disease (short dashed lines) with a variant that is well matched to the vaccine, such as the Alpha variant (indicated in beige), or is a less well matched variant, such as the Delta variant (indicated in red). Increased age (and some other factors, such as an immunocompromised state) are associated with lower initial immune responses to primary vaccination and to booster (indicated by light blue line) compared with those of a healthy, younger individual (indicated by dark blue line).
Besides time since vaccination, several factors can modulate vaccine effectiveness and thus the probability of breakthrough infection. Clearly, different COVID-19 vaccines provide different levels of immunity following immunization and thus have varying effectiveness48,49. For some COVID-19 vaccines, there is evidence that increasing the time interval between the first and second dose can increase immune responses and protection12,66,67,68,69,70. In addition, COVID-19 vaccination is less immunogenic in individuals with various immunocompromising conditions71,72,73. Moreover, there is evidence that, among vaccinated individuals, those with haematological neoplasms experience substantially higher rates of SARS-CoV-2 infection and/or severe COVID-19 (ref.74).
The genetic variant of SARS-CoV-2 to which one is exposed can also affect the degree of protection offered by vaccine-induced immune responses. In vitro studies show reduced neutralization of some virus variants by sera from vaccinated people. For example, sera from vaccinated individuals showed a 3–15-fold reduction in neutralizing titres for the Beta variant of SARS-CoV-2 and 1.4–3-fold lower neutralizing titres for the Delta variant compared with earlier variants of SARS-CoV-2 (refs54,66,75,76,77,78,79). This in vitro evidence is largely consistent with evidence from epidemiological studies. All else equal, several studies suggest that the probability of breakthrough infection is higher with the Delta variant than with the Alpha variant22,24. Such comparisons are challenging and require assumptions and statistical adjustment because, in each location, there was only a short period in which the two variants co-circulated and could be directly compared. There is also evidence from case–control studies in Qatar80 and Israel81 of reduced vaccine effectiveness against the Beta variant compared with the Alpha variant, although another contact tracing-based study43 found vaccine effectiveness against the Beta variant in exposed individuals to be similar to that previously found against the Alpha variant42.
Modelling and experiences with other vaccines suggest that exposure to a higher viral inoculum can reduce vaccine effectiveness and increase the probability of breakthrough infection82,83. If this effect were important for SARS-CoV-2, it could imply that populations employing better non-pharmaceutical interventions (such as masking) that reduce typical viral inoculums would see higher vaccine effectiveness, although any synergy between masking and vaccination is speculative at present. Higher viral exposures could also help explain why the Delta variant causes breakthrough infections more than other variants as, in some studies, infection with the Delta variant has been associated with higher viral loads84,85. In addition, there are several other virological factors that could facilitate Delta variant breakthrough infections, including a shorter incubation time85, which leaves less time for immune memory to respond and higher fusogenicity of the spike protein86,87, which may facilitate fusion of the virus at the cell membrane instead of in the endosome and which may also increase spread of the virus from cell to cell in the lungs, leading to reduced effectiveness of humoural immunity.
In the early months after vaccination, mRNA vaccines had efficacy9,10 (as measured in randomized trials) and effectiveness32,33 (as measured in population-wide observational studies) of well above 90% for a range of disease outcomes (from symptomatic infection to death). These vaccines were also shown to be highly effective irrespective of age group and other factors, although effectiveness against any infection (irrespective of symptoms) was somewhat lower42. Given that the maximum protection from a vaccine is 100%, we can understand that these determinants might have been comparatively unimportant in the presence of the Alpha variant for freshly vaccinated persons, in whom nearly everyone would have achieved an adequate level of response to prevent symptomatic infection, as was the case in randomized trials and early observational studies of the mRNA vaccines. As immunity has waned to some degree, the most noticeable declines of vaccine effectiveness have been for asymptomatic infections, in the milder infection outcomes, in older individuals, in those vaccinated earliest, and likely in the presence of the Delta variant23,25,88. With vaccines that were initially less protective against infection or symptomatic infection, such as adenoviral vectored vaccines, there was evidence of greater efficacy against severe outcomes even in the initial trials8,11.
As it usually takes several days from initial SARS-CoV-2 infection to development of severe disease, it is plausible that this time frame is sufficient for the memory immune response to become effective. The longer time available to mount an effective immune response before severe disease sets in may be the reason for the relatively high vaccine effectiveness against severe disease observed even as time since vaccination passes and with the Delta variant circulation. The greater effectiveness (equivalently, lower degree of vaccine-induced immunity required) for more severe outcomes is consistent with that observed for vaccines against other respiratory infections, such as Streptococcus pneumoniae89,90 and influenza virus2.
If variants, waning immunity and age all contribute to breakthrough infections, what is the relative contribution of each of these? While this is a natural question, we propose that, given observations to date with COVID-19, there is no simple answer, even in principle. Rather, we argue that the data are consistent with a model (Fig. 1) in which the degree of protection depends on the strength of immunity at the moment when an individual is exposed. This level depends on several factors: the initial immune response is lower in older adults and declines in all individuals from a peak in the early weeks after vaccination. Moreover, higher levels of immunity are required to prevent milder disease (as described above) and to protect against the Delta variant compared with the Alpha variant, for any given severity level. This model implies that age sets a lower peak response, time reduces the response and different variants are differentially affected by the response.
While sera from vaccinated individuals neutralize the Delta variant less efficiently than earlier variants, individuals boosted with a third dose of mRNA vaccine neutralize Delta efficiently56,91 even though that third dose encodes the original SARS-CoV-2 spike protein rather than a Delta variant-specific spike protein. Moreover, individuals with a third dose are significantly protected against infection at a time when Delta is circulating92.
The ability of quantity to compensate for quality in immune responses to SARS-CoV-2 variants — and, in particular, the fact that vaccines designed against older variants, although showing somewhat lower neutralization against later variants such as Delta, can nonetheless be protective against infection and severe outcomes with these later variants when they achieve high enough titres92,93,94 — may or may not be a general phenomenon. It is imaginable that future variants of SARS-CoV-2 may arise in which escape from immunity is so complete that boosting with the original spike protein is ineffectual. In this case, a short-term public health response to an increase in severe breakthrough infections would be to reimpose social measures to slow transmission while a medium-term solution would be to develop and rapidly deploy vaccines that more closely match the circulating variant. One advantage that has been touted for mRNA vaccines is the ability to rapidly change the antigen. Such updates could follow the approach by which influenza vaccinations are updated as influenza viruses change antigenically95. A challenge for regulators will be to determine whether vaccines targeting such novel antigenic variants of SARS-CoV-2 will require full human safety and efficacy trials or whether, as for influenza virus vaccination, they can be treated as strain changes to already proven vaccines and given more limited testing to speed their availability.
Impact of breakthrough infections
The nature and scale of response to breakthrough infections with SARS-CoV-2 depends on their severity, distribution in the population and contribution to transmission. At one extreme, mild breakthrough infections that are not very infectious pose little danger to the vaccinated person and little danger of fuelling future surges and, indeed, may boost the individual’s immune responses; such cases would call for little or no public health response beyond monitoring.
Early experience in the era of Alpha, when most vaccinated individuals had received their vaccines only in recent months, showed lower viral loads in those with breakthrough infections42, and measured viral RNA levels were correlated with low antibody levels around the time of infection45. Soon after vaccination and in the era of Alpha, vaccination reduced household transmission to unvaccinated individuals96. Evidence on the infectiousness of Delta variant breakthrough cases remains limited. At the time of writing, it consists largely (though not exclusively97) of viral RNA quantification at a single point in time98,99. A third dose seems to reduce viral loads in breakthrough infections, including with the Delta variant99.
Measuring the infectiousness of breakthrough infections has several subtleties100,101. The most common quantitative measure of viral load is the PCR cycle threshold (Ct), which measures how many cycles of PCR are required to amplify DNA made from viral RNA to a detectable level, so that larger numbers mean smaller amounts of viral RNA. While the Ct value is often taken as a measure of infectiousness, studies have found that, for a given Ct value, the probability of testing positive by other measures of infectiousness, such as antigen tests42 or viral culture102, are lower in vaccinated than in unvaccinated individuals. One interpretation is that more of the viral RNA shed by vaccinated people who are infected is non-viable, so such breakthrough infections may be less infectious even if they have the same Ct as an infection in an unvaccinated person. A further issue is that single-timepoint measures of viral load may depend on host factors other than vaccination and may also reflect the rate of growth or decline in the particular viral variant in the host population103, further complicating interpretation.
Contact tracing-based studies are another approach that more directly measures the infectiousness of breakthrough cases, comparing the probability of infection in contacts of vaccinated index cases versus unvaccinated ones. A preprint study from the UK showed that vaccinated index cases were less likely to infect their contacts. Additionally, it found that the Pfizer vaccine was more protective than the AstraZeneca vaccine and that each vaccine was more protective against transmission of the Alpha variant than of the Delta variant104.
Because vaccination may change the kinetics of viral shedding97 and the relationship between viral load and symptoms, interventions directed at individual people who are infected, such as isolation and contact tracing, may be more or less effective, and thus may need to be modified if breakthrough cases are common and infectious. More generally, the degree of infectiousness of breakthrough cases may inform planning and response to prevent additional surges.
COVID-19 is associated with a plethora of sequelae on patient health and wellbeing105 — some of these are abrupt but others may linger for prolonged periods of time. These may extend beyond the acute pulmonary inflammation and generalized inflammatory response to include additional complications for which the underlying mechanisms are less clear.106 These post-acute sequelae of SARS-CoV-2 infection (PASC, also referred to as long COVID) have been observed in both severe and mild or even in asymptomatic cases45 of SARS-CoV-2 infection but are significantly reduced in breakthrough infections.107
Despite the strong protection that vaccination provides against severe outcomes108, breakthrough infections have been shown to progress to severe illness at non-negligible rates. In late July to early August 2021, at the outset of a surge of cases in Israel, the majority of severe COVID-19 cases were documented among individuals who had been vaccinated with two doses of the Pfizer vaccine109. These cases occurred at a time when most of the highest-risk age groups were ≥5 months past their second vaccine dose, and this was a factor in leading Israel to offer a third dose of the vaccine, which drastically reduced the incidence of severe disease in those who received it, across age groups94.
In children, a severe manifestation of SARS-CoV-2 infection is the multisystem inflammatory syndrome in children (MIS-C), which requires intensive care in the majority of cases. In unvaccinated populations, MIS-C has been shown to occur in around 1 in every 3,200 children post-infection and to mostly occur in previously healthy children110. At the time of writing, there are insufficient data to fully characterize the impact that vaccinating children against COVID-19 has on MIS-C. However, by preventing serious SARS-CoV-2 infection, vaccination of children is expected to substantially reduce the incidence of MIS-C. It remains to be seen whether SARS-CoV-2 breakthrough infections that occur in vaccinated children will have a reduced likelihood of leading to MIS-C compared with SARS-CoV-2 infections in unvaccinated children.
Several individual characteristics have been shown to be associated with higher incidence of severe illness in individuals infected with SARS-CoV-2 infection, including older age; immunosuppression; specific comorbidities, such as chronic cardiovascular, pulmonary, renal, liver and neurological diseases; advanced pregnancy; and heavy smoking. Individuals with such risk factors therefore usually comprise the majority of severe COVID-19 cases. Vaccine effectiveness against severe illness is generally expected to be higher than against infection or mild illness, as it combines the lower likelihood for infection and the lower likelihood of those who are infected having severe complications. Estimating the protective effect of vaccination against severe illness from descriptive population-level statistics is non-trivial. For instance, in a population with lower average vaccine uptake but very high uptake among the key risk groups — namely the elderly and chronically ill — severe cases might still be expected to occur disproportionately among those vaccinated even if vaccine effectiveness is very high.
It would be plausible to assume that the clinical impact of SARS-CoV-2 infection could also differ by the level of immunity prior to exposure, even if this level did not suffice to completely prevent infection altogether. Yet, large retrospective studies that compare severe clinical outcomes of breakthrough cases with primary infection cases, adequately adjusting for individual-level confounders, are yet to be published.
The susceptibility of specific vaccinated population subgroups to breakthrough infections has implications for the prioritization policy during booster vaccination campaigns. Such at-risk population subgroups could also drive differential policy on non-pharmaceutical interventions, such as self-quarantine rules when a vaccinated individual is exposed to infection.
Identifying subgroups at a uniquely high risk for severe breakthrough infections is also key in prioritizing early preventive treatment or prophylaxis, such as monoclonal antibody products, that may be scarce and costly111. Accurate prognostic scores that account for vaccination in identifying, early in their course, those at greatest risk of severe outcomes are especially important for therapeutics that are most effective when given early in infection prior to the appearance of severe disease112,113.
In the context of the debate, in Spring 2021, over the idea of delaying second doses to extend supply, it was argued that breakthrough infections (in individuals with low levels of immunity following a single vaccine dose) would likely accelerate the rise of variants that can escape immunity114. If correct, the same logic could apply to breakthrough infections following a full two-dose series, especially after significant waning. However, one of us has argued previously that the acute nature of infection (probably shortened further by vaccination) makes the emergence of an immune escape variant during an infection very unlikely, at least in an immunocompetent individual115. Likewise, in influenza virus infection, vaccine-derived immunity seems to contribute minimally to the selective pressure for immune escape116. Therefore, it appears that, while each infection poses some risk of generating a variant that is capable of escaping immunity, and a higher incidence and prevalence of infections thus increases the risk, breakthrough infections per se may not be of specific concern regarding the generation or amplification of immune escape variants115. Nevertheless, the issue is complex and speculative, and the outcome may depend on the quantitative details of the comparative susceptibility of vaccinated individuals to infection with and the transmission of different variants.
Unprecedented data but many open questions
In countries that have large supplies, the rollout of COVID-19 vaccines has occurred with unprecedented speed and under unprecedented scrutiny, perhaps best exemplified by the fact that multiple studies have produced a measurement of vaccine effectiveness on each individual day post immunization41,92. At the same time, detailed antibody kinetics have been measured in thousands of individuals47,54,55, providing more data on the temporal patterns of immune responses than for any past vaccine, if not throughout history then at least in the first months of deployment. Also unprecedented — or at least never previously documented — has been the emergence over a timescale of months of variants that, to varying degrees, have reduced susceptibility to immunity from prior natural infection117 and/or from vaccines22,23. The presence of multiple such variants over a short period of calendar time has allowed comparisons of vaccine effectiveness against different variants that have only rarely been possible for other pathogens.
Despite the unprecedented speed and scale of data accumulating on breakthrough infections and related topics, several important questions remain open. For example, although there is evidence that the vaccines against SARS-CoV-2 reduce transmission in households96,118,119 and communities120, it has been argued that sustained high levels of herd immunity against SARS-CoV-2 infection may be an impossible goal for vaccination2 given that it is a mucosal infection without an obligate stage of dissemination through lymph or blood. In this scenario, even with high vaccine coverage, some combination of waning immunity and antigenic variation will produce enough susceptibility in the population to maintain endemic transmission of SARS-CoV-2 for the foreseeable future, likely similar to what is seen for the four other coronaviruses circulating in the human population121. Nevertheless, this situation seems unlikely to produce the same level of disruption that has been seen in the first 1.5 years of the COVID-19 pandemic. Pandemics are rare events in which all or nearly all humans lack exposure to a novel pathogen and are thus at risk for severe disease and transmission, particularly, in this case, those who are older and have certain comorbidities. As with influenza virus122 or even more so as with human coronaviruses, this pandemic pattern may gradually fade into a pattern of milder disease, because virtually everyone will experience multiple exposures through one or more vaccine doses and/or one or more exposures to viral (possibly breakthrough) infection123. On this view, the role of vaccines is not to provide durable herd immunity as with measles or smallpox, but to prevent severe outcomes during the transition to endemicity.
Other key scientific and public health questions arise in the short term. The appropriate balance in tackling Delta variant-driven surges between non-pharmaceutical interventions and booster dose vaccination campaigns is under fierce debate in many countries. Some countries that attempted to drop all non-pharmaceutical interventions after reaching high levels of vaccine coverage were forced to reinstate most (for example, vaccination passes, indoor face masks) in face of massive resurgence23 while applying a population-wide third dose mass-vaccination campaign to avert the need of further restrictions92. Other countries have more gradually relaxed some non-pharmaceutical interventions and performed more gradual and age-dependent third dose vaccination campaigns or did not even experience a strong wave of Delta variant infections. As new variants will likely emerge, and as more countries experience waning immunity to an increasing extent, these debates will likely intensify in view of global shortages in vaccine supply for primary vaccination, which is particularly acute in low-income and lower-middle-income countries124.
Another question is whether there is an ‘instantaneous immune correlate of protection’ — that is, a measurement of individual-level immune responses that can predict, at any moment in time, how protected that individual is against breakthrough infection. Neutralizing antibody titres during the first months after vaccination appear to be well correlated with vaccine effectiveness as measured in randomized trials48,49 and are predictive of the risk of breakthrough infection in individuals45. However, no specific antibody or neutralizing threshold titre has yet been identified that can predict the degree of protection as it changes over time with waning or boosting. Clearly, as time passes, it will be important to design studies to assess the relationship between measurements of immune responses and the risk of reinfection. Such studies are challenging because of the need for relatively frequent samples (for example, serum samples or measurements of immune cells taken near the time of exposure) from large numbers of people, most of whom will not become infected in any short period of time. Innovations in study design can help to make such studies more efficient125 as could lower-cost, less-invasive means of obtaining blood or other biological samples126. Some work also indicates that, if diagnosis is prompt, it may be possible to estimate the level of antibodies present at the time of exposure by obtaining blood on the day of diagnosis or the next day, before antibody levels have appreciably risen in response to the infection45.
At the time of writing, critics of the use of third doses to boost immunity in individuals ≥5 months out from their second dose have noted that the evidence of significant waning has not been observed for all vaccine products and in all age groups. Proponents of boosters for large groups of the population implicitly assume that the documented increasing risk of breakthrough infections in those who are exposed to the Delta variant, are older, were vaccinated earlier, and received certain vaccine products are harbingers of similar declines in younger populations or with future variants. This expectation is consistent with our simple model if levels of protective immunity continue to decline substantially after the first 6 months; this remains to be seen in some groups and for some vaccine products, at least for protection against severe outcomes. Detecting such waning may require especially large sample sizes in the lower-risk age groups. Importantly, for the Pfizer vaccine in Israel, there is now evidence that, at least in the first several weeks after vaccination, a third dose confers an >90% further reduction in the risk of hospitalization and severe disease in each age group compared to two doses94.
Another question is whether a third dose administered months after the second will be qualitatively different from the second and provide enhanced long-term protection against breakthroughs or whether protection levels will return to the pre-boost level (or lower) once again in a matter of months2. More generally, there is a need to set up continuing studies to understand how an individual’s degree of protection against the occurrence and severity of breakthrough infection depends on that individual’s prior history of exposure to, active infection by and vaccination against SARS-CoV-2. Related to these scientific questions is the practical question of how to use limited vaccine supplies to maximize the longevity of effective immune responses; in this regard, growing evidence of higher immunogenicity for two-dose regimens with a longer interval between doses should prompt serious consideration of increasing the standard interval, with additional trials as necessary to meet regulatory requirements. A robust system to monitor duration of protection, impact of variants on vaccine effectiveness, and a simple and fast system that allows quick and easy adaptation of vaccine antigens and dosing intervals in the future is urgently needed.
References
Gotuzzo, E., Yactayo, S. & Córdova, E. Efficacy and duration of immunity after yellow fever vaccination: systematic review on the need for a booster every 10 years. Am. J. Trop. Med. Hyg. 89, 434–444 (2013).
Yewdell, J. W. Individuals cannot rely on COVID-19 herd immunity: durable immunity to viral disease is limited to viruses with obligate viremic spread. PLoS Pathog. 17, e1009509 (2021).
Orenstein, W. A. et al. Field evaluation of vaccine efficacy. Bull. World Health Organ. 63, 1055–1068 (1985).
O’Hagan, J. J., Lipsitch, M. & Hernán, M. A. Estimating the per-exposure effect of infectious disease interventions. Epidemiology 25, 134–138 (2014).
Lipsitch, M., Kahn, R. & Mina, M. J. Antibody testing will enhance the power and accuracy of COVID-19-prevention trials. Nat. Med. 26, 818–819 (2020).
Kahn, R., Schrag, S. J., Verani, J. R. & Lipsitch, M. Identifying and alleviating bias due to differential depletion of susceptible people in post-marketing evaluations of COVID-19 vaccines. Preprint at medRxiv https://doi.org/10.1101/2021.07.15.21260595 (2021).
Mehrotra, D. V. et al. Clinical endpoints for evaluating efficacy in COVID-19 vaccine trials. Ann. Intern. Med. 174, 221–228 (2021).
Voysey, M. et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 397, 99–111 (2020).
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383, 2603–2615 (2020).
Baden, L. R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384, 403–416 (2021).
Sadoff, J. et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N. Engl. J. Med. 384, 2187–2201 (2021).
Voysey, M. et al. Single dose administration, and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet 397, 881–891 (2021).
Lipsitch, M. & Dean, N. E. Understanding COVID-19 vaccine efficacy. Science 370, 763–765 (2020).
Rid, A., Lipsitch, M. & Miller, F. G. The ethics of continuing placebo in SARS-CoV-2 vaccine trials. JAMA 325, 219–220 (2021).
Thomas, S. J. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N. Engl. J. Med. 385, 1761–1773 (2021).
El Sahly, H. M. et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N. Engl. J. Med. 385, 1774–1785 (2021).
Pfizer. BNT162b2 [COMIRNATY (COVID-19 Vaccine, mRNA)] Evaluation of a Booster Dose (Third Dose). Vaccines and Related Biological Products Advisory Committee Briefing Document FDA.gov https://www.fda.gov/media/152161/download (2021).
Moderna. Moderna Highlights New Clinical Data on its COVID-19 Vaccine. Moderna Inc https://investors.modernatx.com/news-releases/news-release-details/moderna-highlights-new-clinical-data-its-covid-19-vaccine (2021).
Shinde, V. et al. Efficacy of NVX-CoV2373 Covid-19 vaccine against the B.1.351 variant. N. Engl. J. Med. 384, 1899–1909 (2021).
Madhi, S. A. et al. Safety and efficacy of the ChAdOx1 nCoV-19 (AZD1222) Covid-19 vaccine against the B.1.351 variant in South Africa. N. Engl. J. Med. https://doi.org/10.1101/2021.02.10.21251247 (2021).
Eyal, N. & Lipsitch, M. How to test SARS-CoV-2 vaccines ethically even after one is available. Clin. Infect. Dis. https://doi.org/10.1093/cid/ciab182 (2021).
Bernal, J. L. et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 385, 585–594 (2021).
Goldberg, Y. et al. Waning immunity of the BNT162b2 vaccine: a nationwide study from Israel. Preprint at medRxiv https://doi.org/10.1101/2021.08.24.21262423 (2021).
Chemaitelly, H. et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa2114114 (2021).
Scobie, H. M. et al. Monitoring incidence of COVID-19 cases, hospitalizations, and deaths, by vaccination status - 13 U.S. Jurisdictions, April 4-July 17, 2021. MMWR 70, 1284–1290 (2021).
Puranik, A. et al. Comparison of two highly-effective mRNA vaccines for COVID-19 during periods of Alpha and Delta variant prevalence. Preprint at medRxiv https://doi.org/10.1101/2021.08.06.21261707 (2021).
Tartof, S. Y. et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet 398, 1407–1416 (2021).
Cerqueira-Silva, T. et al. Influence of age on the effectiveness and duration of protection in vaxzevria and coronavac vaccines. Preprint at medRxiv https://doi.org/10.1101/2021.08.21.21261501 (2021).
Self, W. H. et al. Comparative effectiveness of moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) vaccines in preventing COVID-19 hospitalizations among adults without immunocompromising conditions - United States, March-August 2021. MMWR 70, 1337–1343 (2021).
Sharma, A., Oda, G. & Holodniy, M. COVID-19 vaccine breakthrough infections in veterans health administration. Preprint at medRxiv https://doi.org/10.1101/2021.09.23.21263864 (2021).
Cohn, B. A., Cirillo, P. M., Murphy, C. C., Krigbaum, N. Y. & Wallace, A. W. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science https://doi.org/10.1126/science.abm0620 (2021).
Haas, E. J. et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet 397, 1819–1829 (2021).
Dagan, N. et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N. Engl. J. Med. 384, 1412–1423 (2021).
Roest, S., Hoek, R. A. S. & Manintveld, O. C. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N. Engl. J. Med. 384, 1968–1970 (2021).
Dagan, N. et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat. Med. 27, 1693–1695 (2021).
Thompson, M. G. et al. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers - Eight U.S. Locations, December 2020-March 2021. MMWR 70, 495–500 (2021).
Lipsitch, M., Jha, A. & Simonsen, L. Observational studies and the difficult quest for causality: lessons from vaccine effectiveness and impact studies. Int. J. Epidemiol. 45, 2060–2074 (2016).
Patel, M. K. et al. Evaluation of post-introduction COVID-19 vaccine effectiveness: summary of interim guidance of the World Health Organization. Vaccine 39, 4013–4024 (2021).
Sullivan, S. G., Tchetgen Tchetgen, E. J. & Cowling, B. J. Theoretical basis of the test-negative study design for assessment of influenza vaccine effectiveness. Am. J. Epidemiol. 184, 345–353 (2016).
Chua, H. et al. The use of test-negative controls to monitor vaccine effectiveness: a systematic review of methodology. Epidemiology 31, 43–64 (2020).
Yelin, I. et al. Associations of the BNT162b2 COVID-19 vaccine effectiveness with patient age and comorbidities at daily resolution. Preprint at medRxiv https://doi.org/10.1101/2021.03.16.21253686 (2021).
Regev-Yochay, G. et al. Decreased infectivity following BNT162b2 vaccination: a prospective cohort study in Israel. Lancet Reg. Health Eur. 7, 100150 (2021).
Singer, S. R. et al. Effectiveness of BNT162b2 mRNA COVID-19 vaccine against SARS-CoV-2 variant beta (B.1.351) among persons identified through contact tracing in Israel. Preprint at SSRN https://doi.org/10.2139/ssrn.3904701 (2021).
Lipsitch, M. & Kahn, R. Interpreting vaccine efficacy trial results for infection and transmission. Vaccine 39, 4082–4088 (2021).
Bergwerk, M. et al. Covid-19 breakthrough infections in vaccinated health care workers. N. Engl. J. Med. 385, 1474–1484 (2021).
Hall, V. J. et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet 397, 1725–1735 (2021).
Levin, E. G. et al. Waning immune humoral response to BNT162b2 covid-19 vaccine over 6 months. N. Engl. J. Med. https://doi.org/10.1056/nejmoa2114583 (2021).
Khoury, D. S. et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 27, 1205–1211 (2021).
Earle, K. A. et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 39, 4423–4428 (2021).
Goldblatt, D. et al. A population-based threshold of protection for COVID-19 vaccines. Preprint at Res. Sq. https://doi.org/10.21203/rs.3.rs-832531/v1 (2021).
Gilbert, P. B. et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy trial. Preprint at medRxiv https://doi.org/10.1101/2021.08.09.21261290 (2021).
Plotkin, S. A. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 17, 1055–1065 (2010).
Plotkin, S. A. & Gilbert, P. B. Nomenclature for immune correlates of protection after vaccination. Clin. Infect. Dis. 54, 1615–1617 (2012).
Widge, A. T. et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N. Engl. J. Med. 384, 80–82 (2021).
Naaber, P. et al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. Lancet Reg. Health Eur. 10, 100208 (2021).
Choi, A. et al. Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: an interim analysis. Nat. Med. 27, 2025–2031 (2021).
Amanna, I. J., Carlson, N. E. & Slifka, M. K. Duration of humoral immunity to common viral and vaccine antigens. N. Engl. J. Med. 357, 1903–1915 (2007).
Ellebedy, A. H. et al. Defining antigen-specific plasmablast and memory B cell subsets in human blood after viral infection or vaccination. Nat. Immunol. 17, 1226–1234 (2016).
Wrammert, J. et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature 453, 667–671 (2008).
Turner, J. S. et al. SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans. Nature 595, 421–425 (2021).
Slifka, M. K., Antia, R., Whitmire, J. K. & Ahmed, R. Humoral immunity due to long-lived plasma cells. Immunity 8, 363–372 (1998).
Sheikh-Mohamed, S. et al. A mucosal antibody response is induced by intra-muscular SARS-CoV-2 mRNA vaccination. Preprint at medRxiv https://doi.org/10.1101/2021.08.01.21261297 (2021).
Ray, G. T. et al. Intraseason waning of influenza vaccine effectiveness. Clin. Infect. Dis. 68, 1623–1630 (2019).
Lewnard, J. A. & Grad, Y. H. Vaccine waning and mumps re-emergence in the United States. Sci. Transl Med. 10, eaao5945 (2018).
Lustig, Y. et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir. Med. 9, 999–1009 (2021).
Lustig, Y. et al. Neutralising capacity against Delta (B.1.617.2) and other variants of concern following Comirnaty (BNT162b2, BioNTech/Pfizer) vaccination in health care workers, Israel. Euro Surveill. 26, 2100557 (2021).
Parry, H. M. et al. Extended interval BNT162b2 vaccination enhances peak antibody generation in older people. Preprint at medRxiv https://doi.org/10.1101/2021.05.15.21257017 (2021).
Tauzin, A. et al. Strong humoral immune responses against SARS-CoV-2 spike after BNT162b2 mRNA vaccination with a sixteen-week interval between doses. Preprint at medRxiv https://doi.org/10.1101/2021.09.17.21263532 (2021).
Vinh, D. C. et al. Real-world serologic responses to extended-interval and heterologous COVID-19 mRNA vaccination in frail elderly - interim report from a prospective observational cohort study. Preprint at medRxiv https://doi.org/10.2139/ssrn.3927745 (2021).
Payne, R. P. et al. Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell 184, 5699–5714 (2021).
Kearns, P. et al. Examining the immunological effects of COVID-19 vaccination in patients with conditions potentially leading to diminished immune response capacity – The OCTAVE Trial. SSRN https://doi.org/10.2139/ssrn.3910058 (2021).
Van Oekelen, O. et al. Highly variable SARS-CoV-2 spike antibody responses to two doses of COVID-19 RNA vaccination in patients with multiple myeloma. Cancer Cell 39, 1028–1030 (2021).
Aleman, A. et al. Variable cellular responses to SARS-CoV-2 in fully vaccinated patients with multiple myeloma. Cancer Cell 39, 1442–1444 (2021).
Mittelman, M. et al. Effectiveness of the BNT162b2mRNA Covid-19 vaccine in patients with hematological neoplasms in a nationwide mass vaccination setting. Blood https://doi.org/10.1182/blood.2021013768 (2021).
Planas, D. et al. Sensitivity of infectious SARS-CoV-2 B.1.1.7 and B.1.351 variants to neutralizing antibodies. Nat. Med. 27, 917–924 (2021).
Edara, V. V. et al. Infection- and vaccine-induced antibody binding and neutralization of the B.1.351 SARS-CoV-2 variant. Cell Host Microbe 29, 516–521.e3 (2021).
Shen, X. et al. Neutralization of SARS-CoV-2 variants B.1.429 and B.1.351. N. Engl. J. Med. 384, 2352–2354 (2021).
Carreño, J. M. et al. Reduced neutralizing activity of post-SARS-CoV-2 vaccination serum against variants B.1.617.2, B.1.351, B.1.1.7+E484K and a sub-variant of C.37. Preprint at medRxiv https://doi.org/10.1101/2021.07.21.21260961 (2021).
Wu, K. et al. Serum neutralizing activity elicited by mRNA-1273 vaccine. N. Engl. J. Med. 384, 1468–1470 (2021).
Abu-Raddad, L. J., Chemaitelly, H. & Butt, A. A. Effectiveness of the BNT162b2 Covid-19 vaccine against the B.1.1.7 and B.1.351 variants. N. Engl. J. Med. 385, 187–189 (2021).
Kustin, T. et al. Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat. Med. 27, 1379–1384 (2021).
Gomes, M. G. M. et al. A missing dimension in measures of vaccination impacts. PLoS Pathog. 10, e1003849 (2014).
Langwig, K. E. et al. Limited available evidence supports theoretical predictions of reduced vaccine efficacy at higher exposure dose. Sci. Rep. 9, 3203 (2019).
Luo, C. H. et al. Infection with the SARS-CoV-2 Delta variant is associated with higher infectious virus loads compared to the Alpha variant in both unvaccinated and vaccinated individuals. Preprint at medRxiv https://doi.org/10.1101/2021.08.15.21262077 (2021).
Wang, Y. et al. Transmission, viral kinetics and clinical characteristics of the emergent SARS-CoV-2 Delta VOC in Guangzhou, China. EClinicalMedicine 40, 101129 (2021).
Mlcochova, P. et al. SARS-CoV-2 B.1.617.2 Delta variant replication, sensitivity to neutralising antibodies and vaccine breakthrough. Preprint at bioRxiv https://doi.org/10.1101/2021.05.08.443253 (2021).
Escalera, A., Gonzalez-Reiche, A. S., Aslam, S. & Mena, I. SARS-CoV-2 variants of concern have acquired mutations associated with an increased spike cleavage. Preprint at bioRxiv https://doi.org/10.1101/2021.08.05.455290 (2021).
Tang, P. et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the Delta (B.1.617.2) variant in Qatar. Nat. Med. https://doi.org/10.1038/s41591-021-01583-4 (2021).
Andrews, N. J. et al. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. Lancet Infect. Dis. 14, 839–846 (2014).
Voysey, M. et al. Serotype-specific correlates of protection for pneumococcal carriage: an analysis of immunity in 19 countries. Clin. Infect. Dis. 66, 913–920 (2018).
Falsey, A. R. et al. SARS-CoV-2 neutralization with BNT162b2 vaccine dose 3. N. Engl. J. Med. 385, 1627–1629 (2021).
Bar-On, Y. M. et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N. Engl. J. Med. 385, 1393–1400 (2021).
Reis, B. Y. et al. Effectiveness of BNT162b2 vaccine against delta variant in adolescents. N. Engl. J. Med. 385, 2101–2103 (2021).
Barda, N. et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet https://doi.org/10.1016/s0140-6736(21)02249-2 (2021).
Krammer, F. et al. Influenza. Nat. Rev. Dis. Prim. 4, 3 (2018).
Layan, M. et al. Impact of BNT162b2 vaccination and isolation on SARS-CoV-2 transmission in Israeli households: an observational study. Preprint at bioRxiv https://doi.org/10.1101/2021.07.12.21260377 (2021).
Kissler, S. M. et al. Viral dynamics of SARS-CoV-2 variants in vaccinated and unvaccinated individuals. Preprint at medRxiv https://doi.org/10.1101/2021.02.16.21251535 (2021).
Pouwels, K. B. et al. Impact of Delta on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Preprint at medRxiv https://doi.org/10.1101/2021.08.18.21262237 (2021).
Levine-Tiefenbrun, M. et al. Viral loads of Delta-variant SARS-CoV2 breakthrough infections following vaccination and booster with the BNT162b2 vaccine. Preprint at medRxiv https://doi.org/10.1101/2021.08.29.21262798 (2021).
Kennedy-Shaffer, L., Kahn, R. & Lipsitch, M. Estimating vaccine efficacy against transmission via effect on viral load. Epidemiology 32, 820–828 (2021).
Follmann, D. & Fay, M. Vaccine efficacy at a point in time. Preprint at medRxiv https://doi.org/10.1101/2021.02.04.21251133 (2021).
Shamier, M. C. et al. Virological characteristics of SARS-CoV-2 vaccine breakthrough infections in health care workers. Preprint at medRxiv https://doi.org/10.1101/2021.08.20.21262158 (2021).
Hay, J. A., Kennedy-Shaffer, L. & Mina, M. J. Viral loads observed under competing strain dynamics. Preprint at medRxiv https://doi.org/10.1101/2021.07.27.21261224 (2021).
Eyre, D. W. et al. The impact of SARS-CoV-2 vaccination on Alpha and Delta variant transmission. Preprint at medRxiv https://doi.org/10.1101/2021.09.28.21264260 (2021).
Barda, N. et al. Safety of the BNT162b2 mRNA Covid-19 Vaccine in a nationwide setting. N. Engl. J. Med. 385, 1078–1090 (2021).
Daugherty, S. E. et al. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ 373, n1098 (2021).
Antonelli, M. et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study. Lancet Infect. Dis. https://doi.org/10.1016/s1473-3099(21)00460-6 (2021).
Feldstein, L. R. et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA 325, 1074–1087 (2021).
Ministry of Health. Israel Ministry of Health COVID-19 Dashboard https://datadashboard.health.gov.il/COVID-19/general (2021).
Payne, A. B. et al. Incidence of multisystem inflammatory syndrome in children among US persons infected with SARS-CoV-2. JAMA Netw. Open 4, e2116420 (2021).
O’Brien, M. P. et al. Subcutaneous REGEN-COV antibody combination to prevent Covid-19. N. Engl. J. Med. 385, 1184–1195 (2021).
Weinreich, D. M. et al. REGEN-COV antibody cocktail clinical outcomes study in Covid-19 outpatients. Preprint at medRxiv https://doi.org/10.1101/2021.05.19.21257469 (2021).
Young, B., Tan, T. T. & Leo, Y. S. The place for remdesivir in COVID-19 treatment. Lancet Infect. Dis. 21, 20–21 (2021).
Bieniasz, P. D. The case against delaying severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA vaccine boosting doses. Clin. Infect. Dis. 73, 1321–1323 (2021).
Cobey, S., Larremore, D. B., Grad, Y. H. & Lipsitch, M. Concerns about SARS-CoV-2 evolution should not hold back efforts to expand vaccination. Nat. Rev. Immunol. 21, 330–335 (2021).
Debbink, K. et al. Vaccination has minimal impact on the intrahost diversity of H3N2 influenza viruses. PLoS Pathog. 13, e1006194 (2017).
Faria, N. R. et al. Genomics and epidemiology of the P.1 SARS-CoV-2 lineage in Manaus, Brazil. Science 372, 815–821 (2021).
Harris, R. J. et al. Impact of vaccination on household transmission of SARS-COV-2 in England. Preprint at medRxiv https://doi.org/10.1101/2021.09.28.21264260 (2021).
Salo, J. et al. The indirect effect of mRNA-based Covid-19 vaccination on unvaccinated household members. Preprint at medRxiv https://doi.org/10.1101/2021.05.27.21257896 (2021).
Milman, O. et al. Community-level evidence for SARS-CoV-2 vaccine protection of unvaccinated individuals. Nat. Med. 27, 1367–1369 (2021).
Townsend, J. P. et al. The durability of immunity against reinfection by SARS-CoV-2: a comparative evolutionary study. Lancet Microbe https://doi.org/10.1016/s2666-5247(21)00219-6 (2021).
Simonsen, L. et al. Pandemic versus epidemic influenza mortality: a pattern of changing age distribution. J. Infect. Dis. 178, 53–60 (1998).
Lavine, J. S., Bjornstad, O. N. & Antia, R. Immunological characteristics govern the transition of COVID-19 to endemicity. Science 371, 741–745 (2021).
Krause, P. R. et al. Considerations in boosting COVID-19 vaccine immune responses. Lancet 398, 1377–1380 (2021).
Follmann, D. A. & Dodd, L. Immune correlates analysis using vaccinees from test negative designs. Biostatistics https://doi.org/10.1093/biostatistics/kxaa037 (2020).
Wang, Z. et al. Microneedle patch for the ultrasensitive quantification of protein biomarkers in interstitial fluid. Nat. Biomed. Eng. 5, 64–76 (2021).
Acknowledgements
The authors thank Rebecca Kahn for assistance with the figure. M.L. was supported by the Morris-Singer Fund and by the US National Cancer Institute/NIH Seronet Cooperative Agreement 1U01CA261277. Work in the Krammer laboratory on SARS-CoV-2 is supported by the PARIS/SPARTA studies funded by the NIAID Collaborative Influenza Vaccine Innovation Centers (CIVIC) contract 75N93019C00051, by the Centers of Excellence for Influenza Research and Surveillance (CEIRS, contract # HHSN272201400008C), by the generous support of the JPB Foundation and the Open Philanthropy Project (research grant 2020-215611 (5384), and by anonymous donors. Y.L. is supported by the Nehemia Rubin Excellence in Biomedical Research — The TELEM Program of Chaim Sheba Medical Center.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
M.L. reports institutional grant funding from Pfizer, consulting/honoraria from Bristol Myers Squibb, Janssen, Merck, Sanofi Pasteur, Peter Diamandis/Abundance Platinum, and unpaid advice to One Day Sooner, Pfizer, Janssen, AstraZeneca, Covaxx (United Biomedical), and the Coalition for Epidemic Preparedness Innovations (CEPI). The Icahn School of Medicine at Mount Sinai has filed patent applications relating to SARS-CoV-2 serological assays and NDV-based SARS-CoV-2 vaccines, which list F.K. as co-inventor. Mount Sinai has spun out a company, Kantaro, to market serological tests for SARS-CoV-2. F.K. has consulted for Merck and Pfizer (before 2020) and is currently consulting for Pfizer, Seqirus and Avimex. The Krammer laboratory is also collaborating with Pfizer on animal models of SARS-CoV-2. R.D.B. reports past institutional grants to Clalit Research Institute from Pfizer, outside the submitted work and unrelated to COVID-19, with no direct or indirect personal benefits. G.R.-Y. has received a research grant from Pfizer on an unrelated topic (pneumococcal infections) and has received honoraria from Teva and MSD for presentations given (on fluid contamination and vaccine rollout). Y.L. has received a research grant from Pfizer on an unrelated topic (tick-borne encephalitis).
Additional information
Peer review information
Nature Reviews Immunology thanks J. Yewdell and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lipsitch, M., Krammer, F., Regev-Yochay, G. et al. SARS-CoV-2 breakthrough infections in vaccinated individuals: measurement, causes and impact. Nat Rev Immunol 22, 57–65 (2022). https://doi.org/10.1038/s41577-021-00662-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-021-00662-4
This article is cited by
-
Effects of antivirals on patients with COVID-19 breakthrough
BMC Infectious Diseases (2024)
-
Influence of vaccination on critical COVID-19 patients with acute respiratory failure: a retrospective cohort study
European Journal of Medical Research (2024)
-
Theory and methods of the multiverse: an application for panel-based models
Quality & Quantity (2024)
-
Current Progress, Challenges and Prospects in the Development of COVID-19 Vaccines
Drugs (2024)
-
Immune response stability to the SARS-CoV-2 mRNA vaccine booster is influenced by differential splicing of HLA genes
Scientific Reports (2024)