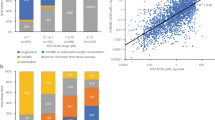
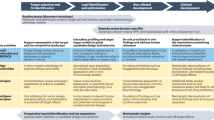
Abstract
Secondary pharmacology screening of investigational small-molecule drugs for potentially adverse off-target activities has become standard practice in pharmaceutical research and development, and regulatory agencies are increasingly requesting data on activity against targets with recognized adverse effect relationships. However, the screening strategies and target panels used by pharmaceutical companies may vary substantially. To help identify commonalities and differences, as well as to highlight opportunities for further optimization of secondary pharmacology assessment, we conducted a broad-ranging survey across 18 companies under the auspices of the DruSafe leadership group of the International Consortium for Innovation and Quality in Pharmaceutical Development. Based on our analysis of this survey and discussions and additional research within the group, we present here an overview of the current state of the art in secondary pharmacology screening. We discuss best practices, including additional safety-associated targets not covered by most current screening panels, and present approaches for interpreting and reporting off-target activities. We also provide an assessment of the safety impact of secondary pharmacology screening, and a perspective on opportunities and challenges in this rapidly developing field.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Jalencas, X. & Mestres, J. On the origins of drug polypharmacology. MedChemComm 4, 80–87 (2013).
Finan, C. et al. The druggable genome and support for target identification and validation in drug development. Sci. Transl Med. 9, eaag1166 (2017).
Dai, S. X., Li, W. X., Li, G. H. & Huang, J. F. Proteome-wide prediction of targets for aspirin: new insight into the molecular mechanism of aspirin. PeerJ 4, e1791 (2016).
Shapiro, P. A promiscuous kinase inhibitor reveals secrets to cancer cell survival. J. Biol. Chem. 294, 8674–8675 (2019).
Bowes, J. et al. Reducing safety-related drug attrition: the use of in vitro pharmacological profiling. Nat. Rev. Drug Discov. 11, 909–922 (2012).
Lynch, J. J. III, Van Vleet, T. R., Mittelstadt, S. W. & Blomme, E. A. G. Potential functional and pathological side effects related to off-target pharmacological activity. J. Pharmacol. Toxicol. Methods 87, 108–126 (2017).
Bendels, S. et al. Safety screening in early drug discovery: an optimized assay panel. J. Pharmacol. Toxicol. Methods 99, 106609 (2019).
Cook, D. et al. Lessons learned from the fate of AstraZeneca’s drug pipeline: a five-dimensional framework. Nat. Rev. Drug Discov. 13, 419–431 (2014).
Guengerich, F. P. Mechanisms of drug toxicity and relevance to pharmaceutical development. Drug Metab. Pharmacokinet. 26, 3–14 (2011).
Weaver, R. J. & Valentin, J. P. Today’s challenges to de-risk and predict drug safety in human “Mind-the-Gap. Toxicol. Sci. 167, 307–321 (2019).
Hishigaki, H. & Kuhara, S. hERGAPDbase: a database documenting hERG channel inhibitory potentials and APD-prolongation activities of chemical compounds. Database 2011, bar017 (2011).
Gintant, G., Sager, P. T. & Stockbridge, N. Evolution of strategies to improve preclinical cardiac safety testing. Nat. Rev. Drug Discov. 15, 457–471 (2016).
Cavero, I. & Guillon, J. M. Safety pharmacology assessment of drugs with biased 5-HT(2B) receptor agonism mediating cardiac valvulopathy. J. Pharmacol. Toxicol. Methods 69, 150–161 (2014).
Keller, D. A., Brennan, R. J. & Leach, K. L. in Antitargets and Drug Safety (eds Urbán, L., Patel, V. F. & Vaz, R. J.) 365–400 (Wiley, 2015).
Hasinoff, B. B. & Patel, D. The lack of target specificity of small molecule anticancer kinase inhibitors is correlated with their ability to damage myocytes in vitro. Toxicol. Appl. Pharmacol. 249, 132–139 (2010).
Hasinoff, B. B. The cardiotoxicity and myocyte damage caused by small molecule anticancer tyrosine kinase inhibitors is correlated with lack of target specificity. Toxicol. Appl. Pharmacol. 244, 190–195 (2010).
Force, T. & Kolaja, K. L. Cardiotoxicity of kinase inhibitors: the prediction and translation of preclinical models to clinical outcomes. Nat. Rev. Drug Discov. 10, 111–126 (2011).
Urbán, L., Patel, V. F. & Vaz, R. J. (eds) Antitargets and Drug Safety (Wiley, 2015).
Deaton, A. M. et al. Rationalizing secondary pharmacology screening using human genetic and pharmacological evidence. Toxicol. Sci. 167, 593–603 (2019).
Dodson, A. et al. Aggregation and analysis of secondary pharmacology data from investigational new drug submissions at the US Food and Drug Administration. J. Pharmacol. Toxicol. Methods 111, 107098 (2021).
Papoian, T. et al. Secondary pharmacology data to assess potential off-target activity of new drugs: a regulatory perspective. Nat. Rev. Drug Discov. 14, 294 (2015).
Papoian, T. et al. Regulatory forum review*: utility of in vitro secondary pharmacology data to assess risk of drug-induced valvular heart disease in humans: regulatory considerations. Toxicol. Pathol. 45, 381–388 (2017).
Safety Testing of Drug Metabolites: Guidance for Industry. (FDA, 2020).
Valentin, J. P. et al. In vitro secondary pharmacological profiling: an IQ-DruSafe industry survey on current practices. J. Pharmacol. Toxicol. Methods 93, 7–14 (2018).
Santos, R. et al. A comprehensive map of molecular drug targets. Nat. Rev. Drug Discov. 16, 19–34 (2017).
Strauss, D. G., Wu, W. W., Li, Z., Koerner, J. & Garnett, C. Translational models and tools to reduce clinical trials and improve regulatory decision making for QTc and proarrhythmia risk (ICH E14/S7B Updates). Clin. Pharmacol. Ther. 109, 319–333 (2021).
Vargas, H. M. et al. Time for a fully integrated nonclinical-clinical risk assessment to streamline QT prolongation liability determinations: a pharma industry perspective. Clin. Pharmacol. Ther. 109, 310–318 (2021).
Harvey, R. D. in Muscarinic Receptors. Handbook of Experimental Pharmacology, Vol. 208 (eds Fryer, A., Christopoulos, A. & Nathanson, N.) 299–316 (Springer, 2012).
Nguyen, T., Thomas, B. F. & Zhang, Y. Overcoming the psychiatric side effects of the cannabinoid CB1 receptor antagonists: current approaches for therapeutics development. Curr. Top. Med. Chem. 19, 1418–1435 (2019).
De Vries, T. J. & Shippenberg, T. S. Neural systems underlying opiate addiction. J. Neurosci. 22, 3321–3325 (2002).
Lucas, J. A., Miller, A. T., Atherly, L. O. & Berg, L. J. The role of Tec family kinases in T cell development and function. Immunol. Rev. 191, 119–138 (2003).
Popa-Nita, O., Marois, L., Paré, G. & Naccache, P. H. Crystal-induced neutrophil activation: X. proinflammatory role of the tyrosine kinase Tec. Arthritis Rheum. 58, 1866–1876 (2008).
Greenwell, I. B., Ip, A. & Cohen, J. B. PI3K inhibitors: understanding toxicity mechanisms and management. Oncology 31, 821–828 (2017).
James, M. O. et al. Therapeutic applications of dichloroacetate and the role of glutathione transferase zeta-1. Pharmacol. Ther. 170, 166–180 (2017).
Freeman-Cook, K. et al. Expanding control of the tumor cell cycle with a CDK2/4/6 inhibitor. Cancer Cell 39, 1404–1421.e11 (2021).
Howell, K. R., Floyd, K. & Law, A. J. PKBγ/AKT3 loss-of-function causes learning and memory deficits and deregulation of AKT/mTORC2 signaling: relevance for schizophrenia. PLoS ONE 12, e0175993 (2017).
Bavetsias, V. & Linardopoulos, S. Aurora kinase inhibitors: current status and outlook. Front. Oncol. 5, 278 (2015).
Brianso, F., Carrascosa, M. C., Oprea, T. I. & Mestres, J. Cross-pharmacology analysis of G protein-coupled receptors. Curr. Top. Med. Chem. 11, 1956–1963 (2011).
Olney, J. W. et al. NMDA antagonist neurotoxicity: mechanism and prevention. Science 254, 1515–1518 (1991).
Kenna, J. G. et al. Can bile salt export pump inhibition testing in drug discovery and development reduce liver injury risk? An international transporter consortium perspective. Clin. Pharmacol. Ther. 104, 916–932 (2018).
Fabian, M. A. et al. A small molecule–kinase interaction map for clinical kinase inhibitors. Nat. Biotechnol. 23, 329–336 (2005).
Hanson, S. M. et al. What makes a kinase promiscuous for inhibitors? Cell Chem. Biol. 26, 390–399 (2019).
Dy, G. K. & Adjei, A. A. Understanding, recognizing, and managing toxicities of targeted anticancer therapies. CA Cancer J. Clin. 63, 249–279 (2013).
Hartmann, J. T., Haap, M., Kopp, H. G. & Lipp, H. P. Tyrosine kinase inhibitors - a review on pharmacology, metabolism and side effects. Curr. Drug Metab. 10, 470–481 (2009).
Grossman, M. & Adler, E. in Protein Kinases — Promising Targets for Anticancer Drug Research (ed. Singh R. K.) Ch. 2 (IntechOpen, 2021).
Shah, D. R., Shah, R. R. & Morganroth, J. Tyrosine kinase inhibitors: their on-target toxicities as potential indicators of efficacy. Drug Saf. 36, 413–426 (2013).
Chen, J. et al. Expression and function of the epidermal growth factor receptor in physiology and disease. Physiol. Rev. 96, 1025–1069 (2016).
Gurule, N. J. & Heasley, L. E. Linking tyrosine kinase inhibitor-mediated inflammation with normal epithelial cell homeostasis and tumor therapeutic responses. Cancer Drug Resist. 1, 118–125 (2018).
Solassol, I., Pinguet, F. & Quantin, X. FDA- and EMA-approved tyrosine kinase inhibitors in advanced EGFR-mutated non-small cell lung cancer: safety, tolerability, plasma concentration monitoring, and management. Biomolecules 9, 668 (2019).
Galanis, A. & Levis, M. Inhibition of c-Kit by tyrosine kinase inhibitors. Haematologica 100, e77–e79 (2015).
Livingstone, E., Zimmer, L., Vaubel, J. & Schadendorf, D. BRAF, MEK and KIT inhibitors for melanoma: adverse events and their management. Chin. Clin. Oncol. 3, 29 (2014).
Schmidinger, M. Understanding and managing toxicities of vascular endothelial growth factor (VEGF) inhibitors. EJC Suppl. 11, 172–191 (2013).
Jonker, D. J. et al. A phase I study to determine the safety, pharmacokinetics and pharmacodynamics of a dual VEGFR and FGFR inhibitor, brivanib, in patients with advanced or metastatic solid tumors. Ann. Oncol. 22, 1413–1419 (2011).
Park, S. et al. Biomarker-driven phase 2 umbrella trial study for patients with recurrent small cell lung cancer failing platinum-based chemotherapy. Cancer 126, 4002–4012 (2020).
Tirronen, A. et al. The ablation of VEGFR-1 signaling promotes pressure overload-induced cardiac dysfunction and sudden death. Biomolecules 11, 452 (2021).
Lamore, S. D. et al. Deconvoluting kinase inhibitor induced cardiotoxicity. Toxicol. Sci. 158, 213–226 (2017).
Jagasia, M. et al. ROCK2 inhibition with belumosudil (KD025) for the treatment of chronic graft-versus-host disease. J. Clin. Oncol. 39, 1888–1898 (2021).
Hartmann, S., Ridley, A. J. & Lutz, S. The function of Rho-associated kinases ROCK1 and ROCK2 in the pathogenesis of cardiovascular disease. Front. Pharmacol. 6, 276 (2015).
Zuhl, A. M. et al. Chemoproteomic profiling reveals that cathepsin D off-target activity drives ocular toxicity of β-secretase inhibitors. Nat. Commun. 7, 13042 (2016).
Martin, K. et al. Pharmacological inhibition of MALT1 protease leads to a progressive IPEX-like pathology. Front. Immunol. 11, 745 (2020).
Artero, A., Tarín, J. J. & Cano, A. The adverse effects of estrogen and selective estrogen receptor modulators on hemostasis and thrombosis. Semin. Thromb. Hemost. 38, 797–807 (2012).
Delgado, B. J. & Lopez-Ojeda, W. Estrogen (StatPearls, 2024).
Jia, M., Dahlman-Wright, K. & Gustafsson, J. A. Estrogen receptor alpha and beta in health and disease. Best Pract. Res. Clin. Endocrinol. Metab. 29, 557–568 (2015).
DIRECTIVE 2010/63/EU OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 22 September 2010 on the protection of animals used for scientific purposes. OJEU https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:276:0033:0079:en:PDF (2010).
FDA Modernization Act of 2021. https://www.congress.gov/bill/117th-congress/house-bill/2565?s=3&r=1 (2021).
Jenkinson, S., Schmidt, F., Rosenbrier Ribeiro, L., Delaunois, A. & Valentin, J. P. A practical guide to secondary pharmacology in drug discovery. J. Pharmacol. Toxicol. Methods 105, 106869 (2020).
Armstrong, D. et al. in Pharmaceutical Sciences Encyclopedia (eds Gad S. C. et al.) 1–29 (Wiley, 2010).
Redfern, W. et al. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc. Res. 58, 32–45 (2003).
Rosenbrier Ribeiro, L. & Ian Storer, R. A semi-quantitative translational pharmacology analysis to understand the relationship between in vitro ENT1 inhibition and the clinical incidence of dyspnoea and bronchospasm. Toxicol. Appl. Pharmacol. 317, 41–50 (2017).
Redfern, W. S. et al. Safety pharmacology–a progressive approach. Fundam. Clin. Pharmacol. 16, 161–173 (2002).
European Medicines Agency. ICH Topic S 7 A: Safety pharmacology studies for human pharmaceuticals. European Medicines Agency ema.europa.eu/en/documents/scientific-guideline/ich-s-7-safety-pharmacology-studies-human-pharmaceuticals-step-5_en.pdf (2001).
ICH Expert Working Group. ICH harmonised tripartite guideline: The non-clinical evaluation of the potential for delayed ventricular repolarization (QT interval prolongation) by human pharmaceuticals. ICH S7B guideline https://database.ich.org/sites/default/files/S7B_Guideline.pdf (2005).
Committee for Medicinal Products for Human Use. ICH guideline M4 (R4) on common technical document (CTD) for the registration of pharmaceuticals for human use - organisation of CTD. European Medicines Agency https://www.ema.europa.eu/documents/scientific-guideline/ich-guideline-m4-r4-common-technical-document-ctd-registration-pharmaceuticals-human-use_en.pdf (2021).
International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. ICH E14/S7B Implementation Working Group: Clinical and nonclinical evaluation of QT/QTc interval prolongation and proarrhythmic potential — Questions and Answers. ICH https://database.ich.org/sites/default/files/E14-S7B_QAs_Step4_2022_0221.pdf (2022).
U.S. Department of Health and Human Services, Food and Drug Administration & Center for Drug Evaluation and Research. Assessment of Abuse Potential of Drugs: Guidance for Industry. FDA https://www.fda.gov/media/116739/download (2017).
Harding, S. D. et al. The IUPHAR/BPS guide to PHARMACOLOGY in 2022: curating pharmacology for COVID-19, malaria and antibacterials. Nucleic Acids Res. 50, D1282–D1294 (2022).
Harmer, A. R., Valentin, J. P. & Pollard, C. E. On the relationship between block of the cardiac Na+ channel and drug-induced prolongation of the QRS complex. Br. J. Pharmacol. 164, 260–273 (2011).
Mellor, H. R., Bell, A. R., Valentin, J. P. & Roberts, R. R. Cardiotoxicity associated with targeting kinase pathways in cancer. Toxicol. Sci. 120, 14–32 (2011).
Sameshima, T. et al. Small-scale panel comprising diverse gene family targets to evaluate compound promiscuity. Chem. Res. Toxicol. 33, 154–161 (2020).
Simon, I. A. et al. Ligand selectivity hotspots in serotonin GPCRs. Trends Pharmacol. Sci. 44, 978–990 (2023).
Center for Drug Evaluation and Research. Guidance for Industry: Suicidal Ideation and Behavior: prospective assessment of occurrence in clinical trials. FDA fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-suicidal-ideation-and-behavior-prospective-assessment-occurrence-clinical-trials (2012).
Urban, L. et al. Translation of off-target effects: prediction of ADRs by integrated experimental and computational approach. Toxicol. Res. 3, 433–444 (2014).
Center for Drug Evaluation and Research. Assessment of Pressor Effects of Drugs Guidance for Industry. FDA fda.gov/regulatory-information/search-fda-guidance-documents/assessment-pressor-effects-drugs-guidance-industry (2022).
International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. Testing for Carcinogenicity of Pharmaceuticals S1B(R1). ICH Database database.ich.org/sites/default/files/ICHS1B%28R1%29_Step4_Presentation_2022_0809.pdf (2022).
Carss, K. J. et al. Using human genetics to improve safety assessment of therapeutics. Nat. Rev. Drug Discov. 22, 145–162 (2023).
Whitebread, S. et al. Secondary pharmacology: screening and interpretation of off-target activities – focus on translation. Drug Discov. Today 21, 1232–1242 (2016).
Paolini, G. V., Shapland, R. H., van Hoorn, W. P., Mason, J. S. & Hopkins, A. L. Global mapping of pharmacological space. Nat. Biotechnol. 24, 805–815 (2006).
Hresko, R. C. & Hruz, P. W. HIV protease inhibitors act as competitive inhibitors of the cytoplasmic glucose binding site of GLUTs with differing affinities for GLUT1 and GLUT4. PLoS ONE 6, e25237 (2011).
Conn, P. J., Christopoulos, A. & Lindsley, C. W. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat. Rev. Drug Discov. 8, 41–54 (2009).
Fischer, G., Rossmann, M. & Hyvönen, M. Alternative modulation of protein-protein interactions by small molecules. Curr. Opin Biotechnol. 35, 78–85 (2015).
Jones, L. H. et al. Targeted protein degraders: a call for collective action to advance safety assessment. Nat. Rev. Drug Discov. 21, 401–402 (2022).
Valeur, E. et al. New modalities for challenging targets in drug discovery. Angew. Chem. Int. Ed. 56, 10294–10323 (2017).
Prachayasittikul, V. et al. Exploring the epigenetic drug discovery landscape. Expert Opin Drug Discov. 12, 345–362 (2017).
Blanco, M. J. & Gardinier, K. M. New chemical modalities and strategic thinking in early drug discovery. ACS Med. Chem. Lett. 11, 228–231 (2020).
Sutton, C. W. The role of targeted chemical proteomics in pharmacology. Br. J. Pharmacol. 166, 457–475 (2012).
van Esbroeck, A. C. M. et al. Activity-based protein profiling reveals off-target proteins of the FAAH inhibitor BIA 10-2474. Science 356, 1084–1087 (2017).
Freeth, J. & Soden, J. New advances in cell microarray technology to expand applications in target deconvolution and off-target screening. SLAS Discov. 25, 223–230 (2020).
Hasselgren, C. et al. in Chemoinformatics for Drug Discovery (ed. Bajorath, J.) 267–290 (Wiley, 2013).
Raies, A. B. & Bajic, V. B. In silico toxicology: computational methods for the prediction of chemical toxicity. Wiley Interdiscip. Rev. Comput. Mol. Sci. 6, 147–172 (2016).
Ietswaart, R. et al. Machine learning guided association of adverse drug reactions with in vitro target-based pharmacology. EBioMedicine 57, 102837 (2020).
Vamathevan, J. et al. Applications of machine learning in drug discovery and development. Nat. Rev. Drug Discov. 18, 463–477 (2019).
Scott, C., Dodson, A., Saulnier, M., Snyder, K. & Racz, R. Analysis of secondary pharmacology assays received by the US Food and Drug Administration. J. Pharmacol. Toxicol. Methods 117, 107205 (2022).
Valentin, J. P. & Leishman, D. 2000–2023 over two decades of ICH S7A: has the time come for a revamp? Regul. Toxicol. Pharmacol. 139, 105368 (2023).
Valentin, J. P., Sibony, A., Rosseels, M. L. & Delaunois, A. “Appraisal of state-of-the-art” the 2021 distinguished service award of the safety pharmacology society: reflecting on the past to tackle challenges ahead. J. Pharmacol. Toxicol. Methods 123, 107269 (2023).
Pottel, J. et al. The activities of drug inactive ingredients on biological targets. Science 369, 403–413 (2020).
Sipes, N. S. et al. Profiling 976 ToxCast chemicals across 331 enzymatic and receptor signaling assays. Chem. Res. Toxicol. 26, 878–895 (2013).
Bolden, J. E. et al. Inducible in vivo silencing of Brd4 identifies potential toxicities of sustained BET protein inhibition. Cell Rep. 8, 1919–1929 (2014).
Wagoner, M. et al. Bromodomain and extraterminal (BET) domain inhibitors induce a loss of intestinal stem cells and villous atrophy. Toxicol. Lett. 229, S75–S76 (2014).
Abbruzzese, G. et al. A European observational study to evaluate the safety and the effectiveness of safinamide in routine clinical practice: the SYNAPSES trial. J. Parkinsons Dis. 11, 187–198 (2021).
Blackwell, B. & Mabbitt, L. A. Tyramine in cheese related to hypertensive crises after monoamine-oxidase inhibition. Lancet 1, 938–940 (1965).
Finberg, J. P. & Rabey, J. M. Inhibitors of MAO-A and MAO-B in psychiatry and neurology. Front. Pharmacol. 7, 340 (2016).
Gross, M. E. et al. Phase 2 trial of monoamine oxidase inhibitor phenelzine in biochemical recurrent prostate cancer. Prostate Cancer Prostatic Dis. 24, 61–68 (2021).
Woolley, M. L., Marsden, C. A. & Fone, K. C. 5-HT6 receptors. Curr. Drug. Targets CNS Neurol. Disord. 3, 59–79 (2004).
Boyce, M. et al. Effect of netazepide, a gastrin/CCK2 receptor antagonist, on gastric acid secretion and rabeprazole-induced hypergastrinaemia in healthy subjects. Br. J. Clin. Pharmacol. 79, 744–755 (2015).
Boyce, M., Warrington, S. & Black, J. Netazepide, a gastrin/CCK2 receptor antagonist, causes dose-dependent, persistent inhibition of the responses to pentagastrin in healthy subjects. Br. J. Clin. Pharmacol. 76, 689–698 (2013).
Dufresne, M., Seva, C. & Fourmy, D. Cholecystokinin and gastrin receptors. Physiol. Rev. 86, 805–847 (2006).
Horinouchi, Y. et al. Reduced anxious behavior in mice lacking the CCK2 receptor gene. Eur. Neuropsychopharmacol. 14, 157–161 (2004).
Moore, A. R. et al. Netazepide, a gastrin receptor antagonist, normalises tumour biomarkers and causes regression of type 1 gastric neuroendocrine tumours in a nonrandomised trial of patients with chronic atrophic gastritis. PLoS ONE 8, e76462 (2013).
Wang, H., Wong, P. T., Spiess, J. & Zhu, Y. Z. Cholecystokinin-2 (CCK2) receptor-mediated anxiety-like behaviors in rats. Neurosci. Biobehav. Rev. 29, 1361–1373 (2005).
Zanoveli, J. M., Netto, C. F., Guimarães, F. S. & Zangrossi, H. Jr Systemic and intra-dorsal periaqueductal gray injections of cholecystokinin sulfated octapeptide (CCK-8s) induce a panic-like response in rats submitted to the elevated T-maze. Peptides 25, 1935–1941 (2004).
Falkai, P. et al. The efficacy and safety of cariprazine in the early and late stage of schizophrenia: a post hoc analysis of three randomized, placebo-controlled trials. CNS Spectr. 28, 104–111 (2021).
Guma, E. et al. Role of D3 dopamine receptors in modulating neuroanatomical changes in response to antipsychotic administration. Sci. Rep. 9, 7850 (2019).
Guo, K. et al. Safety profile of antipsychotic drugs: analysis based on a provincial spontaneous reporting systems database. Front. Pharmacol. 13, 848472 (2022).
Heidbreder, C. A. et al. The role of central dopamine D3 receptors in drug addiction: a review of pharmacological evidence. Brain Res. Rev. 49, 77–105 (2005).
Periclou, A. et al. Relationship between plasma concentrations and clinical effects of cariprazine in patients with schizophrenia or bipolar mania. Clin. Transl Sci. 13, 362–371 (2020).
Hodge, R. J. & Nunez, D. J. Therapeutic potential of Takeda-G-protein-receptor-5 (TGR5) agonists. Hope or hype? Diabetes Obes. Metab. 18, 439–443 (2016).
McNeil, B. D. et al. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 519, 237–241 (2015).
Grimes, J. et al. MrgX2 is a promiscuous receptor for basic peptides causing mast cell pseudo-allergic and anaphylactoid reactions. Pharmacol. Res. Perspect. 7, e00547 (2019).
Barrett, J. et al. Tachykinin receptors (version 2019.4). in IUPHAR/BPS Guide to Pharmacology CITE. https://doi.org/10.2218/gtopdb/F62/2019.4 (2019).
Smits, G. J. & Lefebvre, R. A. Tachykinin receptors involved in the contractile effect of the natural tachykinins in the rat gastric fundus. J. Auton. Pharmacol. 14, 383–392 (1994).
Valero, M. S. et al. Contractile effect of tachykinins on rabbit small intestine. Acta Pharmacol. Sin. 32, 487–494 (2011).
Vilain, P., Emonds-Alt, X., Le Fur, G. & Brelière, J. C. Tachykinin-induced contractions of the guinea pig ileum longitudinal smooth muscle: tonic and phasic muscular activities. Can. J. Physiol. Pharmacol. 75, 587–590 (1997).
Amenyogbe, E. et al. A review on sex steroid hormone estrogen receptors in mammals and fish. Int. J. Endocrinol. 2020, 5386193 (2020).
Scarpin, K. M., Graham, J. D., Mote, P. A. & Clarke, C. L. Progesterone action in human tissues: regulation by progesterone receptor (PR) isoform expression, nuclear positioning and coregulator expression. Nucl. Recept. Signal. 7, e009 (2009).
Spitz, I. M. Progesterone receptor antagonists. Curr. Opin Investig. Drugs 7, 882–890 (2006).
Chen, J. Y. et al. Two distinct actions of retinoid-receptor ligands. Nature 382, 819–822 (1996).
Chung, S. S. et al. Pharmacological activity of retinoic acid receptor alpha-selective antagonists in vitro and in vivo. ACS Med. Chem. Lett. 4, 446–450 (2013).
Scimemi, A. Structure, function, and plasticity of GABA transporters. Front. Cell Neurosci. 8, 161 (2014).
Zafar, S. & Jabeen, I. Structure, function, and modulation of γ-aminobutyric acid transporter 1 (GAT1) in neurological disorders: a pharmacoinformatic prospective. Front. Chem. 6, 397 (2018).
Frosina, G., Marubbi, D., Marcello, D., Vecchio, D. & Daga, A. The efficacy and toxicity of ATM inhibition in glioblastoma initiating cells-driven tumor models. Crit. Rev. Oncol. Hematol. 138, 214–222 (2019).
Majd, N. K. et al. The promise of DNA damage response inhibitors for the treatment of glioblastoma. Neuro-Oncol. Adv. 3, vdab015 (2021).
Pizzamiglio, L. et al. New role of ATM in controlling GABAergic tone during development. Cereb. Cortex 26, 3879–3888 (2016).
Tassinari, V. et al. Atrophy, oxidative switching and ultrastructural defects in skeletal muscle of the ataxia telangiectasia mouse model. J. Cell Sci. 132, jcs223008 (2019).
Bhushan, B. et al. Dual role of epidermal growth factor receptor in liver injury and regeneration after acetaminophen overdose in mice. Toxicol. Sci. 155, 363–378 (2017).
Kirchner, S. in Polypharmacology in Drug Discovery (ed. Peters, J.-U.) Ch. 4 (Wiley, 2012).
Horta, E., Bongiorno, C., Ezzeddine, M. & Neil, E. C. Neurotoxicity of antibodies in cancer therapy: a review. Clin. Neurol. Neurosurg. 188, 105566 (2020).
Huang, J. et al. Safety profile of epidermal growth factor receptor tyrosine kinase inhibitors: a disproportionality analysis of FDA adverse event reporting system. Sci. Rep. 10, 4803 (2020).
Miroddi, M. et al. Systematic review and meta-analysis of the risk of severe and life-threatening thromboembolism in cancer patients receiving anti-EGFR monoclonal antibodies (cetuximab or panitumumab). Int. J. Cancer 139, 2370–2380 (2016).
Ohmori, T. et al. Molecular and clinical features of EGFR-TKI-associated lung injury. Int. J. Mol. Sci. 22, 792 (2021).
Rizzo, A. et al. Anti-EGFR monoclonal antibodies in advanced biliary tract cancer: a systematic review and meta-analysis. In Vivo 34, 479–488 (2020).
Shah, R. R. & Shah, D. R. Safety and tolerability of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors in oncology. Drug Saf. 42, 181–198 (2019).
Tischer, B., Huber, R., Kraemer, M. & Lacouture, M. E. Dermatologic events from EGFR inhibitors: the issue of the missing patient voice. Support. Care Cancer 25, 651–660 (2017).
Baruch, A. et al. Antibody-mediated activation of the FGFR1/Klothoβ complex corrects metabolic dysfunction and alters food preference in obese humans. Proc. Natl Acad. Sci. USA 117, 28992–29000 (2020).
Chae, Y. K. et al. Inhibition of the fibroblast growth factor receptor (FGFR) pathway: the current landscape and barriers to clinical application. Oncotarget 8, 16052–16074 (2017).
Gile, J. J. et al. FGFR inhibitor toxicity and efficacy in cholangiocarcinoma: multicenter single-institution cohort experience. JCO Precis. Oncol. https://doi.org/10.1200/PO.21.00064 (2021).
Kommalapati, A., Tella, S. H., Borad, M., Javle, M. & Mahipal, A. FGFR inhibitors in oncology: insight on the management of toxicities in clinical practice. Cancers 13, 2968 (2021).
Mahipal, A., Tella, S. H., Kommalapati, A., Yu, J. & Kim, R. Prevention and treatment of FGFR inhibitor-associated toxicities. Crit. Rev. Oncol. Hematol. 155, 103091 (2020).
Sonoda, J., Chen, M. Z. & Baruch, A. FGF21-receptor agonists: an emerging therapeutic class for obesity-related diseases. Horm. Mol. Biol. Clin. Investig. 30, 20170002 (2017).
Tassi, E. et al. Blood pressure control by a secreted FGFBP1 (fibroblast growth factor-binding protein). Hypertension 71, 160–167 (2018).
Wu, A.-L. et al. Antibody-mediated activation of FGFR1 induces FGF23 production and hypophosphatemia. PLoS ONE 8, e57322 (2013).
Xie, Y. et al. FGF/FGFR signaling in health and disease. Signal Transduct. Target. Ther. 5, 181 (2020).
Gómez-Sintes, R. et al. Neuronal apoptosis and reversible motor deficit in dominant-negative GSK-3 conditional transgenic mice. EMBO J. 26, 2743–2754 (2007).
Hurcombe, J. A. et al. Podocyte GSK3 is an evolutionarily conserved critical regulator of kidney function. Nat. Commun. 10, 403 (2019).
Boucher, J. et al. Differential roles of insulin and IGF-1 receptors in adipose tissue development and function. Diabetes 65, 2201–2213 (2016).
Cai, W. et al. Insulin regulates astrocyte gliotransmission and modulates behavior. J. Clin. Investig. 128, 2914–2926 (2018).
Srivastava, S. P. & Goodwin, J. E. Cancer biology and prevention in diabetes. Cells 9, 1380 (2020).
Bharate, J. B. et al. Rational design, synthesis and biological evaluation of pyrimidine-4,6-diamine derivatives as type-II inhibitors of FLT3 selective against c-KIT. Sci. Rep. 8, 3722 (2018).
Omdal, R., Skoie, I. M. & Grimstad, T. Fatigue is common and severe in patients with mastocytosis. Int. J. Immunopathol. Pharmacol. 32, 2058738418803252 (2018).
Openshaw, R. L. et al. Map2k7 haploinsufficiency induces brain imaging endophenotypes and behavioral phenotypes relevant to schizophrenia. Schizophr. Bull. 46, 211–223 (2020).
Cocco, E., Scaltriti, M. & Drilon, A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat. Rev. Clin. Oncol. 15, 731–747 (2018).
Drilon, A. TRK inhibitors in TRK fusion-positive cancers. Ann. Oncol. 30, viii23–viii30 (2019).
Gambella, A. et al. NTRK fusions in central nervous system tumors: a rare, but worthy target. Int. J. Mol. Sci. 21, 753 (2020).
Han, S.-Y. TRK inhibitors: tissue-agnostic anti-cancer drugs. Pharmaceuticals 14, 632 (2021).
Rohrberg, K. S. & Lassen, U. Detecting and targeting NTRK fusions in cancer in the era of tumor agnostic oncology. Drugs 81, 445–452 (2021).
Sanchez-Ortiz, E. et al. TrkA gene ablation in basal forebrain results in dysfunction of the cholinergic circuitry. J. Neurosci. 32, 4065–4079 (2012).
Chong, C. R., Ong, G. J. & Horowitz, J. D. Emerging drugs for the treatment of angina pectoris. Expert Opin Emerg. Drugs 21, 365–376 (2016).
Heinemann-Yerushalmi, L. et al. BCKDK regulates the TCA cycle through PDC in the absence of PDK family during embryonic development. Dev. Cell 56, 1182–1194 (2021).
Stakišaitis, D. et al. The importance of gender-related anticancer research on mitochondrial regulator sodium dichloroacetate in preclinical studies in vivo. Cancers 11, 1210 (2019).
Wang, H. et al. Deletion of PDK1 in oligodendrocyte lineage cells causes white matter abnormality and myelination defect in the central nervous system. Neurobiol. Dis. 148, 105212 (2021).
Drullinsky, P. R. & Hurvitz, S. A. Mechanistic basis for PI3K inhibitor antitumor activity and adverse reactions in advanced breast cancer. Breast Cancer Res. Treat. 181, 233–248 (2020).
Gustafson, D., Fish, J. E., Lipton, J. H. & Aghel, N. Mechanisms of cardiovascular toxicity of BCR-ABL1 tyrosine kinase inhibitors in chronic myelogenous leukemia. Curr. Hematol. Malig. Rep. 15, 20–30 (2020).
Nunnery, S. E. & Mayer, I. A. Management of toxicity to isoform α-specific PI3K inhibitors. Ann. Oncol. 30, x21–x26 (2019).
Yap, T. A., Bjerke, L., Clarke, P. A. & Workman, P. Drugging PI3K in cancer: refining targets and therapeutic strategies. Curr. Opin Pharmacol. 23, 98–107 (2015).
Chen, Y. et al. Focal adhesion kinase promotes hepatic stellate cell activation by regulating plasma membrane localization of TGFβ receptor 2. Hepatol. Commun. 4, 268–283 (2020).
Dawson, J. C., Serrels, A., Stupack, D. G., Schlaepfer, D. D. & Frame, M. C. Targeting FAK in anticancer combination therapies. Nat. Rev. Cancer 21, 313–324 (2021).
Guidetti, G. F., Torti, M. & Canobbio, I. Focal adhesion kinases in platelet function and thrombosis. Arterioscler. Thromb. Vasc. Biol. 39, 857–868 (2019).
Lassiter, D. G. et al. FAK tyrosine phosphorylation is regulated by AMPK and controls metabolism in human skeletal muscle. Diabetologia 61, 424–432 (2018).
Peng, X. et al. Cardiac developmental defects and eccentric right ventricular hypertrophy in cardiomyocyte focal adhesion kinase (FAK) conditional knockout mice. Proc. Natl Acad. Sci. USA 105, 6638–6643 (2008).
Sorkin, M. et al. Novel strategies to attenuate skin fibrosis: targeted inhibition of focal adhesion kinase in dermal fibroblasts. J. Am. Coll. Surg. 211, S127 (2010).
Weng, Y. et al. Liver epithelial focal adhesion kinase modulates fibrogenesis and hedgehog signaling. JCI Insight 5, e141217 (2020).
Zhang, J. & Hochwald, S. N. The role of FAK in tumor metabolism and therapy. Pharmacol. Ther. 142, 154–163 (2014).
Zhao, X.-K. et al. Focal adhesion kinase regulates hepatic stellate cell activation and liver fibrosis. Sci. Rep. 7, 4032 (2017).
Greathouse, K. M., Henderson, B. W., Gentry, E. G. & Herskowitz, J. H. Fasudil or genetic depletion of ROCK1 or ROCK2 induces anxiety-like behaviors. Behav. Brain Res. 373, 112083 (2019).
Kusuhara, S. & Nakamura, M. Ripasudil hydrochloride hydrate in the treatment of glaucoma: safety, efficacy, and patient selection. Clin. Ophthalmol. 14, 1229–1236 (2020).
Li, J. et al. Renal protective effects of empagliflozin via inhibition of EMT and aberrant glycolysis in proximal tubules. JCI Insight 5, e129034 (2020).
McLeod, R. et al. First-in-human study of AT13148, a dual ROCK-AKT inhibitor in patients with solid tumors. Clin. Cancer Res. 26, 4777–4784 (2020).
Sunamura, S. et al. Different roles of myocardial ROCK1 and ROCK2 in cardiac dysfunction and postcapillary pulmonary hypertension in mice. Proc. Natl Acad. Sci. USA 115, E7129–E7138 (2018).
Pappu, R. Essential tole for the RHO-KINASES in intestinal stem cell viability and maintenance of organ homeostasis [abstract T.126]. Federation of Clinical Immunology Societies Meeting 2019 (2019).
Zheng, K. et al. miR-135a-5p mediates memory and synaptic impairments via the Rock2/Adducin1 signaling pathway in a mouse model of Alzheimer’s disease. Nat. Commun. 12, 1903 (2021).
De Kock, L. et al. De novo variant in tyrosine kinase SRC causes thrombocytopenia: case report of a second family. Platelets 30, 931–934 (2019).
Li, J. et al. Heat-induced epithelial barrier dysfunction occurs via C-Src kinase and P120ctn expression regulation in the lungs. Cell. Physiol. Biochem. 48, 237–250 (2018).
Revilla, N. et al. Clinical and biological assessment of the largest family with SRC‐RT due to p. E527K gain‐of‐function variant [abstract]. Res. Pract. Thromb. Haemost. 5 (suppl. 2), 145–146 (2021).
Yo, S., Thenganatt, J., Lipton, J. & Granton, J. Incident pulmonary arterial hypertension associated with Bosutinib. Pulm. Circ. 10, 1–4 (2020).
Yurttas, N. O. & Eskazan, A. E. Tyrosine kinase inhibitor-associated platelet dysfunction: does this need to have a significant clinical impact? Clin. Appl. Thromb. Hemost. 25, https://doi.org/10.1177/1076029619866925 (2019).
Garcia-Serna, R., Vidal, D., Remez, N. & Mestres, J. Large-scale predictive drug safety: from structural alerts to biological mechanisms. Chem. Res. Toxicol. 28, 1875–1887 (2015).
Compilation of CDER NME and new biologic approvals 1985–2022. FDA fda.gov/media/135307/download (2022).
Acknowledgements
The authors thank the following individuals for their valuable contributions: K. A. Henderson from Amgen, C. J. B. Larner from AstraZeneca, A. Fekete from Novartis, E. Pawluk from UCB Biopharma and the entire membership of the IQ-DruSafe In vitro Secondary Pharmacology Working Group. J.-P.V., R.J.B. and S.J. are the Chair and Co-Chairs of the IQ-DruSafe In vitro Secondary Pharmacology Working Group, respectively.
Author information
Authors and Affiliations
Contributions
R.J.B., S.J., A.B., A.D., B.D., M.P., M.R., L.R.R., F.S., A.S., Y.T., V.T.S., D.A., A.L., S.W.M., R.N., R.P., S.R., J.M.V. and J.-P.V. researched data for the article. R.J.B., S.J., A.B., A.D., B.D., M.P., M.R., L.R.R., F.S., A.S., Y.T., V.T.S., A.L., S.W.M., R.P., S.R., J.M.V. and J.-P.V. reviewed and/or edited the manuscript before submission. R.J.B., S.J., A.B., A.D., B.D., M.P., M.R., L.R.R., F.S., A.S., Y.T., V.T.S., D.A., A.L., S.W.M., R.P., S.R. and J.-P.V. made a substantial contribution to discussion of content. R.J.B., S.J., A.B., A.D., B.D, M.P., L.R.R., F.S., A.S., Y.T., V.T.S., S.W.M., S.R. and J.-P.V. wrote the article.
Corresponding author
Ethics declarations
Competing interests
A.B., A.D., B.D., M.P., M.R., L.R.R., F.S., A.S., Y.T., V.T.S., A.L., S.W.M., R.N., R.P., S.R. and J.-P.V. are employees of pharmaceutical companies. R.J.B. is a Director/Trustee and Scientific Advisory Board member of Lhasa Ltd., which manages the Effiris consortium. S.J. is an employee of a CRO providing screening services to pharmaceutical companies. D.A., R.J.B., S.J., A.B., A.D., M.P., M.R., L.R.R., F.S., A.S., Y.T., V.T.S., A.L., S.W.M., R.N., R.P., S.R. and J.-P.V. hold shares, share rights and/or stock options in pharmaceutical companies. J.M.V. declares no competing interests.
Peer review
Peer review information
Nature Reviews Drug Discovery thanks Wolfgang Jarolimek and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
CiPA Initiative: https://cipaproject.org/
CredibleMeds QTDrugs Lists: https://www.crediblemeds.org/druglist
Effiris Consortium: https://www.lhasalimited.org/effiris-a-secondary-pharmacology-model-suite-powered-by-privacy-preserving-data-sharing/
Elsevier PharmaPendium: https://www.pharmapendium.com
International Consortium for Innovation and Quality in Pharmaceutical Development (IQ): https://iqconsortium.org
International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH): https://www.ich.org
NC3Rs: https://www.nc3rs.org.uk/who-we-are/3rs
Pistoia Alliance: https://www.pistoiaalliance.org/
The International Union of Basic and Clinical Pharmacology (IUPHAR): https://www.guidetopharmacology.org/
Who are the top 10 pharmaceutical companies in the world? (2023): https://www.proclinical.com/blogs/2023-7/the-top-10-pharmaceutical-companies-in-the-world-2023
Supplementary information
Glossary
- Adverse drug reactions
-
Harmful, unintended results caused by the act of taking a medication. An adverse drug reaction is a special type of adverse event in which a causative relationship can be demonstrated.
- Adverse event
-
(AE). Any untoward medical occurrence in a patient or clinical investigation subject administered a pharmaceutical product, which does not necessarily have a causal relationship with this treatment.
- Assay hit rate
-
The number of compounds showing activity in a specific assay as a proportion of the total number of compounds evaluated in the assay.
- Compound promiscuity rate
-
Promiscuity is defined as the specific interaction of a small molecule with multiple biological targets (as opposed to nonspecific binding events) and represents the molecular basis of polypharmacology. The promiscuity rate of a compound refers to the number of off-target hits as a proportion of the total number of targets assayed.
- DruSafe
-
A leadership group of the International Consortium for Innovation and Quality in Pharmaceutical Development with the mission to advance nonclinical safety sciences and impact the global regulatory environment.
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use
-
(ICH). An initiative that brings together regulatory authorities and the pharmaceutical industry to discuss scientific and technical aspects of pharmaceutical product development and registration. The mission of the ICH is to promote public health by achieving greater harmonization through the development of technical guidelines and requirements for pharmaceutical product registration.
- New drug application
-
A document through which drug sponsors formally propose that the US Food and Drug Administration (FDA) approve a new pharmaceutical for sale and marketing in the USA.
- New molecular entities
-
New drugs containing active ingredients not previously approved by the FDA or marketed as drugs in the USA.
- Off-target activity
-
Pharmacological activity of a drug against a target other than its intended therapeutic target.
- Primary pharmacology studies
-
These studies aim to investigate the mode of action and/or effects of a substance in relation to its desired therapeutic target.
- Safety pharmacology studies
-
These studies aim to investigate the potential undesirable pharmacodynamic effects of a substance on physiological functions in relation to exposure in the therapeutic range and above. Safety pharmacodynamic effects may result from activity at the primary molecular target, secondary targets or nonspecific interactions.
- Secondary pharmacology studies
-
These studies aim to investigate the mode of action and/or effects of a substance not related to its desired therapeutic target.
- Side effects
-
Unintended pharmacological effects of a drug, frequently used to describe adverse effects, but this term may also apply to additional beneficial consequences.
- The International Consortium for Innovation and Quality in Pharmaceutical Development
-
(IQ). A not-for-profit organization of pharmaceutical and biotechnology companies with a mission of advancing science and technology to augment the capability of member companies to develop transformational solutions that benefit patients, regulators and the broader research and development community.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brennan, R.J., Jenkinson, S., Brown, A. et al. The state of the art in secondary pharmacology and its impact on the safety of new medicines. Nat Rev Drug Discov (2024). https://doi.org/10.1038/s41573-024-00942-3
Accepted:
Published:
DOI: https://doi.org/10.1038/s41573-024-00942-3