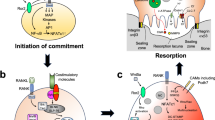
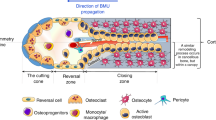
Abstract
In terrestrial vertebrates, bone tissue constitutes the ‘osteoimmune’ system, which functions as a locomotor organ and a mineral reservoir as well as a primary lymphoid organ where haematopoietic stem cells are maintained. Bone and mineral metabolism is maintained by the balanced action of bone cells such as osteoclasts, osteoblasts and osteocytes, yet subverted by aberrant and/or prolonged immune responses under pathological conditions. However, osteoimmune interactions are not restricted to the unidirectional effect of the immune system on bone metabolism. In recent years, we have witnessed the discovery of effects of bone cells on immune regulation, including the function of osteoprogenitor cells in haematopoietic stem cell regulation and osteoblast-mediated suppression of haematopoietic malignancies. Moreover, the dynamic reciprocal interactions between bone and malignancies in remote organs have attracted attention, extending the horizon of osteoimmunology. Here, we discuss emerging concepts in the osteoimmune dialogue in health and disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Okamoto, K. et al. Osteoimmunology: the conceptual framework unifying the immune and skeletal systems. Physiol. Rev. 97, 1295–1349 (2017).
Horton, J. E., Raisz, L. G., Simmons, H. A., Oppenheim, J. J. & Mergenhagen, S. E. Bone resorbing activity in supernatant fluid from cultured human peripheral blood leukocytes. Science 177, 793–795 (1972).
Arron, J. R. & Choi, Y. Bone versus immune system. Nature 408, 535–536 (2000).
Takayanagi, H. et al. T cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature 408, 600–605 (2000).
Takayanagi, H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 7, 292–304 (2007).
Bouillon, R. & Suda, T. Vitamin D: calcium and bone homeostasis during evolution. Bonekey Rep. 3, 480 (2014).
Weiss, M. C. et al. The physiology and habitat of the last universal common ancestor. Nat. Microbiol. 1, 16116 (2016).
Costello, M. J. & Chaudhary, C. Marine biodiversity, biogeography, deep-sea gradients, and conservation. Curr. Biol. 27, 2051 (2017).
You, X. et al. Mudskipper genomes provide insights into the terrestrial adaptation of amphibious fishes. Nat. Commun. 5, 5594 (2014). This report suggests that the immune system rapidly evolved during the water-to-land transition to provide defence against terrestrial pathogens.
Ishii, A., Kawasaki, M., Matsumoto, M., Tochinai, S. & Seya, T. Phylogenetic and expression analysis of amphibian Xenopus Toll-like receptors. Immunogenetics 59, 281–293 (2007).
Kong, Y. Y. et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397, 315–323 (1999).
Tsukasaki, M. et al. LOX fails to substitute for RANKL in osteoclastogenesis. J. Bone Miner. Res. 32, 434–439 (2017).
Hikosaka, Y. et al. The cytokine RANKL produced by positively selected thymocytes fosters medullary thymic epithelial cells that express autoimmune regulator. Immunity 29, 438–450 (2008).
Mueller, C. G. & Hess, E. Emerging functions of RANKL in lymphoid tissues. Front. Immunol. 3, 261 (2012).
Onder, L. et al. Lymphatic endothelial cells control initiation of lymph node organogenesis. Immunity 47, 80–92 (2017).
Nagashima, K. et al. Identification of subepithelial mesenchymal cells that induce IgA and diversify gut microbiota. Nat. Immunol. 18, 675–682 (2017).
Loser, K. et al. Epidermal RANKL controls regulatory T cell numbers via activation of dendritic cells. Nat. Med. 12, 1372–1379 (2006).
Habbeddine, M. et al. Receptor activator of NF-κB orchestrates activation of antiviral memory CD8 T cells in the spleen marginal zone. Cell Rep. 21, 2515–2527 (2017).
Bando, J. K. et al. The tumor necrosis factor superfamily member RANKL suppresses effector cytokine production in group 3 innate lymphoid cells. Immunity 48, 1208–1219 (2018).
Fata, J. E. et al. The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell 103, 41–50 (2000).
Sigl, V. et al. RANKL/RANK control Brca1 mutation-driven mammary tumors. Cell Res. 26, 761–774 (2016).
Gonzalez-Suarez, E. et al. RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature 468, 103–107 (2010).
Nolan, E. et al. RANK ligand as a potential target for breast cancer prevention in BRCA1-mutation carriers. Nat. Med. 22, 933–939 (2016).
Duheron, V. et al. Receptor activator of NF-kappaB (RANK) stimulates the proliferation of epithelial cells of the epidermo-pilosebaceous unit. Proc. Natl Acad. Sci. USA 108, 5342–5347 (2011).
Luo, J. L. et al. Nuclear cytokine-activated IKKalpha controls prostate cancer metastasis by repressing Maspin. Nature 446, 690–694 (2007).
Rao, S. et al. RANK rewires energy homeostasis in lung cancer cells and drives primary lung cancer. Genes Dev. 31, 2099–2112 (2017).
Schramek, D. et al. Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer. Nature 468, 98–102 (2010).
Joshi, P. A. et al. Progesterone induces adult mammary stem cell expansion. Nature 465, 803–807 (2010).
Hanada, R. et al. Central control of fever and female body temperature by RANKL/RANK. Nature 462, 505–509 (2009).
Kiechl, S. et al. Blockade of receptor activator of nuclear factor-κB (RANKL) signaling improves hepatic insulin resistance and prevents development of diabetes mellitus. Nat. Med. 19, 358–363 (2013).
Kondegowda, N. G. et al. Osteoprotegerin and denosumab stimulate human beta cell proliferation through inhibition of the receptor activator of NF-κB ligand pathway. Cell Metab. 22, 77–85 (2015).
Shoji-Matsunaga, A. et al. Osteocyte regulation of orthodontic force-mediated tooth movement via RANKL expression. Sci. Rep. 7, 8753 (2017).
Nakashima, T. et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 17, 1231–1234 (2011).
Xiong, J. et al. Matrix-embedded cells control osteoclast formation. Nat. Med. 17, 1235–1241 (2011).
Fujiwara, Y. et al. RANKL (receptor activator of NFκB ligand) produced by osteocytes is required for the increase in B cells and bone loss caused by estrogen deficiency in mice. J. Biol. Chem. 291, 24838–24850 (2016).
Tan, W. et al. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature 470, 548–553 (2011).
Panizo, S. et al. RANKL increases vascular smooth muscle cell calcification through a RANK-BMP4-dependent pathway. Circ. Res. 104, 1041–1048 (2009).
Shimamura, M. et al. OPG/RANKL/RANK axis is a critical inflammatory signaling system in ischemic brain in mice. Proc. Natl Acad. Sci. USA 111, 8191–8196 (2014).
Jones, D. H. et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature 440, 692–696 (2006).
Guerrini, M. M. et al. Inhibition of the TNF family cytokine RANKL prevents autoimmune inflammation in the central nervous system. Immunity 43, 1174–1185 (2015).
Venkatesh, B. et al. Elephant shark genome provides unique insights into gnathostome evolution. Nature 505, 174–179 (2014).
Koga, T. et al. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature 428, 758–763 (2004).
Takayanagi, H. et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 3, 889–901 (2002).
Shinohara, M. et al. Tyrosine kinases Btk and Tec regulate osteoclast differentiation by linking RANK and ITAM signals. Cell 132, 794–806 (2008).
Asagiri, M. et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J. Exp. Med. 202, 1261–1269 (2005).
Negishi-Koga, T. et al. Immune complexes regulate bone metabolism through FcRγ signalling. Nat. Commun. 6, 6637 (2015). This paper shows that immune complexes directly promote osteoclastogenesis.
Morrison, S. J. & Scadden, D. T. The bone marrow niche for haematopoietic stem cells. Nature 505, 327–334 (2014).
Orkin, S. H. & Zon, L. I. Hematopoiesis: an evolving paradigm for stem cell biology. Cell 132, 631–644 (2008).
Kapp, F. G. et al. Protection from UV light is an evolutionarily conserved feature of the haematopoietic niche. Nature 558, 445–448 (2018). This study suggests that higher levels of ultraviolet light in the terrestrial environment might be an evolutionary pressure that drives HSC homing to the bone marrow.
Adams, G. B. et al. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature 439, 599–603 (2006).
Takubo, K. et al. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell 7, 391–402 (2010).
Spencer, J. A. et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 508, 269–273 (2014).
Calvi, L. M. et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425, 841–846 (2003).
Zhang, J. et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425, 836–841 (2003).
Visnjic, D. et al. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood 103, 3258–3264 (2004).
Greenbaum, A. et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 495, 227–230 (2013). This is one of the studies showing the role of osteoprogenitors in HSC maintenance.
Ding, L., Saunders, T. L., Enikolopov, G. & Morrison, S. J. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481, 457–462 (2012).
Ding, L. & Morrison, S. J. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 495, 231–235 (2013).
Omatsu, Y. et al. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity 33, 387–399 (2010).
Xu, C. et al. Stem cell factor is selectively secreted by arterial endothelial cells in bone marrow. Nat. Commun. 9, 2449 (2018).
Asada, N. et al. Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat. Cell Biol. 19, 214–223 (2017).
Zhou, B. O., Yue, R., Murphy, M. M., Peyer, J. G. & Morrison, S. J. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 15, 154–168 (2014).
Mizoguchi, T. et al. Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Dev. Cell 29, 340–349 (2014).
Yu, V. W. et al. Specific bone cells produce DLL4 to generate thymus-seeding progenitors from bone marrow. J. Exp. Med. 212, 759–774 (2015).
Miyaura, C. et al. Increased B-lymphopoiesis by interleukin 7 induces bone loss in mice with intact ovarian function: similarity to estrogen deficiency. Proc. Natl Acad. Sci. USA 94, 9360–9365 (1997).
Lee, S. K. et al. Interleukin-7 influences osteoclast function in vivo but is not a critical factor in ovariectomy-induced bone loss. J. Bone Miner. Res. 21, 695–702 (2006).
Terashima, A. et al. Sepsis-induced osteoblast ablation causes immunodeficiency. Immunity 44, 1434–1443 (2016). This paper reveals the role of reciprocal interactions between osteoblasts and immune cells during sepsis.
Himburg, H. A. et al. Dickkopf-1 promotes hematopoietic regeneration via direct and niche-mediated mechanisms. Nat. Med. 23, 91–99 (2017).
Rankin, E. B. et al. The HIF signaling pathway in osteoblasts directly modulates erythropoiesis through the production of EPO. Cell 149, 63–74 (2012).
Raaijmakers, M. H. et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature 464, 852–857 (2010). This was the first in vivo evidence for the involvement of osteoblastic cells in the development of haematologic malignancy.
Kode, A. et al. Leukaemogenesis induced by an activating β-catenin mutation in osteoblasts. Nature 506, 240–244 (2014). The authors show that activation of β-catenin in osteoblasts leads to the development of leukaemia.
Kode, A. et al. FoxO1-dependent induction of acute myeloid leukemia by osteoblasts in mice. Leukemia 30, 1–13 (2016).
Dong, L. et al. Leukaemogenic effects of Ptpn11 activating mutations in the stem cell microenvironment. Nature 539, 304–308 (2016). This report shows that the activating mutations of SHP2 in osteoprogenitors results in the development of acute myeloid leukaemia.
Menendez, P. et al. Bone marrow mesenchymal stem cells from infants with MLL-AF4+ acute leukemia harbor and express the MLL-AF4 fusion gene. J. Exp. Med. 206, 3131–3141 (2009).
Blau, O. et al. Chromosomal aberrations in bone marrow mesenchymal stroma cells from patients with myelodysplastic syndrome and acute myeloblastic leukemia. Exp. Hematol. 35, 221–229 (2007).
Tefferi, A. & Vardiman, J. W. Myelodysplastic syndromes. N. Engl. J. Med. 361, 1872–1885 (2009).
Flynn, C. M. & Kaufman, D. S. Donor cell leukemia: insight into cancer stem cells and the stem cell niche. Blood 109, 2688–2692 (2007).
Hawkins, E. D. et al. T cell acute leukaemia exhibits dynamic interactions with bone marrow microenvironments. Nature 538, 518–522 (2016).
Krevvata, M. et al. Inhibition of leukemia cell engraftment and disease progression in mice by osteoblasts. Blood 124, 2834–2846 (2014).
Krause, D. S. et al. Differential regulation of myeloid leukemias by the bone marrow microenvironment. Nat. Med. 19, 1513–1517 (2013).
Shono, Y. et al. Bone marrow graft-versus-host disease: early destruction of hematopoietic niche after MHC-mismatched hematopoietic stem cell transplantation. Blood 115, 5401–5411 (2010).
D’Amico, L. et al. Dickkopf-related protein 1 (Dkk1) regulates the accumulation and function of myeloid derived suppressor cells in cancer. J. Exp. Med. 213, 827–840 (2016). This study shows that bone–immune interactions contribute to extraskeletal tumour growth.
Engblom, C. et al. Osteoblasts remotely supply lung tumors with cancer-promoting SiglecFhigh neutrophils. Science 358, eaal5081 (2017). The authors show that osteoblasts supply lung tumours with pathogenic neutrophils, which promote tumour growth in the lung.
Sreehari, S., Naik, D. R. & Eapen, M. Osteopetrosis: a rare cause of anemia. Hematol. Rep. 3, e1 (2011).
Reeves, J. D., August, C. S., Humbert, J. R. & Weston, W. L. Host defense in infantile osteopetrosis. Pediatrics 64, 202–206 (1979).
Gerritsen, E. J. et al. Autosomal recessive osteopetrosis: variability of findings at diagnosis and during the natural course. Pediatrics 93, 247–253 (1994).
Kollet, O. et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat. Med. 12, 657–664 (2006).
Shivtiel, S. et al. CD45 regulates retention, motility, and numbers of hematopoietic progenitors, and affects osteoclast remodeling of metaphyseal trabecules. J. Exp. Med. 205, 2381–2395 (2008).
Miyamoto, K. et al. Osteoclasts are dispensable for hematopoietic stem cell maintenance and mobilization. J. Exp. Med. 208, 2175–2181 (2011).
Rao, M., Supakorndej, T., Schmidt, A. P. & Link, D. C. Osteoclasts are dispensable for hematopoietic progenitor mobilization by granulocyte colony-stimulating factor in mice. Exp. Hematol. 43, 110–114 (2015).
Mansour, A. et al. Osteoclasts promote the formation of hematopoietic stem cell niches in the bone marrow. J. Exp. Med. 209, 537–549 (2012).
Lymperi, S., Ersek, A., Ferraro, F., Dazzi, F. & Horwood, N. J. Inhibition of osteoclast function reduces hematopoietic stem cell numbers in vivo. Blood 117, 1540–1549 (2011).
Mansour, A. et al. Osteoclast activity modulates B cell development in the bone marrow. Cell Res. 21, 1102–1115 (2011).
Teufel, S. et al. Inhibition of bone remodeling in young mice by bisphosphonate displaces the plasma cell niche into the spleen. J. Immunol. 193, 223–233 (2014).
Charles, J. F. et al. Inflammatory arthritis increases mouse osteoclast precursors with myeloid suppressor function. J. Clin. Invest. 122, 4592–4605 (2012).
Yamazaki, S. et al. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell 147, 1146–1158 (2011).
Matsumoto, T. & Abe, M. TGF-β-related mechanisms of bone destruction in multiple myeloma. Bone 48, 129–134 (2011).
Sato, M. et al. Osteocytes regulate primary lymphoid organs and fat metabolism. Cell Metab. 18, 749–758 (2013).
Cain, C. J. et al. Absence of sclerostin adversely affects B cell survival. J. Bone Miner. Res. 27, 1451–1461 (2012).
Fulzele, K. et al. Myelopoiesis is regulated by osteocytes through Gsα-dependent signaling. Blood 121, 930–939 (2013).
Firestein, G. S. Evolving concepts of rheumatoid arthritis. Nature 423, 356–361 (2003).
Kong, Y. Y. et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 402, 304–309 (1999).
Okada, Y. et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature 506, 376–381 (2014).
Firestein, G. S. & Zvaifler, N. J. How important are T cells in chronic rheumatoid synovitis? Arthritis Rheum. 33, 768–773 (1990).
Kotake, S. et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J. Clin. Invest. 103, 1345–1352 (1999).
Sato, K. et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J. Exp. Med. 203, 2673–2682 (2006).
Nistala, K. et al. Interleukin-17-producing T cells are enriched in the joints of children with arthritis, but have a reciprocal relationship to regulatory T cell numbers. Arthritis Rheum. 58, 875–887 (2008).
Hirota, K. et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J. Exp. Med. 204, 2803–2812 (2007).
Danks, L. et al. RANKL expressed on synovial fibroblasts is primarily responsible for bone erosions during joint inflammation. Ann. Rheum. Dis. 75, 1187–1195 (2016).
Ciucci, T. et al. Bone marrow Th17 TNFα cells induce osteoclast differentiation, and link bone destruction to IBD. Gut 64, 1072–1081 (2015).
Komatsu, N. et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat. Med. 20, 62–68 (2014). This study reveals that Foxp3 + T cells convert into T H 17 cells under arthritic conditions.
Edwards, J. C. et al. Efficacy of B cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N. Engl. J. Med. 350, 2572–2581 (2004).
Harre, U. et al. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J. Clin. Invest. 122, 1791–1802 (2012).
Harre, U. et al. Glycosylation of immunoglobulin G determines osteoclast differentiation and bone loss. Nat. Commun. 6, 6651 (2015).
Krishnamurthy, A. et al. Identification of a novel chemokine-dependent molecular mechanism underlying rheumatoid arthritis-associated autoantibody-mediated bone loss. Ann. Rheum. Dis. 75, 721–729 (2016).
Pfeifle, R. et al. Regulation of autoantibody activity by the IL-23-TH17 axis determines the onset of autoimmune disease. Nat. Immunol. 18, 104–113 (2017).
Rao, D. A. et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature 542, 110–114 (2017).
Ogura, H. et al. Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity 29, 628–636 (2008).
Sawa, S. et al. Autoimmune arthritis associated with mutated interleukin (IL)-6 receptor gp130 is driven by STAT3/IL-7-dependent homeostatic proliferation of CD4+ T cells. J. Exp. Med. 203, 1459–1470 (2006).
Hirota, K. et al. Autoimmune Th17 cells induced synovial stromal and innate lymphoid cell secretion of the cytokine GM-CSF to initiate and augment autoimmune arthritis. Immunity 48, 1220–1232 (2018).
Armaka, M., Ospelt, C., Pasparakis, M. & Kollias, G. The p55TNFR-IKK2-Ripk3 axis orchestrates arthritis by regulating death and inflammatory pathways in synovial fibroblasts. Nat. Commun. 9, 618 (2018).
Stephenson, W. et al. Single-cell RNA-seq of rheumatoid arthritis synovial tissue using low-cost microfluidic instrumentation. Nat. Commun. 9, 791 (2018).
Mizoguchi, F. et al. Functionally distinct disease-associated fibroblast subsets in rheumatoid arthritis. Nat. Commun. 9, 789 (2018).
Rauber, S. et al. Resolution of inflammation by interleukin-9-producing type 2 innate lymphoid cells. Nat. Med. 23, 938–944 (2017).
Omata, Y. et al. Group 2 innate lymphoid cells attenuate inflammatory arthritis and protect from bone destruction in mice. Cell Rep. 24, 169–180 (2018).
Chowdhury, K. et al. Synovial IL-9 facilitates neutrophil survival, function and differentiation of Th17 cells in rheumatoid arthritis. Arthritis Res. Ther. 20, 18 (2018).
Genovese, M. C. et al. LY2439821, a humanized anti-interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: a phase I randomized, double-blind, placebo-controlled, proof-of-concept study. Arthritis Rheum. 62, 929–939 (2010).
Hueber, W. et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci. Transl Med. 2, 52ra72 (2010).
Baeten, D. et al. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double-blind, placebo-controlled trial. Lancet 382, 1705–1713 (2013).
McInnes, I. B. et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 386, 1137–1146 (2015).
Hajishengallis, G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 15, 30–44 (2015).
Hajishengallis, G. et al. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe 10, 497–506 (2011).
Maekawa, T. et al. Porphyromonas gingivalis manipulates complement and TLR signaling to uncouple bacterial clearance from inflammation and promote dysbiosis. Cell Host Microbe 15, 768–778 (2014).
Hajishengallis, G., Darveau, R. P. & Curtis, M. A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 10, 717–725 (2012).
Ukai, T., Hara, Y. & Kato, I. Effects of T cell adoptive transfer into nude mice on alveolar bone resorption induced by endotoxin. J. Periodontal Res. 31, 414–422 (1996).
Yamaguchi, M. et al. T cells are able to promote lipopolysaccharide-induced bone resorption in mice in the absence of B cells. J. Periodontal Res. 43, 549–555 (2008).
Teng, Y. T. et al. Functional human T cell immunity and osteoprotegerin ligand control alveolar bone destruction in periodontal infection. J. Clin. Invest. 106, R59–R67 (2000).
Baker, P. J., Howe, L., Garneau, J. & Roopenian, D. C. T cell knockout mice have diminished alveolar bone loss after oral infection with Porphyromonas gingivalis. FEMS Immunol. Med. Microbiol. 34, 45–50 (2002).
Baker, P. J. et al. CD4(+) T cells and the proinflammatory cytokines gamma interferon and interleukin-6 contribute to alveolar bone loss in mice. Infect. Immun. 67, 2804–2809 (1999).
Jiao, Y. et al. Induction of bone loss by pathobiont-mediated Nod1 signaling in the oral cavity. Cell Host Microbe 13, 595–601 (2013).
Abe, T. & Hajishengallis, G. Optimization of the ligature-induced periodontitis model in mice. J. Immunol. Methods 394, 49–54 (2013).
Tsukasaki, M. et al. Host defense against oral microbiota by bone-damaging T cells. Nat. Commun. 9, 701 (2018). This study provides insights on the origin and biological significance of inflammatory bone destruction.
Dutzan, N. et al. On-going mechanical damage from mastication drives homeostatic Th17 cell responses at the oral barrier. Immunity 46, 133–147 (2017).
Yasuhara, R. et al. Lysine-specific gingipain promotes lipopolysaccharide- and active-vitamin D3-induced osteoclast differentiation by degrading osteoprotegerin. Biochem. J. 419, 159–166 (2009).
Akiyama, T. et al. Porphyromonas gingivalis-derived lysine gingipain enhances osteoclast differentiation induced by tumor necrosis factor-α and interleukin-1β but suppresses that by interleukin-17A: importance of proteolytic degradation of osteoprotegerin by lysine gingipain. J. Biol. Chem. 289, 15621–15630 (2014).
Koide, M. et al. Osteoprotegerin-deficient male mice as a model for severe alveolar bone loss: comparison with RANKL-overexpressing transgenic male mice. Endocrinology 154, 773–782 (2013).
Dutzan, N. et al. A dysbiotic microbiome triggers TH17 cells to mediate oral mucosal immunopathology in mice and humans. Sci. Transl Med. 10, eaat0797 (2018). This paper shows that patients with genetic defects in T H 17 cell development were protected from periodontitis-induced bone loss.
Okui, T., Aoki, Y., Ito, H., Honda, T. & Yamazaki, K. The presence of IL-17+/FOXP3+ double-positive cells in periodontitis. J. Dent. Res. 91, 574–579 (2012).
Kobayashi, T. et al. Assessment of interleukin-6 receptor inhibition therapy on periodontal condition in patients with rheumatoid arthritis and chronic periodontitis. J. Periodontol. 85, 57–67 (2014).
Moutsopoulos, N. M. et al. Interleukin-12 and interleukin-23 blockade in leukocyte adhesion deficiency type 1. N. Engl. J. Med. 376, 1141–1146 (2017). The authors show that inhibition of IL-23/T H 17 cell pathway can ameliorate severe periodontitis in leukocyte adhesion defect type 1 patients.
Ogütcen-Toller, M. et al. Intractable bimaxillary osteomyelitis in osteopetrosis: review of the literature and current therapy. J. Oral Maxillofac. Surg. 68, 167–175 (2010).
Otto, S. et al. Tooth extraction in patients receiving oral or intravenous bisphosphonate administration: a trigger for BRONJ development? J. Craniomaxillofac. Surg. 43, 847–854 (2015).
Aguirre, J. I. et al. Oncologic doses of zoledronic acid induce osteonecrosis of the jaw-like lesions in rice rats (Oryzomys palustris) with periodontitis. J. Bone Miner. Res. 27, 2130–2143 (2012).
Soutome, S. et al. Factors affecting development of medication-related osteonecrosis of the jaw in cancer patients receiving high-dose bisphosphonate or denosumab therapy: is tooth extraction a risk factor? PLOS ONE 13, e0201343 (2018).
Reisz, R. R., Scott, D. M., Pynn, B. R. & Modesto, S. P. Osteomyelitis in a Paleozoic reptile: ancient evidence for bacterial infection and its evolutionary significance. Naturwissenschaften 98, 551–555 (2011).
Maruyama, K. et al. Nociceptors boost the resolution of fungal osteoinflammation via the TRP channel-CGRP-Jdp2 axis. Cell Rep. 19, 2730–2742 (2017).
Lee, M. S. J. et al. Plasmodium products persist in the bone marrow and promote chronic bone loss. Sci. Immunol. 2, eaam8093 (2017).
Smith-Guzmán, N. E. The skeletal manifestation of malaria: an epidemiological approach using documented skeletal collections. Am. J. Phys. Anthropol. 158, 624–635 (2015).
Kurihara, N., Reddy, S. V., Menaa, C., Anderson, D. & Roodman, G. D. Osteoclasts expressing the measles virus nucleocapsid gene display a pagetic phenotype. J. Clin. Invest. 105, 607–614 (2000).
Kurihara, N. et al. Expression of measles virus nucleocapsid protein in osteoclasts induces Paget’s disease-like bone lesions in mice. J. Bone Miner. Res. 21, 446–455 (2006).
Raynaud-Messina, B. et al. Bone degradation machinery of osteoclasts: an HIV-1 target that contributes to bone loss. Proc. Natl Acad. Sci. USA 115, E2556–E2565 (2018).
Lee, J. W. et al. The HIV co-receptor CCR5 regulates osteoclast function. Nat. Commun. 8, 2226 (2017).
Maltby, S. et al. Osteoblasts are rapidly ablated by virus-induced systemic inflammation following lymphocytic choriomeningitis virus or pneumonia virus of mice infection in mice. J. Immunol. 200, 632–642 (2018).
Lukens, J. R. et al. Critical role for inflammasome-independent IL-1β production in osteomyelitis. Proc. Natl Acad. Sci. USA 111, 1066–1071 (2014).
Lukens, J. R. et al. Dietary modulation of the microbiome affects autoinflammatory disease. Nature 516, 246–249 (2014).
Chitu, V. et al. PSTPIP2 deficiency in mice causes osteopenia and increased differentiation of multipotent myeloid precursors into osteoclasts. Blood 120, 3126–3135 (2012).
Bader-Meunier, B., Van Nieuwenhove, E., Breton, S. & Wouters, C. Bone involvement in monogenic autoinflammatory syndromes. Rheumatology 57, 606–618 (2018).
Akitsu, A. et al. IL-1 receptor antagonist-deficient mice develop autoimmune arthritis due to intrinsic activation of IL-17-producing CCR2(+)Vγ6(+)γδ T cells. Nat. Commun. 6, 7464 (2015).
Ueki, Y. et al. Mutations in the gene encoding c-Abl-binding protein SH3BP2 cause cherubism. Nat. Genet. 28, 125–126 (2001).
Ueki, Y. et al. Increased myeloid cell responses to M-CSF and RANKL cause bone loss and inflammation in SH3BP2 “cherubism” mice. Cell 128, 71–83 (2007).
Yoshitaka, T. et al. Enhanced TLR-MYD88 signaling stimulates autoinflammation in SH3BP2 cherubism mice and defines the etiology of cherubism. Cell Rep. 8, 1752–1766 (2014).
Hero, M. et al. Anti-tumor necrosis factor treatment in cherubism — clinical, radiological and histological findings in two children. Bone 52, 347–353 (2013).
Farr, J. N. et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat. Med. 23, 1072–1079 (2017). This paper shows that removal of senescent cells can prevent age-related bone loss.
Cenci, S. et al. Estrogen deficiency induces bone loss by enhancing T cell production of TNF-alpha. J. Clin. Invest. 106, 1229–1237 (2000).
Pacifici, R. Role of T cells in ovariectomy induced bone loss — revisited. J. Bone Miner. Res. 27, 231–239 (2012).
Li, J. Y. et al. IL-17A is increased in humans with primary hyperparathyroidism and mediates PTH-induced bone loss in mice. Cell Metab. 22, 799–810 (2015).
Lee, S. K. et al. T lymphocyte-deficient mice lose trabecular bone mass with ovariectomy. J. Bone Miner. Res. 21, 1704–1712 (2006).
Uy, H. L. et al. Effects of parathyroid hormone (PTH)-related protein and PTH on osteoclasts and osteoclast precursors in vivo. Endocrinology 136, 3207–3212 (1995).
Lopes, E. B. P., Filiberti, A., Husain, S. A. & Humphrey, M. B. Immune contributions to osteoarthritis. Curr. Osteoporos. Rep. 15, 593–600 (2017).
Sherlock, J. P. et al. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4-CD8- entheseal resident T cells. Nat. Med. 18, 1069–1076 (2012).
Brennan, T. A. et al. Mast cell inhibition as a therapeutic approach in fibrodysplasia ossificans progressiva (FOP). Bone 109, 259–266 (2018).
Convente, M. R. et al. Depletion of mast cells and macrophages impairs heterotopic ossification in an Acvr1R206H mouse model of fibrodysplasia ossificans progressiva. J. Bone Miner. Res. 33, 269–282 (2018).
Torossian, F. et al. Macrophage-derived oncostatin M contributes to human and mouse neurogenic heterotopic ossifications. JCI Insight 2, e96034 (2017).
Sage, A. P., Tintut, Y. & Demer, L. L. Regulatory mechanisms in vascular calcification. Nat. Rev. Cardiol. 7, 528–536 (2010).
Moore, K. J., Sheedy, F. J. & Fisher, E. A. Macrophages in atherosclerosis: a dynamic balance. Nat. Rev. Immunol. 13, 709–721 (2013).
Al-Aly, Z. et al. Aortic Msx2-Wnt calcification cascade is regulated by TNF-alpha-dependent signals in diabetic Ldlr−/− mice. Arterioscler. Thromb. Vasc. Biol. 27, 2589–2596 (2007).
Yu, M. et al. Regulatory T cells are expanded by Teriparatide treatment in humans and mediate intermittent PTH-induced bone anabolism in mice. EMBO Rep. 19, 156–171 (2018).
Terauchi, M. et al. T lymphocytes amplify the anabolic activity of parathyroid hormone through Wnt10b signaling. Cell Metab. 10, 229–240 (2009).
Li, J. Y. et al. The sclerostin-independent bone anabolic activity of intermittent PTH treatment is mediated by T cell-produced Wnt10b. J. Bone Miner. Res. 29, 43–54 (2014).
Tyagi, A. M. et al. The microbial metabolite butyrate stimulates bone formation via T regulatory cell-mediated regulation of WNT10B expression. Immunity 49, 1116–1131 (2018).
Ono, T. et al. IL-17-producing γδ T cells enhance bone regeneration. Nat. Commun. 7, 10928 (2016).
Acknowledgements
We thank K. Okamoto, T. Nitta, N. Komatsu and A. Terashima for providing helpful discussions during preparation of this manuscript. This work was supported in part by a grant for the Grants-in-Aid for Specially Promoted Research (15H05703) and the Research Fellowship for Young Scientists (18J00744) from the Japan Society for the Promotion of Science (JSPS).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Catalogue of Life: http://www.catalogueoflife.org
OrthoDB: http://www.orthodb.org
The World’s Register of Marine Species: http://www.marinespecies.org
Glossary
- Osteoclast
-
Multinucleated cell of haematopoietic origin that resorbs bone via acid decalcification and proteolytic degradation.
- Osteoblasts
-
Specialized mesenchymal cells that create bone by secreting bone matrix proteins and promoting mineralization.
- Osteocytes
-
Bone matrix-embedded cells that originate from osteoblasts, functioning as a commander of bone metabolism by controlling osteoblasts and osteoclasts.
- Myeloid-derived suppressor cells
-
(MDSCs). Immature myeloid cells that have the capacity to suppress T cell responses.
- Bone marrow mesenchymal stromal cells
-
Bone marrow cells of mesenchymal origin that contain heterogeneous populations of cells, including mesenchymal stem cells.
- Pathogen-associated molecular patterns
-
(PAMPs). Microorganism-derived molecular structures that are recognized by pattern recognition receptors expressed on innate immune cells.
- Danger-associated molecular patterns
-
(DAMPs). Cell-derived endogenous molecules that are released upon tissue damage and stimulate pattern recognition receptors expressed on innate immune cells.
Rights and permissions
About this article
Cite this article
Tsukasaki, M., Takayanagi, H. Osteoimmunology: evolving concepts in bone–immune interactions in health and disease. Nat Rev Immunol 19, 626–642 (2019). https://doi.org/10.1038/s41577-019-0178-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-019-0178-8
This article is cited by
-
Artesunate-loaded thermosensitive chitosan hydrogel promotes osteogenesis of maxillary tooth extraction through regulating T lymphocytes in type 2 diabetic rats
BMC Oral Health (2024)
-
A xonotlite nanofiber bioactive 3D-printed hydrogel scaffold based on osteo-/angiogenesis and osteoimmune microenvironment remodeling accelerates vascularized bone regeneration
Journal of Nanobiotechnology (2024)
-
Monocyte alteration in elderly hip fracture healing: monocyte promising role in bone regeneration
Immunity & Ageing (2024)
-
Recent advances of NFATc1 in rheumatoid arthritis-related bone destruction: mechanisms and potential therapeutic targets
Molecular Medicine (2024)
-
Development of an optogenetics tool, Opto-RANK, for control of osteoclast differentiation using blue light
Scientific Reports (2024)