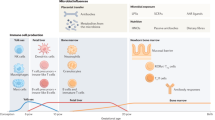
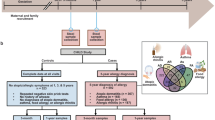
Abstract
Allergies, including asthma, food allergy and atopic dermatitis, are increasing in prevalence, particularly in westernized countries. Although a detailed mechanistic explanation for this increase is lacking, recent evidence indicates that, in addition to genetic predisposition, lifestyle changes owing to modernization have an important role. Such changes include increased rates of birth by caesarean delivery, increased early use of antibiotics, a westernized diet and the associated development of obesity, and changes in indoor and outdoor lifestyle and activity patterns. Most of these factors directly and indirectly impact the formation of a diverse microbiota, which includes bacterial, viral and fungal components; the microbiota has a leading role in shaping (early) immune responses. This default programme is markedly disturbed under the influence of environmental and lifestyle risk factors. Here, we review the most important allergy risk factors associated with changes in our exposure to the microbial world and the application of this knowledge to allergy prevention strategies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hill, D. A. & Spergel, J. M. The atopic march: critical evidence and clinical relevance. Ann. Allergy Asthma Immunol. 120, 131–137 (2018).
Belgrave, D. C. M. et al. Developmental profiles of eczema, wheeze, and rhinitis: two population-based birth cohort studies. PLoS Med. 11, e1001748 (2014).
Saunes, M. et al. Early eczema and the risk of childhood asthma: a prospective, population-based study. BMC Pediatr. 12, 168 (2012).
Kobyletzki, L. B. von et al. Eczema in early childhood is strongly associated with the development of asthma and rhinitis in a prospective cohort. BMC Dermatol. 12, 11 (2012).
Alduraywish, S. A. et al. The march from early life food sensitization to allergic disease: a systematic review and meta-analyses of birth cohort studies. Allergy 71, 77–89 (2016).
Brough, H. A. et al. Peanut allergy. Effect of environmental peanut exposure in children with filaggrin loss-of-function mutations. J. Allergy Clin. Immunol. 134, 867–875.e1 (2014).
Bønnelykke, K. et al. Meta-analysis of genome-wide association studies identifies ten loci influencing allergic sensitization. Nat. Genet. 45, 902–906 (2013).
Torgerson, T. R. et al. Severe food allergy as a variant of IPEX syndrome caused by a deletion in a noncoding region of the FOXP3 gene. Gastroenterology 132, 1705–1717 (2007).
Ober, C. & Yao, T.-C. The genetics of asthma and allergic disease: a 21st century perspective. Immunol. Rev. 242, 10–30 (2011).
Round, J. L. & Mazmanian, S. K. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl Acad. Sci. USA 107, 12204–12209 (2010).
Dasgupta, S., Erturk-Hasdemir, D., Ochoa-Reparaz, J., Reinecker, H.-C. & Kasper, D. L. Plasmacytoid dendritic cells mediate anti-inflammatory responses to a gut commensal molecule via both innate and adaptive mechanisms. Cell Host Microbe 15, 413–423 (2014).
Atarashi, K. et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500, 232–236 (2013).
Atarashi, K. et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331, 337–341 (2011).
Chng, K. R. et al. Whole metagenome profiling reveals skin microbiome-dependent susceptibility to atopic dermatitis flare. Nat. Microbiol. 1, 16106 (2016).
Meylan, P. et al. Skin colonization by Staphylococcus aureus precedes the clinical diagnosis of atopic dermatitis in infancy. J. Invest. Dermatol. 137, 2497–2504 (2017).
Tauber, M. et al. Staphylococcus aureus density on lesional and nonlesional skin is strongly associated with disease severity in atopic dermatitis. J. Allergy Clin. Immunol. 137, 1272–1274.e3 (2016).
McKenzie, C., Tan, J., Macia, L. & Mackay, C. R. The nutrition-gut microbiome-physiology axis and allergic diseases. Immunol. Rev. 278, 277–295 (2017).
Wang, S. et al. MyD88 adaptor-dependent microbial sensing by regulatory T cells promotes mucosal tolerance and enforces commensalism. Immunity 43, 289–303 (2015).
Round, J. L. et al. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 332, 974–977 (2011).
Noval Rivas, M. et al. Regulatory T cell reprogramming toward a Th2-cell-like lineage impairs oral tolerance and promotes food allergy. Immunity 42, 512–523 (2015). This article provides a mechanistic link between oral tolerance, development of food allergy, Treg cells and the microbiota.
Hill, D. A. et al. Commensal bacteria-derived signals regulate basophil hematopoiesis and allergic inflammation. Nat. Med. 18, 538–546 (2012).
Tamburini, S., Shen, N., Wu, H. C. & Clemente, J. C. The microbiome in early life: implications for health outcomes. Nat. Med. 22, 713–722 (2016).
Kemter, A. M. & Nagler, C. R. Influences on allergic mechanisms through gut, lung, and skin microbiome exposures. J. Clin. Invest. 130, 1483–1492 (2019).
Arrieta, M.-C. et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci. Transl Med. 7, 307ra152 (2015). This study reports links between microbial colonization during early infancy, metabolic alterations and risk of childhood asthma.
Huang, Y. J. et al. The microbiome in allergic disease: current understanding and future opportunities-2017 PRACTALL document of the American Academy of Allergy, Asthma & Immunology and the European Academy of Allergy and Clinical Immunology. J. Allergy Clin. Immunol. 139, 1099–1110 (2017).
Berni Canani, R. et al. Gut microbiota composition and butyrate production in children affected by non-IgE-mediated cow’s milk allergy. Sci. Rep. 8, 12500 (2018).
Wang, M. et al. Reduced diversity in the early fecal microbiota of infants with atopic eczema. J. Allergy Clin. Immunol. 121, 129–134 (2008).
Abrahamsson, T. R. et al. Low diversity of the gut microbiota in infants with atopic eczema. J. Allergy Clin. Immunol. 129, 434–440 (2012).
Byrd, A. L., Belkaid, Y. & Segre, J. A. The human skin microbiome. Nat. Rev. Microbiol. 16, 143–155 (2018).
Paller, A. S. et al. The microbiome in patients with atopic dermatitis. J. Allergy Clin. Immunol. 143, 26–35 (2019).
Huang, Y. J. & Boushey, H. A. The microbiome in asthma. J. Allergy Clin. Immunol. 135, 25–30 (2015).
Nowak-Wegrzyn, A., Szajewska, H. & Lack, G. Food allergy and the gut. Nat. Rev. Gastroenterol. Hepatol. 14, 241–257 (2017).
Sampson, H. A. et al. Mechanisms of food allergy. J. Allergy Clin. Immunol. 141, 11–19 (2018).
Cadwell, K. The virome in host health and disease. Immunity 42, 805–813 (2015).
Freer, G. et al. The virome and its major component, anellovirus, a convoluted system molding human immune defenses and possibly affecting the development of asthma and respiratory diseases in childhood. Front. Microbiol. 9, 686 (2018).
Lim, E. S. et al. Early life dynamics of the human gut virome and bacterial microbiome in infants. Nat. Med. 21, 1228–1234 (2015). These investigators characterize the gut virome and bacterial microbiota in a longitudinal cohort of healthy infant twins and show that the infant microbiota is highly dynamic.
Lim, E. S., Wang, D. & Holtz, L. R. The bacterial microbiome and virome milestones of infant development. Trends Microbiol. 24, 801–810 (2016).
Stewart, C. J. et al. Bacterial and fungal viability in the preterm gut: NEC and sepsis. Arch. Dis. Child. Fetal Neonatal Ed. 98, F298–F303 (2013).
Findley, K. et al. Topographic diversity of fungal and bacterial communities in human skin. Nature 498, 367–370 (2013).
Limon, J. J., Skalski, J. H. & Underhill, D. M. Commensal fungi in health and disease. Cell Host Microbe 22, 156–165 (2017).
Jo, J.-H. et al. Diverse human skin fungal communities in children converge in adulthood. J. Invest. Dermatol. 136, 2356–2363 (2016).
Kong, H. H. & Morris, A. The emerging importance and challenges of the human mycobiome. Virulence 8, 310–312 (2017).
Gensollen, T., Iyer, S. S., Kasper, D. L. & Blumberg, R. S. How colonization by microbiota in early life shapes the immune system. Science 352, 539–544 (2016).
Gomez de Agüero, M. et al. The maternal microbiota drives early postnatal innate immune development. Science 351, 1296–1302 (2016).
Eggesbø, M., Botten, G., Stigum, H., Nafstad, P. & Magnus, P. Is delivery by cesarean section a risk factor for food allergy? J. Allergy Clin. Immunol. 112, 420–426 (2003).
Koplin, J. J. et al. Do factors known to alter infant microbial exposures alter the risk of food allergy and eczema in a population-based infant study? J. Allergy Clin. Immunol. 129, AB231 (2012).
Laubereau, B. et al. Caesarean section and gastrointestinal symptoms, atopic dermatitis, and sensitisation during the first year of life. Arch. Dis. Child. 89, 993–997 (2004).
Thavagnanam, S., Fleming, J., Bromley, A., Shields, M. D. & Cardwell, C. R. A meta-analysis of the association between caesarean section and childhood asthma. Clin. Exp. Allergy 38, 629–633 (2008).
Kristensen, K. & Henriksen, L. Cesarean section and disease associated with immune function. J. Allergy Clin. Immunol. 137, 587–590 (2016).
Tollånes, M. C., Moster, D., Daltveit, A. K. & Irgens, L. M. Cesarean section and risk of severe childhood asthma: a population-based cohort study. J. Pediatr. 153, 112–116 (2008).
Cho, C. E. & Norman, M. Cesarean section and development of the immune system in the offspring. Am. J. Obstet. Gynecol. 208, 249–254 (2013).
Bager, P., Wohlfahrt, J. & Westergaard, T. Caesarean delivery and risk of atopy and allergic disease: meta-analyses. Clin. Exp. Allergy 38, 634–642 (2008).
Roduit, C. et al. Asthma at 8 years of age in children born by caesarean section. Thorax 64, 107–113 (2009).
Pyrhönen, K., Näyhä, S., Hiltunen, L. & Läärä, E. Caesarean section and allergic manifestations: insufficient evidence of association found in population-based study of children aged 1 to 4 years. Acta Paediatr. 102, 982–989 (2013).
Dominguez-Bello, M. G. et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl Acad. Sci. USA 107, 11971–11975 (2010).
Romero, R. et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome 2, 4 (2014).
Gajer, P. et al. Temporal dynamics of the human vaginal microbiota. Sci. Transl Med. 4, 132ra52 (2012).
Miller, L. et al. Depomedroxyprogesterone-induced hypoestrogenism and changes in vaginal flora and epithelium. Obstet. Gynecol. 96, 431–439 (2000).
Chu, D. M. et al. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat. Med. 23, 314–326 (2017).
Penders, J. et al. Establishment of the intestinal microbiota and its role for atopic dermatitis in early childhood. J. Allergy Clin. Immunol. 132, 601–607.e8 (2013).
Pistiner, M., Gold, D. R., Abdulkerim, H., Hoffman, E. & Celedón, J. C. Birth by cesarean section, allergic rhinitis, and allergic sensitization among children with a parental history of atopy. J. Allergy Clin. Immunol. 122, 274–279 (2008).
Lee, S.-Y. et al. Additive effect between IL-13 polymorphism and cesarean section delivery/prenatal antibiotics use on atopic dermatitis. A birth cohort study (COCOA). PLoS ONE 9, e96603 (2014).
Liao, S.-L. et al. Caesarean section is associated with reduced perinatal cytokine response, increased risk of bacterial colonization in the airway, and infantile wheezing. Sci. Rep. 7, 9053 (2017).
Dominguez-Bello, M. G. et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat. Med. 22, 250–253 (2016). This proof-of-principle study shows that exposure of newborns born by caesarean delivery to maternal vaginal microbiota partially restores their gastrointestinal microbiota.
Cox, L. M. et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 158, 705–721 (2014).
Stokholm, J. et al. Prevalence and predictors of antibiotic administration during pregnancy and birth. PLoS ONE 8, e82932 (2013).
Bailey, L. C. et al. Association of antibiotics in infancy with early childhood obesity. JAMA Pediatr. 168, 1063–1069 (2014).
Saari, A., Virta, L. J., Sankilampi, U., Dunkel, L. & Saxen, H. Antibiotic exposure in infancy and risk of being overweight in the first 24 months of life. Pediatrics 135, 617–626 (2015).
Mikkelsen, K. H., Knop, F. K., Frost, M., Hallas, J. & Pottegård, A. Use of antibiotics and risk of type 2 diabetes: a population-based case-control study. J. Clin. Endocrinol. Metab. 100, 3633–3640 (2015).
Risnes, K. R., Belanger, K., Murk, W. & Bracken, M. B. Antibiotic exposure by 6 months and asthma and allergy at 6 years: findings in a cohort of 1,401 US children. Am. J. Epidemiol. 173, 310–318 (2011).
Hoskin-Parr, L., Teyhan, A., Blocker, A. & Henderson, A. J. W. Antibiotic exposure in the first two years of life and development of asthma and other allergic diseases by 7.5 yr: a dose-dependent relationship. Pediatr. Allergy Immunol. 24, 762–771 (2013).
Hviid, A., Svanström, H. & Frisch, M. Antibiotic use and inflammatory bowel diseases in childhood. Gut 60, 49–54 (2011).
Shaw, S. Y., Blanchard, J. F. & Bernstein, C. N. Association between the use of antibiotics and new diagnoses of Crohn’s disease and ulcerative colitis. Am. J. Gastroenterol. 106, 2133–2142 (2011).
Kronman, M. P., Zaoutis, T. E., Haynes, K., Feng, R. & Coffin, S. E. Antibiotic exposure and IBD development among children: a population-based cohort study. Pediatrics 130, e794–e803 (2012).
Metsälä, J. et al. Mother’s and offspring’s use of antibiotics and infant allergy to cow’s milk. Epidemiology 24, 303–309 (2013).
Wu, P. et al. Relative importance and additive effects of maternal and infant risk factors on childhood asthma. PLoS ONE 11, e0151705 (2016).
Kozyrskyj, A. L., Ernst, P. & Becker, A. B. Increased risk of childhood asthma from antibiotic use in early life. Chest 131, 1753–1759 (2007).
Cho, I. et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 488, 621–626 (2012).
Pérez-Cobas, A. E. et al. Gut microbiota disturbance during antibiotic therapy: a multi-omic approach. Gut 62, 1591–1601 (2013).
Schulfer, A. F. et al. Intergenerational transfer of antibiotic-perturbed microbiota enhances colitis in susceptible mice. Nat. Microbiol. 3, 234–242 (2018). This aticle shows that antibiotic exposure shapes the maternal gut microbiota and this effect extends to their offspring, with both ecological and long-term disease consequences.
Murk, W., Risnes, K. R. & Bracken, M. B. Prenatal or early-life exposure to antibiotics and risk of childhood asthma: a systematic review. Pediatrics 127, 1125–1138 (2011).
Heintze, K. & Petersen, K.-U. The case of drug causation of childhood asthma: antibiotics and paracetamol. Eur. J. Clin. Pharmacol. 69, 1197–1209 (2013).
Ivanov, I. I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009).
Stefka, A. T. et al. Commensal bacteria protect against food allergen sensitization. Proc. Natl Acad. Sci. USA 111, 13145–13150 (2014).
Noverr, M. C., Falkowski, N. R., McDonald, R. A., McKenzie, A. N. & Huffnagle, G. B. Development of allergic airway disease in mice following antibiotic therapy and fungal microbiota increase: role of host genetics, antigen, and interleukin-13. Infect. Immun. 73, 30–38 (2005).
Gonzalez-Perez, G. et al. Maternal antibiotic treatment impacts development of the neonatal intestinal microbiome and antiviral immunity. J. Immunol. 196, 3768–3779 (2016).
Semic-Jusufagic, A. et al. Assessing the association of early life antibiotic prescription with asthma exacerbations, impaired antiviral immunity, and genetic variants in 17q21: a population-based birth cohort study. Lancet Respir. Med. 2, 621–630 (2014).
Lodge, C. J. et al. Breastfeeding and asthma and allergies: a systematic review and meta-analysis. Acta Paediatr. 104, 38–53 (2015).
Heinrich, J. Modulation of allergy risk by breast feeding. Curr. Opin. Clin. Nutr. Metab. Care 20, 217–221 (2017).
Stewart, C. J. et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature 562, 583–588 (2018). This is an extensive characterization of the microbiota in early life in a large multicentre population and provides the background for future mechanistic studies.
van den Elsen, L. W. J., Garssen, J., Burcelin, R. & Verhasselt, V. Shaping the gut microbiota by breastfeeding: the gateway to allergy prevention? Front. Pediatr. 7, 47 (2019).
Bäckhed, F. et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 17, 690–703 (2015). This article shows that the gut microbiota of infants born by caesarean delivery has less resemblance to their mother’s microbiota than vaginally delivered infants and describes breastfeeding cessation as being required for maturation into an adult-like microbiota.
Alderete, T. L. et al. Associations between human milk oligosaccharides and infant body composition in the first 6 mo of life. Am. J. Clin. Nutr. 102, 1381–1388 (2015).
Bode, L. et al. Overcoming the limited availability of human milk oligosaccharides: Challenges and opportunities for research and application. Nutr. Rev. 74, 635–644 (2016).
Seppo, A. E., Autran, C. A., Bode, L. & Järvinen, K. M. Human milk oligosaccharides and development of cow’s milk allergy in infants. J. Allergy Clin. Immunol. 139, 708–711.e5 (2017).
Pannaraj, P. S. et al. Shared and distinct features of human milk and infant stool viromes. Front. Microbiol. 9, 1162 (2018).
Rekima, A. et al. A role for early oral exposure to house dust mite allergens through breast milk in IgE-mediated food allergy susceptibility. J. Allergy Clin. Immunol. 145, 1416–1429.e11 (2020).
Jartti, T. & Gern, J. E. Role of viral infections in the development and exacerbation of asthma in children. J. Allergy Clin. Immunol. 140, 895–906 (2017).
Jackson, D. J. et al. Evidence for a causal relationship between allergic sensitization and rhinovirus wheezing in early life. Am. J. Respir. Crit. Care Med. 185, 281–285 (2012).
Kusel, M. M. H. et al. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J. Allergy Clin. Immunol. 119, 1105–1110 (2007).
Geerdink, R. J., Pillay, J., Meyaard, L. & Bont, L. Neutrophils in respiratory syncytial virus infection. A target for asthma prevention. J. Allergy Clin. Immunol. 136, 838–847 (2015).
Gavala, M. L., Bashir, H. & Gern, J. E. Virus/allergen interactions in asthma. Curr. Allergy Asthma Rep. 13, 298–307 (2013).
Krishnamoorthy, N. et al. Early infection with respiratory syncytial virus impairs regulatory T cell function and increases susceptibility to allergic asthma. Nat. Med. 18, 1525–1530 (2012).
Edwards, M. R. et al. Viral infections in allergy and immunology. How allergic inflammation influences viral infections and illness. J. Allergy Clin. Immunol. 140, 909–920 (2017). Edwards et al. discuss evidence for a reciprocal inverse correlation between innate interferons and TH2 cell mediators and how biologics targeting TH2 cell mediators contribute in this regard.
Rupani, H. et al. Toll-like receptor 7 is reduced in severe asthma and linked to an altered MicroRNA profile. Am. J. Respir. Crit. Care Med. 194, 26–37 (2016).
Gill, M. A. et al. Enhanced plasmacytoid dendritic cell antiviral responses after omalizumab. J. Allergy Clin. Immunol. 141, 1735–1743.e9 (2018).
Fujimura, K. E. et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat. Med. 22, 1187–1191 (2016).
Durack, J. et al. Delayed gut microbiota development in high-risk for asthma infants is temporarily modifiable by Lactobacillus supplementation. Nat. Commun. 9, 707 (2018). This study shows that early-life gut microbial development is distinct but plastic in infants at high risk of asthma.
Milani, C. et al. The first microbial colonizers of the human gut. composition, activities, and health implications of the infant gut microbiota. Microbiol. Mol. Biol. Rev. 81, e00036-17 (2017).
Bunyavanich, S. & Berin, M. C. Food allergy and the microbiome. Current understandings and future directions. J. Allergy Clin. Immunol. 144, 1468–1477 (2019).
Russell, S. L. et al. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep. 13, 440–447 (2012).
Geuking, M. B. et al. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity 34, 794–806 (2011).
Smith, P. M. et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573 (2013).
Feehley, T. et al. Healthy infants harbor intestinal bacteria that protect against food allergy. Nat. Med. 25, 448–453 (2019).
Abdel-Gadir, A. et al. Microbiota therapy acts via a regulatory T cell MyD88/RORγt pathway to suppress food allergy. Nat. Med. 25, 1164–1174 (2019). Using a model of food allergy, these authors show that microbiota therapy can suppress food allergy via innate signalling pathways.
Tan, J. et al. The role of short-chain fatty acids in health and disease. Adv. Immunol. 121, 91–119 (2014).
Roduit, C. et al. High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy 74, 799–809 (2019).
Sandin, A., Bråbäck, L., Norin, E. & Björkstén, B. Faecal short chain fatty acid pattern and allergy in early childhood. Acta Paediatr. 98, 823–827 (2009).
Bunyavanich, S. et al. Early-life gut microbiome composition and milk allergy resolution. J. Allergy Clin. Immunol. 138, 1122–1130 (2016).
Thio, C. L.-P., Chi, P.-Y., Lai, A. C.-Y. & Chang, Y.-J. Regulation of type 2 innate lymphoid cell-dependent airway hyperreactivity by butyrate. J. Allergy Clin. Immunol. 142, 1867–1883.e12 (2018).
Lewis, G. et al. Dietary fiber-induced microbial short chain fatty acids suppress ILC2-dependent airway inflammation. Front. Immunol. 10, 2051 (2019).
Furusawa, Y. et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504, 446–450 (2013). This article reports a mechanistic link between dietary fibre, commensal microorganisms, production of SCFAs and differentiation of Treg cells.
Trompette, A. et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 20, 159–166 (2014). In this study, the effects of dietary fibre on microbiota-mediated effector responses in the lung (allergic airway diseases) and haematopoiesis are described.
Ege, M. J. et al. Exposure to environmental microorganisms and childhood asthma. N. Engl. J. Med. 364, 701–709 (2011). This is a cross-sectional study on the hygiene hypothesis: environmental microorganisms and asthma prevention.
Hagner, S. et al. Farm-derived Gram-positive bacterium Staphylococcus sciuri W620 prevents asthma phenotype in HDM- and OVA-exposed mice. Allergy 68, 322–329 (2013).
Conrad, M. L. et al. Maternal TLR signaling is required for prenatal asthma protection by the nonpathogenic microbe Acinetobacter lwoffii F78. J. Exp. Med. 206, 2869–2877 (2009).
Debarry, J. et al. Acinetobacter lwoffii and Lactococcus lactis strains isolated from farm cowsheds possess strong allergy-protective properties. J. Allergy Clin. Immunol. 119, 1514–1521 (2007).
Stein, K. et al. Endosomal recognition of Lactococcus lactis G121 and its RNA by dendritic cells is key to its allergy-protective effects. J. Allergy Clin. Immunol. 139, 667–678.e5 (2017).
Renz, H., Brandtzaeg, P. & Hornef, M. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nat. Rev. Immunol. 12, 9–23 (2011).
Ruokolainen, L. et al. Significant disparities in allergy prevalence and microbiota between the young people in Finnish and Russian Karelia. Clin. Exp. Allergy 47, 665–674 (2017).
Seeley, J. J. & Ghosh, S. Molecular mechanisms of innate memory and tolerance to LPS. J. Leukoc. Biol. 101, 107–119 (2017).
Brand, S. et al. Epigenetic regulation in murine offspring as a novel mechanism for transmaternal asthma protection induced by microbes. J. Allergy Clin. Immunol. 128, 618–625.e7 (2011).
Haahtela, T. A biodiversity hypothesis. Allergy 74, 1445–1456 (2019).
Kyburz, A. et al. Transmaternal Helicobacter pylori exposure reduces allergic airway inflammation in offspring through regulatory T cells. J. Allergy Clin. Immunol. 143, 1496–1512.e11 (2019).
Matysiak-Budnik, T. et al. Helicobacter pylori increases the epithelial permeability to a food antigen in human gastric biopsies. Am. J. Gastroenterol. 99, 225–232 (2004).
Skevaki, C. et al. Influenza-derived peptides cross-react with allergens and provide asthma protection. J. Allergy Clin. Immunol. 142, 804–814 (2018). This is the first demonstration of virus-induced T cell-mediated heterologous immune responses to allergens with implications for asthma protection.
Culley, F. J., Pennycook, A. M. J., Tregoning, J. S., Hussell, T. & Openshaw, P. J. M. Differential chemokine expression following respiratory virus infection reflects Th1- or Th2-biased immunopathology. J. Virol. 80, 4521–4527 (2006).
Yang, J.-Y. et al. Enteric viruses ameliorate gut inflammation via toll-like receptor 3 and toll-like receptor 7-mediated interferon-β production. Immunity 44, 889–900 (2016).
Neil, J. A. & Cadwell, K. The intestinal virome and immunity. J. Immunol. 201, 1615–1624 (2018).
Machiels, B. et al. A gammaherpesvirus provides protection against allergic asthma by inducing the replacement of resident alveolar macrophages with regulatory monocytes. Nat. Immunol. 18, 1310–1320 (2017). These investigators show that a murid herpesvirus may inhibit experimental asthma by replacing alveolar macrophages with regulatory monocytes and thus contributing to training of lung immune responses.
De Vlaminck, I. et al. Temporal response of the human virome to immunosuppression and antiviral therapy. Cell 155, 1178–1187 (2013).
Pifferi, M. et al. Associations between nasal torquetenovirus load and spirometric indices in children with asthma. J. Infect. Dis. 192, 1141–1148 (2005).
Maggi, F. & Bendinelli, M. Immunobiology of the Torque teno viruses and other anelloviruses. Curr. Top. Microbiol. Immunol. 331, 65–90 (2009).
Li, L. et al. AIDS alters the commensal plasma virome. J. Virol. 87, 10912–10915 (2013).
Tiew, P. Y. et al. The mycobiome in health and disease. emerging concepts, methodologies and challenges. Mycopathologia 185, 207–231 (2020).
Li, X. et al. Response to fungal dysbiosis by gut-resident CX3CR1+ mononuclear phagocytes aggravates allergic airway disease. Cell Host Microbe 24, 847–856.e4 (2018).
Bacher, P. et al. Human anti-fungal Th17 immunity and pathology rely on cross-reactivity against Candida albicans. Cell 176, 1340–1355.e15 (2019). These authors identify heterologous immunity to a single ubiquitous member of the microbiota as the culprit in human antifungal TH17 cell responses, particularly in the context of airway inflammation.
Rayner, S. et al. Identification of small RNAs in extracellular vesicles from the commensal yeast Malassezia sympodialis. Sci. Rep. 7, 39742 (2017).
Sparber, F. et al. The skin commensal yeast Malassezia triggers a type 17 response that coordinates anti-fungal immunity and exacerbates skin inflammation. Cell Host Microbe 25, 389–403.e6 (2019). This article highlights the importance of Malassezia-induced type 17 responses in driving antifungal immunity and skin inflammation.
Krause, R. et al. Mycobiome in the lower respiratory tract - a clinical perspective. Front. Microbiol. 7, 2169 (2016).
Tipton, L., Ghedin, E. & Morris, A. The lung mycobiome in the next-generation sequencing era. Virulence 8, 334–341 (2017).
Fraczek, M. G. et al. Corticosteroid treatment is associated with increased filamentous fungal burden in allergic fungal disease. J. Allergy Clin. Immunol. 142, 407–414 (2018).
Skalski, J. H. et al. Expansion of commensal fungus Wallemia mellicola in the gastrointestinal mycobiota enhances the severity of allergic airway disease in mice. PLoS Pathog. 14, e1007260 (2018).
Goldman, D. L. et al. Lower airway microbiota and mycobiota in children with severe asthma. J. Allergy Clin. Immunol. 141, 808–811.e7 (2018).
Mac Aogáin, M. et al. Distinct “immunoallertypes” of disease and high frequencies of sensitization in non-cystic fibrosis bronchiectasis. Am. J. Respir. Crit. Care Med. 199, 842–853 (2019).
Richardson, M., Bowyer, P. & Sabino, R. The human lung and aspergillus: you are what you breathe in? Med. Mycol. 57, S145–S154 (2019).
Kauth, M. & Heine, H. Allergy protection by cowshed bacteria - recent findings and future prospects. Pediatr. Allergy Immunol. 27, 340–347 (2016).
Hesselmar, B. et al. Pacifier cleaning practices and risk of allergy development. Pediatrics 131, e1829–e1837 (2013).
Alm, J. S., Swartz, J., Lilja, G., Scheynius, A. & Pershagen, G. Atopy in children of families with an anthroposophic lifestyle. Lancet 353, 1485–1488 (1999).
Hesselmar, B., Hicke-Roberts, A. & Wennergren, G. Allergy in children in hand versus machine dishwashing. Pediatrics 135, e590–e597 (2015).
Gern, J. E. Promising candidates for allergy prevention. J. Allergy Clin. Immunol. 136, 23–28 (2015).
Morisset, M., Aubert-Jacquin, C., Soulaines, P., Moneret-Vautrin, D.-A. & Dupont, C. A non-hydrolyzed, fermented milk formula reduces digestive and respiratory events in infants at high risk of allergy. Eur. J. Clin. Nutr. 65, 175–183 (2011).
Kukkonen, A. K., Savilahti, E. M., Haahtela, T., Savilahti, E. & Kuitunen, M. Ovalbumin-specific immunoglobulins A and G levels at age 2 years are associated with the occurrence of atopic disorders. Clin. Exp. Allergy 41, 1414–1421 (2011).
Kuitunen, M. et al. Probiotics prevent IgE-associated allergy until age 5 years in cesarean-delivered children but not in the total cohort. J. Allergy Clin. Immunol. 123, 335–341 (2009).
Marschan, E. et al. Probiotics in infancy induce protective immune profiles that are characteristic for chronic low-grade inflammation. Clin. Exp. Allergy 38, 611–618 (2008).
Osborn, D. A. & Sinn, J. K. H. Prebiotics in infants for prevention of allergy. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD006474.pub3 (2013).
Osborn, D. A. & Sinn, J. K. Probiotics in infants for prevention of allergic disease and food hypersensitivity. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD006475.pub2 (2007).
Grüber, C. et al. Reduced occurrence of early atopic dermatitis because of immunoactive prebiotics among low-atopy-risk infants. J. Allergy Clin. Immunol. 126, 791–797 (2010).
Prescott, S. L. et al. Early markers of allergic disease in a primary prevention study using probiotics: 2.5-year follow-up phase. Allergy 63, 1481–1490 (2008).
Forsberg, A., West, C. E., Prescott, S. L. & Jenmalm, M. C. Pre- and probiotics for allergy prevention: time to revisit recommendations? Clin. Exp. Allergy 46, 1506–1521 (2016).
Muraro, A. et al. EAACI food allergy and anaphylaxis guidelines. Primary prevention of food allergy. Allergy 69, 590–601 (2014).
Braegger, C. et al. Supplementation of infant formula with probiotics and/or prebiotics: a systematic review and comment by the ESPGHAN committee on nutrition. J. Pediatr. Gastroenterol. Nutr. 52, 238–250 (2011).
Fiocchi, A. et al. World Allergy Organization-McMaster University guidelines for allergic disease prevention (GLAD-P). Probiotics. World Allergy Organ. J. 8, 4 (2015).
Niers, L. E. M. et al. Identification of strong interleukin-10 inducing lactic acid bacteria which down-regulate T helper type 2 cytokines. Clin. Exp. Allergy 35, 1481–1489 (2005).
Niers, L. E. M. et al. Selection of probiotic bacteria for prevention of allergic diseases: immunomodulation neonatal dendritic cells. Clin. Exp. Immunol. 149, 344–352 (2007).
Braat, H. et al. Lactobacillus rhamnosus induces peripheral hyporesponsiveness in stimulated CD4+ T cells via modulation of dendritic cell function. Am. J. Clin. Nutr. 80, 1618–1625 (2004).
Rigby, R. J., Knight, S. C., Kamm, M. A. & Stagg, A. J. Production of interleukin (IL)-10 and IL-12 by murine colonic dendritic cells in response to microbial stimuli. Clin. Exp. Immunol. 139, 245–256 (2005).
Kim, J. Y., Choi, Y. O. & Ji, G. E. Effect of oral probiotics (Bifidobacterium lactis AD011 and Lactobacillus acidophilus AD031) administration on ovalbumin-induced food allergy mouse model. J. Microbiol. Biotechnol. 18, 1393–1400 (2008).
So, J.-S. et al. Lactobacillus casei potentiates induction of oral tolerance in experimental arthritis. Mol. Immunol. 46, 172–180 (2008).
Hacini-Rachinel, F. et al. Oral probiotic control skin inflammation by acting on both effector and regulatory T cells. PLoS ONE 4, e4903 (2009).
Elazab, N. et al. Probiotic administration in early life, atopy, and asthma. A meta-analysis of clinical trials. Pediatrics 132, e666–e676 (2013).
Kalliomäki, M., Salminen, S., Poussa, T., Arvilommi, H. & Isolauri, E. Probiotics and prevention of atopic disease. 4-year follow-up of a randomised placebo-controlled trial. Lancet 361, 1869–1871 (2003).
Karimi, K., Inman, M. D., Bienenstock, J. & Forsythe, P. Lactobacillus reuteri-induced regulatory T cells protect against an allergic airway response in mice. Am. J. Respir. Crit. Care Med. 179, 186–193 (2009).
Hoarau, C., Lagaraine, C., Martin, L., Velge-Roussel, F. & Lebranchu, Y. Supernatant of Bifidobacterium breve induces dendritic cell maturation, activation, and survival through a Toll-like receptor 2 pathway. J. Allergy Clin. Immunol. 117, 696–702 (2006).
Forsythe, P., Inman, M. D. & Bienenstock, J. Oral treatment with live Lactobacillus reuteri inhibits the allergic airway response in mice. Am. J. Respir. Crit. Care Med. 175, 561–569 (2007).
Briskey, D. et al. Probiotics modify tight-junction proteins in an animal model of nonalcoholic fatty liver disease. Ther. Adv. Gastroenterol. 9, 463–472 (2016).
Goldin, B. R. & Gorbach, S. L. Clinical indications for probiotics: an overview. Clin. Infect. Dis. 46 (Suppl. 2), S96-S100 (2008).
Berni Canani, R. et al. Lactobacillus rhamnosus GG-supplemented formula expands butyrate-producing bacterial strains in food allergic infants. ISME J. 10, 742–750 (2016).
Kerperien, J. et al. Non-digestible oligosaccharides modulate intestinal immune activation and suppress cow’s milk allergic symptoms. Pediatr. Allergy Immunol. 25, 747–754 (2014).
Moro, G. et al. A mixture of prebiotic oligosaccharides reduces the incidence of atopic dermatitis during the first six months of age. Arch. Dis. Child. 91, 814–819 (2006).
Arslanoglu, S. et al. Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first two years of life. J. Nutr. 138, 1091–1095 (2008).
Edwards, M. R. et al. The potential of anti-infectives and immunomodulators as therapies for asthma and asthma exacerbations. Allergy 73, 50–63 (2018). This review article from the EAACI Anti-infectives in Asthma and Asthma Exacerbations Task Force summarizes the potential of anti-infectives and immunomodulators in asthma.
Ichinohe, T. et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl Acad. Sci. USA 108, 5354–5359 (2011).
Holt, P. G., Strickland, D. H. & Sly, P. D. Virus infection and allergy in the development of asthma. What is the connection? Curr. Opin. Allergy Clin. Immunol. 12, 151–157 (2012).
DesRoches, A., Infante-Rivard, C., Paradis, L., Paradis, J. & Haddad, E. Peanut allergy: is maternal transmission of antigens during pregnancy and breastfeeding a risk factor? J. Investig. Allergol. Clin. Immunol. 20, 289–294 (2010).
Frank, L., Marian, A., Visser, M., Weinberg, E. & Potter, P. C. Exposure to peanuts in utero and in infancy and the development of sensitization to peanut allergens in young children. Pediatr. Allergy Immunol. 10, 27–32 (1999).
Sicherer, S. H. & Burks, A. W. Maternal and infant diets for prevention of allergic diseases. Understanding menu changes in 2008. J. Allergy Clin. Immunol. 122, 29–33 (2008).
Maslova, E. et al. Peanut and tree nut consumption during pregnancy and allergic disease in children-should mothers decrease their intake? Longitudinal evidence from the Danish National Birth Cohort. J. Allergy Clin. Immunol. 130, 724–732 (2012).
Du Toit, G. et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. J N. Engl. J. Med. 372, 803–813 (2015).
Du Toit, G. et al. Effect of avoidance on peanut allergy after early peanut consumption. N. Engl. J. Med. 374, 1435–1443 (2016). This is a hallmark study on the role of avoidance of peanut allergens in the development of peanut allergy, a novel concept for oral tolerance.
Turcanu, V. et al. Immune mechanisms of food allergy and its prevention by early intervention. Curr. Opin. Immunol. 48, 92–98 (2017).
Peters, R. L., Neeland, M. R. & Allen, K. J. Primary prevention of food allergy. Curr. Allergy Asthma Rep. 17, 52 (2017).
Bellach, J. et al. Randomized placebo-controlled trial of hen’s egg consumption for primary prevention in infants. J. Allergy Clin. Immunol. 139, 1591–1599.e2 (2017).
Natsume, O. et al. Two-step egg introduction for prevention of egg allergy in high-risk infants with eczema (PETIT). A randomised, double-blind, placebo-controlled trial. Lancet 389, 276–286 (2017).
Palmer, D. J., Sullivan, T. R., Gold, M. S., Prescott, S. L. & Makrides, M. Randomized controlled trial of early regular egg intake to prevent egg allergy. J. Allergy Clin. Immunol. 139, 1600–1607.e2 (2017).
Perkin, M. R. et al. Randomized trial of introduction of allergenic foods in breast-fed infants. N. Engl. J. Med. 374, 1733–1743 (2016).
Mazzocchi, A., Venter, C., Maslin, K. & Agostoni, C. The role of nutritional aspects in food allergy: prevention and management. Nutrients 9, 850 (2017).
Claesson, M. J. et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 488, 178–184 (2012).
Høst, A. et al. Dietary products used in infants for treatment and prevention of food allergy. Joint Statement of the European Society for Paediatric Allergology and Clinical Immunology (ESPACI) Committee on Hypoallergenic Formulas and the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) Committee on Nutrition. Arch. Dis. Child. 81, 80–84 (1999).
Hill, D. J., Murch, S. H., Rafferty, K., Wallis, P. & Green, C. J. The efficacy of amino acid-based formulas in relieving the symptoms of cow’s milk allergy: A systematic review. Clin. Exp. Allergy 37, 808–822 (2007).
Chafen, J. J. S. et al. Diagnosing and managing common food allergies: a systematic review. JAMA 303, 1848–1856 (2010).
Silva, D. de et al. Primary prevention of food allergy in children and adults. Syst. Rev. Allergy 69, 581–589 (2014).
Allen, C. W., Campbell, D. E. & Kemp, A. S. Food allergy: is strict avoidance the only answer? Pediatr. Allergy Immunol. 20, 415–422 (2009).
Boyle, R. J. et al. Hydrolysed formula and risk of allergic or autoimmune disease: systematic review and meta-analysis. BMJ 352, i974 (2016).
Boyle, R. J. et al. Prebiotic-supplemented partially hydrolysed cow’s milk formula for the prevention of eczema in high-risk infants: a randomized controlled trial. Allergy 71, 701–710 (2016).
Vandenplas, Y. et al. Should partial hydrolysates be used as starter infant formula? A working group consensus. J. Pediatr. Gastroenterol. Nutr. 62, 22–35 (2016).
Wong, G. W. K. & Chow, C. M. Childhood asthma epidemiology: insights from comparative studies of rural and urban populations. Pediatr. Pulmonol. 43, 107–116 (2008).
Abreo, A., Gebretsadik, T., Stone, C. A. & Hartert, T. V. The impact of modifiable risk factor reduction on childhood asthma development. Clin. Transl Med. 7, 15 (2018).
Pelkonen, A. S. et al. Allergy in children: practical recommendations of the Finnish Allergy Programme 2008-2018 for prevention, diagnosis, and treatment. Pediatr. Allergy Immunol. 23, 103–116 (2012).
Tanno, L. K., Haahtela, T., Calderon, M. A., Cruz, A. & Demoly, P. Implementation gaps for asthma prevention and control. Respir. Med. 130, 13–19 (2017).
Haahtela, T., Valovirta, E., Bousquet, J. & Mäkelä, M. The Finnish Allergy Programme 2008–2018 works. Eur. Respir. J. 49, 1700470 (2017).
Burki, T. K. Asthma control. learning from Finland’s success. Lancet Respir. Med. 7, 207–208 (2019).
Acknowledgements
H.R. is supported by the Universities of Giessen and Marburg Lung Center, the German Center for Lung Research (82DZL00502/A2), and the Deutsche Forschungsgemeinschaft funded-SFB 1021 (C04). C.S. is supported by the Universities of Giessen and Marburg Lung Center, the German Center for Lung Research, University Hospital of Giessen and Marburg research funding according to article 2, section 3 cooperation agreement, the Foundation for Pathobiochemistry and Molecular Diagnostics, and the Deutsche Forschungsgemeinschaft-funded SFB 1021 (C04), KFO 309 (P10) and SK 317/1-1 (project number 428518790).
Author information
Authors and Affiliations
Contributions
C.S. wrote and edited the manuscript. H.R. helped revise and edit the manuscript.
Corresponding author
Ethics declarations
Competing interests
C.S. has received consultancy fees and research funding from Hycor Biomedical and Thermo Fisher Scientific, consultancy fees from Bencard Allergie and research funding from Mead Johnson Nutrition. H.R. has received research support from Mead Johnson Nutrition and Beckman Coulter, has received speaker’s honoraria from Allergopharma, Novartis, Thermo Fisher, Danone, Mead Johnson Nutrition and Bencard Allergie, and has been a consultant for Bencard Allergie and Secarna Pharmaceuticals (co-founder).
Additional information
Peer review information
Nature Reviews Immunology thanks K. C. Nadeau, V. Verhass and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Centers for Disease Control and Prevention: most recent asthma data: https://www.cdc.gov/asthma/most_recent_data.htm
Glossary
- Short-chain fatty acids
-
(SCFAs). Subgroup of fatty acids with only two to six carbon atoms. Important representatives are butyrate, acetate and propionate.
- Dysbiosis
-
A condition in which there is a qualitative and quantitative imbalance of bacterial communities that constitute the microbiota, which could represent a predisposition factor for several diseases. Dysbiosis refers mostly to the intestinal microbiota, but it can also occur at other body sites and in other habitats.
- Human milk oligosaccharides
-
(HMOs). Complex sugar molecules that are present in human breast milk in relatively high concentrations; their composition shows high interindividual and intraindividual variability.
- Group 2 innate lymphoid cells
-
(ILC2s). Derived from lymphoid progenitors, these cells lack B and T cell receptors and produce type 2 cytokines such as IL-4, IL-5 and IL-9
- Inflammasome
-
Cytosolic multiprotein oligomer of the innate immune system responsible for the activation of the inflammatory responses.
- Asthma endotypes
-
Forms of asthma with distinct mechanistic pathways that have therapeutic and prognostic implications.
- Neutrophil extracellular traps
-
Networks of extracellular fibres, produced by neutrophils, which bind to pathogens and thus allow neutrophils to kill them with minimal host damage. The extracellular fibril matrix is composed of decondensed chromatin.
- Plasmacytoid dendritic cell
-
Derived from bone marrow haematopoietic stem cells, this type of immune cell circulates in the blood and in peripheral lymphoid organs and is known to secrete large quantities of type I interferon following viral infection.
Rights and permissions
About this article
Cite this article
Renz, H., Skevaki, C. Early life microbial exposures and allergy risks: opportunities for prevention. Nat Rev Immunol 21, 177–191 (2021). https://doi.org/10.1038/s41577-020-00420-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-020-00420-y
This article is cited by
-
28 NICUs participating in a quality improvement collaborative targeting early-onset sepsis antibiotic use
Journal of Perinatology (2024)
-
Environmental Interventions for Preventing Atopic Diseases
Current Allergy and Asthma Reports (2024)
-
Balancing Act of the Intestinal Antimicrobial Proteins on Gut Microbiota and Health
Journal of Microbiology (2024)
-
Gut microbiota in COVID-19: key microbial changes, potential mechanisms and clinical applications
Nature Reviews Gastroenterology & Hepatology (2023)
-
The airway microbiota of neonates colonized with asthma-associated pathogenic bacteria
Nature Communications (2023)