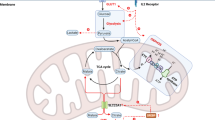
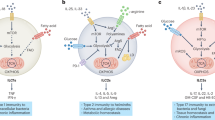
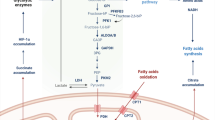
Abstract
Natural killer (NK) cells are lymphocytes with important roles in innate and adaptive immune responses to tumours and viral infection. However, in certain chronic diseases, including obesity and cancer, NK cell functional responses are impaired. Recently, research has highlighted the importance of NK cell metabolism in facilitating robust NK cell effector functions. This Review describes our current understanding of mouse and human NK cell metabolism and the key signalling pathways that mediate metabolic responses in NK cells. Furthermore, it explores how defects in metabolism can contribute to the generation of dysfunctional NK cells in chronic disease. Finally, the potential for new therapeutic strategies targeting cellular metabolism is discussed.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Loftus, R. M. & Finlay, D. K. Immunometabolism: cellular metabolism turns immune regulator. J. Biol. Chem. 291, 1–10 (2016).
O’Neill, L. A., Kishton, R. J. & Rathmell, J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 16, 553–565 (2016).
Campbell, K. S. & Hasegawa, J. Natural killer cell biology: an update and future directions. J. Allergy Clin. Immunol. 132, 536–544 (2013).
Caligiuri, M. A. Human natural killer cells. Blood 112, 461–469 (2008).
Bjorkstrom, N. K. et al. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. J. Exp. Med. 208, 13–21 (2011).
Sun, J. C., Beilke, J. N. & Lanier, L. L. Adaptive immune features of natural killer cells. Nature 457, 557–561 (2009).
Cerwenka, A. & Lanier, L. L. Natural killer cell memory in infection, inflammation and cancer. Nat. Rev. Immunol. 16, 112–123 (2016).
O’Leary, J. G., Goodarzi, M., Drayton, D. L. & von Andrian, U. H. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat. Immunol. 7, 507–516 (2006).
Crouse, J., Xu, H. C., Lang, P. A. & Oxenius, A. NK cells regulating T cell responses: mechanisms and outcome. Trends Immunol. 36, 49–58 (2015).
Keppel, M. P., Saucier, N., Mah, A. Y., Vogel, T. P. & Cooper, M. A. Activation-specific metabolic requirements for NK cell IFN-gamma production. J. Immunol. 194, 1954–1962 (2015). This study shows that acute activation of mouse NK cells does not result in changes in NK cell metabolism and reveals differential requirements for metabolic pathways for the function of cytokine-stimulated versus receptor-stimulated NK cells.
Marcais, A. et al. The metabolic checkpoint kinase mTOR is essential for IL-15 signaling during the development and activation of NK cells. Nat. Immunol. 15, 749–757 (2014). This study demonstrates the importance of the metabolic regulator mTORC1 for mouse NK cell development and activation.
Donnelly, R. P. et al. mTORC1-dependent metabolic reprogramming is a prerequisite for NK cell effector function. J. Immunol. 193, 4477–4484 (2014). This study reveals the importance of mTORC1 and elevated rates of glycolysis and OXPHOS for cytokine-induced mouse NK cell effector function.
Keating, S. E. et al. Metabolic reprogramming supports IFN-gamma production by CD56bright NK cells. J. Immunol. 196, 2552–2560 (2016). This study is the first to show metabolic increases in cytokine-stimulated human NK cells and to link metabolism to human NK cell effector function.
Viel, S. et al. TGF-beta inhibits the activation and functions of NK cells by repressing the mTOR pathway. Sci. Signal. 9, ra19 (2016).
Assmann, N. et al. Srebp-controlled glucose metabolism is essential for NK cell functional responses. Nat. Immunol. 18, 1197–1206 (2017). This study shows that cytokine-activated NK cells use the CMS rather than the TCA cycle to drive OXPHOS and reveals SREBP as a central regulator of NK cell metabolic and functional responses.
O’Sullivan, T. E., Johnson, L. R., Kang, H. H. & Sun, J. C. BNIP3- and BNIP3L-mediated mitophagy promotes the generation of natural killer cell memory. Immunity 43, 331–342 (2015). This study shows the importance of mitophagy in maintaining mitochondrial fitness in NK cells and for the generation NK cell memory-like cells in MCMV-infected mice.
Jensen, H., Potempa, M., Gotthardt, D. & Lanier, L. L. Cutting edge: IL-2-induced expression of the amino acid transporters SLC1A5 and CD98 is a prerequisite for NKG2D-mediated activation of human NK cells. J. Immunol. 199, 1967–1972 (2017).
Felices, M. et al. Continuous treatment with IL-15 exhausts human NK cells via a metabolic defect. JCI Insight 3, 96219 (2018).
Salzberger, W. et al. Tissue-resident NK cells differ in their expression profile of the nutrient transporters Glut1, CD98 and CD71. PLOS ONE 13, e0201170 (2018).
Mah, A. Y. et al. Glycolytic requirement for NK cell cytotoxicity and cytomegalovirus control. JCI Insight 2, 95128 (2017). This study shows that MCMV-infected mice treated with the glycolytic inhibitor 2DG have impaired clearance of NK cell target cells and increased viral burden.
Loftus, R. M. et al. Amino acid-dependent cMyc expression is essential for NK cell metabolic and functional responses in mice. Nat. Commun. 9, 2341 (2018). This study reveals the importance of MYC and SLC7A5 in the control of mouse NK cell metabolic and functional responses.
O’Connor, R. S. et al. The CPT1a inhibitor, etomoxir induces severe oxidative stress at commonly used concentrations. Sci. Rep. 8, 6289 (2018).
Yao, C.-H. et al. Identifying off-target effects of etomoxir reveals that carnitine palmitoyltransferase I is essential for cancer cell proliferation independent of β-oxidation. PLOS Biol. 16, e2003782 (2018).
Raud, B. et al. Etomoxir actions on regulatory and memory T cells are independent of Cpt1a-mediated fatty acid oxidation. Cell Metab. 28, 504–515 (2018).
Michelet, X. et al. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat. Immunol. 19, 1330–1340 (2018). This study shows that NK cell metabolism and function are disrupted during obesity owing to PPAR-mediated inactivation of mTORC1 and MYC signalling.
Chiossone, L. et al. Maturation of mouse NK cells is a 4-stage developmental program. Blood 113, 5488–5496 (2009).
The Tabula Muris Consortium et al. Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature 562, 367–372 (2018).
Schafer, J. R. et al. Education-dependent activation of glycolysis promotes the cytolytic potency of licensed human natural killer cells. J. Allergy Clin. Immunol. 143, 346–358 (2019).
Pfeifer, C. et al. Natural killer cell education is associated with a distinct glycolytic profile. Front. Immunol. 9, 3020 (2018).
Yang, C. et al. mTORC1 and mTORC2 differentially promote natural killer cell development. eLife 7, e35619 (2018).
Nandagopal, N., Ali, A. K., Komal, A. K. & Lee, S. H. The critical role of IL-15-PI3K-mTOR pathway in natural killer cell effector functions. Front. Immunol. 5, 187 (2014).
Abel, A. M. et al. IQ domain-containing GTPase-activating protein 1 regulates cytoskeletal reorganization and facilitates NKG2D-mediated mechanistic target of rapamycin complex 1 activation and cytokine gene translation in natural killer cells. Front. Immunol. 9, 1168 (2018).
Wang, F. et al. Crosstalks between mTORC1 and mTORC2 variagate cytokine signaling to control NK maturation and effector function. Nat. Commun. 9, 4874 (2018).
Finlay, D. K. et al. PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. J. Exp. Med. 209, 2441–2453 (2012).
Sinclair, L. V. et al. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat. Immunol. 14, 500–508 (2013).
Horton, J. D., Goldstein, J. L. & Brown, M. S. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 109, 1125–1131 (2002).
Collins, P. L. et al. Gene regulatory programs conferring phenotypic identities to human NK cells. Cell 176, 348–360 (2019).
Castro, W. et al. The transcription factor Rfx7 limits metabolism of NK cells and promotes their maintenance and immunity. Nat. Immunol. 19, 809–820 (2018).
Arase, H., Mocarski, E. S., Campbell, A. E., Hill, A. B. & Lanier, L. L. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science 296, 1323–1326 (2002).
Rolf, J. et al. AMPKalpha1: a glucose sensor that controls CD8 T cell memory. Eur. J. Immunol. 43, 889–896 (2013).
Araki, K. et al. mTOR regulates memory CD8 T cell differentiation. Nature 460, 108–112 (2009).
Pearce, E. L. et al. Enhancing CD8 T cell memory by modulating fatty acid metabolism. Nature 460, 103–107 (2009).
Schlums, H. et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity 42, 443–456 (2015).
Lee, J. et al. Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity 42, 431–442 (2015).
Cichocki, F. et al. ARID5B regulates metabolic programming in human adaptive NK cells. J. Exp. Med. 215, 2379–2395 (2018). This study shows alterations in NK cell metabolism in adaptive human NK cells that involve epigenetic modifications and the epigenetic modifier ARID5B.
Liu, L. L. et al. Critical role of CD2 co-stimulation in adaptive natural killer cell responses revealed in NKG2C-deficient humans. Cell Rep. 15, 1088–1099 (2016).
Romee, R. et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci. Transl Med. 8, 357ra123 (2016).
Romee, R. et al. Cytokine activation induces human memory-like NK cells. Blood 120, 4751–4760 (2012).
Cooper, M. A. et al. Cytokine-induced memory-like natural killer cells. Proc. Natl Acad. Sci. USA 106, 1915–1919 (2009).
Ni, J., Miller, M., Stojanovic, A., Garbi, N. & Cerwenka, A. Sustained effector function of IL-12/15/18-preactivated NK cells against established tumors. J. Exp. Med. 209, 2351–2365 (2012).
Pahl, J. H. W. et al. CD16A activation of NK cells promotes NK cell proliferation and memory-like cytotoxicity against cancer cells. Cancer Immunol. Res. 6, 517–527 (2018).
Smith, A. G., Sheridan, P. A., Harp, J. B. & Beck, M. A. Diet-induced obese mice have increased mortality and altered immune responses when infected with influenza virus. J. Nutr. 137, 1236–1243 (2007).
Lynch, L. A. et al. Are natural killer cells protecting the metabolically healthy obese patient? Obesity 17, 601–605 (2009).
O’Shea, D., Cawood, T. J., O’Farrelly, C. & Lynch, L. Natural killer cells in obesity: impaired function and increased susceptibility to the effects of cigarette smoke. PLOS ONE 5, e8660 (2010).
Tobin, L. M. et al. NK cells in childhood obesity are activated, metabolically stressed, and functionally deficient. JCI Insight 2, 94939 (2017).
Netter, P., Anft, M. & Watzl, C. Termination of the activating NK cell immunological synapse is an active and regulated process. J. Immunol. 199, 2528–2535 (2017).
Abarca-Rojano, E. et al. Re-organization of mitochondria at the NK cell immune synapse. Immunol. Lett. 122, 18–25 (2009).
Carpen, O., Virtanen, I. & Saksela, E. Ultrastructure of human natural killer cells: nature of the cytolytic contacts in relation to cellular secretion. J. Immunol. 128, 2691–2697 (1982).
Cong, J. et al. Dysfunction of natural killer cells by FBP1-induced inhibition of glycolysis during lung cancer progression. Cell Metab. 28, 243–255 (2018).
Caras, I. et al. Evidence for immune defects in breast and lung cancer patients. Cancer Immunol. Immunother. 53, 1146–1152 (2004).
Hirayama, A. et al. Quantitative metabolome profiling of colon and stomach cancer microenvironment by capillary electrophoresis time-of-flight mass spectrometry. Cancer Res. 69, 4918–4925 (2009).
Urasaki, Y., Heath, L. & Xu, C. W. Coupling of glucose deprivation with impaired histone H2B monoubiquitination in tumors. PLOS ONE 7, e36775 (2012).
Ho, P. C. et al. Phosphoenolpyruvate is a metabolic checkpoint of anti-tumor T cell responses. Cell 162, 1217–1228 (2015).
Chang, C. H. et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 162, 1229–1241 (2015).
Potzl, J. et al. Reversal of tumor acidosis by systemic buffering reactivates NK cells to express IFN-gamma and induces NK cell-dependent lymphoma control without other immunotherapies. Int. J. Cancer 140, 2125–2133 (2017).
Brand, A. et al. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab. 24, 657–671 (2016).
Harmon, C. et al. Lactate-mediated acidification of tumor microenvironment induces apoptosis of liver-resident NK cells in colorectal liver metastasis. Cancer Immunol. Res. https://doi.org/10.1158/2326-6066.CIR-18-0481 (2018).
Massague, J. TGFbeta in cancer. Cell 134, 215–230 (2008).
Ivanovic, V. et al. Elevated plasma levels of transforming growth factor-beta 1 (TGF-beta 1) in patients with advanced breast cancer: association with disease progression. Eur. J. Cancer 39, 454–461 (2003).
Zaiatz-Bittencourt, V., Finlay, D. K. & Gardiner, C. M. Canonical TGF-beta signaling pathway represses human NK cell metabolism. J. Immunol. 200, 3934–3941 (2018).
Li, D., Long, W., Huang, R., Chen, Y. & Xia, M. 27-hydroxycholesterol inhibits sterol regulatory element-binding protein 1 activation and hepatic lipid accumulation in mice. Obesity 26, 713–722 (2018).
Adams, C. M. et al. Cholesterol and 25-hydroxycholesterol inhibit activation of SREBPs by different mechanisms, both involving SCAP and Insigs. J. Biol. Chem. 279, 52772–52780 (2004).
Babiker, A. et al. Elimination of cholesterol in macrophages and endothelial cells by the sterol 27-hydroxylase mechanism. Comparison with high density lipoprotein-mediated reverse cholesterol transport. J. Biol. Chem. 272, 26253–26261 (1997).
Javitt, N. B. Breast cancer and (25R)-26-hydroxycholesterol. Steroids 104, 61–64 (2015).
Wu, Q. et al. 27-Hydroxycholesterol promotes cell-autonomous, ER-positive breast cancer growth. Cell Rep. 5, 637–645 (2013).
Eibinger, G. et al. On the role of 25-hydroxycholesterol synthesis by glioblastoma cell lines. Implications for chemotactic monocyte recruitment. Exp. Cell Res. 319, 1828–1838 (2013).
Diczfalusy, U. et al. Marked upregulation of cholesterol 25-hydroxylase expression by lipopolysaccharide. J. Lipid Res. 50, 2258–2264 (2009).
Park, K. & Scott, A. L. Cholesterol 25-hydroxylase production by dendritic cells and macrophages is regulated by type I interferons. J. Leukoc. Biol. 88, 1081–1087 (2010).
Mondanelli, G., Ugel, S., Grohmann, U. & Bronte, V. The immune regulation in cancer by the amino acid metabolizing enzymes ARG and IDO. Curr. Opin. Pharmacol. 35, 30–39 (2017).
Munn, D. H. & Mellor, A. L. IDO in the tumor microenvironment: inflammation, counter-regulation, and tolerance. Trends Immunol. 37, 193–207 (2016).
Frumento, G. et al. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J. Exp. Med. 196, 459–468 (2002).
Della Chiesa, M. et al. The tryptophan catabolite L-kynurenine inhibits the surface expression of NKp46- and NKG2D-activating receptors and regulates NK-cell function. Blood 108, 4118–4125 (2006).
Sinclair, L. V., Neyens, D., Ramsay, G., Taylor, P. M. & Cantrell, D. A. Single cell analysis of kynurenine and System L amino acid transport in T cells. Nat. Commun. 9, 1981 (2018).
Maus, M. V., Grupp, S. A., Porter, D. L. & June, C. H. Antibody-modified T cells: CARs take the front seat for hematologic malignancies. Blood 123, 2625–2635 (2014).
Cheng, M., Chen, Y., Xiao, W., Sun, R. & Tian, Z. NK cell-based immunotherapy for malignant diseases. Cell. Mol. Immunol. 10, 230–252 (2013).
Still, E. R. & Yuneva, M. O. Hopefully devoted to Q: targeting glutamine addiction in cancer. Br. J. Cancer 116, 1375–1381 (2017).
Zhu, L., Ploessl, K., Zhou, R., Mankoff, D. & Kung, H. F. Metabolic imaging of glutamine in cancer. J. Nucl. Med. 58, 533–537 (2017).
Dunphy, M. P. S. et al. In vivo PET assay of tumor glutamine flux and metabolism: in-human trial of (18)F-(2S,4R)-4-fluoroglutamine. Radiology 287, 667–675 (2018).
Lieberman, B. P. et al. PET imaging of glutaminolysis in tumors by 18F-(2S,4R)4-fluoroglutamine. J. Nucl. Med. 52, 1947–1955 (2011).
Parameswaran, R. et al. Repression of GSK3 restores NK cell cytotoxicity in AML patients. Nat. Commun. 7, 11154 (2016).
Cichocki, F. et al. GSK3 inhibition drives maturation of NK cells and enhances their antitumor activity. Cancer Res. 77, 5664–5675 (2017).
Saetersmoen, M. L., Hammer, Q., Valamehr, B., Kaufman, D. S. & Malmberg, K. J. Off-the-shelf cell therapy with induced pluripotent stem cell-derived natural killer cells. Semin. Immunopathol. 41, 59–68 (2018).
Li, Y., Hermanson, D. L., Moriarity, B. S. & Kaufman, D. S. Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell 23, 181–192 (2018).
Suck, G. et al. NK-92: an ‘off-the-shelf therapeutic’ for adoptive natural killer cell-based cancer immunotherapy. Cancer Immunol. Immunother. 65, 485–492 (2016).
Buck, M. D. et al. Mitochondrial dynamics controls T cell fate through metabolic programming. Cell 166, 63–76 (2016).
Wang, R. et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 35, 871–882 (2011).
Preston, G. C. et al. Single cell tuning of Myc expression by antigen receptor signal strength and interleukin-2 in T lymphocytes. EMBO J. 34, 2008–2024 (2015).
Verbist, K. C. et al. Metabolic maintenance of cell asymmetry following division in activated T lymphocytes. Nature 532, 389–393 (2016).
Kidani, Y. et al. Sterol regulatory element-binding proteins are essential for the metabolic programming of effector T cells and adaptive immunity. Nat. Immunol. 14, 489–499 (2013).
Swamy, M. et al. Glucose and glutamine fuel protein O-GlcNAcylation to control T cell self-renewal and malignancy. Nat. Immunol. 17, 712–720 (2016).
Acknowledgements
The authors acknowledge funding from the following sources: European Commission (EU Framework Programme for Research and Innovation H2020, H2020 Priority Excellent Science and H2020 European Research Council) and Science Foundation Ireland to D.K.F. and Irish Cancer Society to K.L.O’B.
Reviewer information
Nature Reviews Immunology thanks J. Miller and other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
O’Brien, K.L., Finlay, D.K. Immunometabolism and natural killer cell responses. Nat Rev Immunol 19, 282–290 (2019). https://doi.org/10.1038/s41577-019-0139-2
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-019-0139-2
This article is cited by
-
Excess of body weight is associated with accelerated T-cell senescence in hospitalized COVID-19 patients
Immunity & Ageing (2024)
-
CIS deletion by CRISPR/Cas9 enhances human primary natural killer cell functions against allogeneic glioblastoma
Journal of Experimental & Clinical Cancer Research (2023)
-
Side effects of Pfizer/BioNTech (BNT162b2) COVID-19 vaccine reported by the Birzeit University community
BMC Infectious Diseases (2023)
-
Exploiting autophagy balance in T and NK cells as a new strategy to implement adoptive cell therapies
Molecular Cancer (2023)
-
The TNFα/TNFR2 axis mediates natural killer cell proliferation by promoting aerobic glycolysis
Cellular & Molecular Immunology (2023)