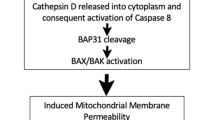
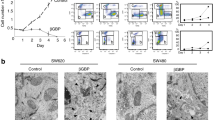
Abstract
Immunogenic cell death (ICD), which results from insufficient cellular adaptation to specific stressors, occupies a central position in the development of novel anticancer treatments. Several therapeutic strategies to elicit ICD — either as standalone approaches or as means to convert immunologically cold tumours that are insensitive to immunotherapy into hot and immunotherapy-sensitive lesions — are being actively pursued. However, the development of ICD-inducing treatments is hindered by various obstacles. Some of these relate to the intrinsic complexity of cancer cell biology, whereas others arise from the use of conventional therapeutic strategies that were developed according to immune-agnostic principles. Moreover, current discovery platforms for the development of novel ICD inducers suffer from limitations that must be addressed to improve bench-to-bedside translational efforts. An improved appreciation of the conceptual difference between key factors that discriminate distinct forms of cell death will assist the design of clinically viable ICD inducers.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
López-Otín, C. & Kroemer, G. Hallmarks of health. Cell 184, 33–63 (2021).
Mehrotra, P. & Ravichandran, K. S. Drugging the efferocytosis process: concepts and opportunities. Nat. Rev. Drug Discov. 21, 601–620 (2022).
Boada-Romero, E., Martinez, J., Heckmann, B. L. & Green, D. R. The clearance of dead cells by efferocytosis. Nat. Rev. Mol. Cell Biol. 21, 398–414 (2020).
Kroemer, G., Galassi, C., Zitvogel, L. & Galluzzi, L. Immunogenic cell stress and death. Nat. Immunol. 23, 487–500 (2022).
Björkström, N. K., Strunz, B. & Ljunggren, H. G. Natural killer cells in antiviral immunity. Nat. Rev. Immunol. 22, 112–123 (2022).
Kyrysyuk, O. & Wucherpfennig, K. W. Designing cancer immunotherapies that engage T cells and NK cells. Annu. Rev. Immunol. 41, 17–38 (2023).
Woodland, D. L. & Kohlmeier, J. E. Migration, maintenance and recall of memory T cells in peripheral tissues. Nat. Rev. Immunol. 9, 153–161 (2009).
Galluzzi, L., Yamazaki, T. & Kroemer, G. Linking cellular stress responses to systemic homeostasis. Nat. Rev. Mol. Cell Biol. 19, 731–745 (2018).
Dunn, G. P., Old, L. J. & Schreiber, R. D. The three Es of cancer immunoediting. Annu. Rev. Immunol. 22, 329–360 (2004).
Chen, R., Ishak, C. A. & De Carvalho, D. D. Endogenous retroelements and the viral mimicry response in cancer therapy and cellular homeostasis. Cancer Discov. 11, 2707–2725 (2021).
Galluzzi, L., Humeau, J., Buqué, A., Zitvogel, L. & Kroemer, G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat. Rev. Clin. Oncol. 17, 725–741 (2020).
McLaughlin, M. et al. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat. Rev. Cancer 20, 203–217 (2020).
Petroni, G., Buqué, A., Coussens, L. M. & Galluzzi, L. Targeting oncogene and non-oncogene addiction to inflame the tumour microenvironment. Nat. Rev. Drug Discov. 21, 440–462 (2022).
Cytlak, U. M. et al. Immunomodulation by radiotherapy in tumour control and normal tissue toxicity. Nat. Rev. Immunol. 22, 124–138 (2022).
Voorwerk, L. et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat. Med. 25, 920–928 (2019).
Antonia, S. J. et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N. Engl. J. Med. 379, 2342–2350 (2018).
Campbell, M. T. et al. Pilot study of tremelimumab with and without cryoablation in patients with metastatic renal cell carcinoma. Nat. Commun. 12, 6375 (2021).
Røssevold, A. H. et al. Atezolizumab plus anthracycline-based chemotherapy in metastatic triple-negative breast cancer: the randomized, double-blind phase 2b ALICE trial. Nat. Med. 28, 2573–2583 (2022).
Thibaudin, M. et al. First-line durvalumab and tremelimumab with chemotherapy in RAS-mutated metastatic colorectal cancer: a phase 1b/2 trial. Nat. Med. 29, 2087–2098 (2023).
Kroemer, G., Chan, T. A., Eggermont, A. M. M. & Galluzzi, L. Immunosurveillance in clinical cancer management. CA Cancer J. Clin. https://doi.org/10.3322/caac.21818 (2023).
Bruni, D., Angell, H. K. & Galon, J. The immune contexture and immunoscore in cancer prognosis and therapeutic efficacy. Nat. Rev. Cancer 20, 662–680 (2020).
Jhunjhunwala, S., Hammer, C. & Delamarre, L. Antigen presentation in cancer: insights into tumour immunogenicity and immune evasion. Nat. Rev. Cancer 21, 298–312 (2021).
Krysko, D. V. et al. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 12, 860–875 (2012).
Zindel, J. & Kubes, P. DAMPs, PAMPs, and LAMPs in immunity and sterile inflammation. Annu. Rev. Pathol. 15, 493–518 (2020).
Singleton, D. C., Macann, A. & Wilson, W. R. Therapeutic targeting of the hypoxic tumour microenvironment. Nat. Rev. Clin. Oncol. 18, 751–772 (2021).
Lucca, L. E. & Dominguez-Villar, M. Modulation of regulatory T cell function and stability by co-inhibitory receptors. Nat. Rev. Immunol. 20, 680–693 (2020).
Vitale, I. et al. Apoptotic cell death in disease — current understanding of the NCCD 2023. Cell Death Differ. 30, 1097–1154 (2023).
Stockwell, B. R. Ferroptosis turns 10: emerging mechanisms, physiological functions, and therapeutic applications. Cell 185, 2401–2421 (2022).
Weinlich, R., Oberst, A., Beere, H. M. & Green, D. R. Necroptosis in development, inflammation and disease. Nat. Rev. Mol. Cell Biol. 18, 127–136 (2017).
Conrad, M., Angeli, J. P., Vandenabeele, P. & Stockwell, B. R. Regulated necrosis: disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 15, 348–366 (2016).
Edinger, A. L. & Thompson, C. B. Death by design: apoptosis, necrosis and autophagy. Curr. Opin. Cell Biol. 16, 663–669 (2004).
Panaretakis, T. et al. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J. 28, 578–590 (2009).
Wiernicki, B. et al. Cancer cells dying from ferroptosis impede dendritic cell-mediated anti-tumor immunity. Nat. Commun. 13, 3676 (2022). This article demonstrates that ferroptosis is not always immunogenic per se and actively inhibits the immunogenicity of apoptotic cell death.
Obeid, M. et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 13, 54–61 (2007).
Ahrens, S. et al. F-actin is an evolutionarily conserved damage-associated molecular pattern recognized by DNGR-1, a receptor for dead cells. Immunity 36, 635–645 (2012).
Kepp, O. et al. ATP and cancer immunosurveillance. EMBO J. 40, e108130 (2021).
Fucikova, J., Spisek, R., Kroemer, G. & Galluzzi, L. Calreticulin and cancer. Cell Res. 31, 5–16 (2021).
Elliott, M. R. et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461, 282–286 (2009).
Chekeni, F. B. et al. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature 467, 863–867 (2010).
Ma, Y. et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity 38, 729–741 (2013).
Ghiringhelli, F. et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat. Med. 15, 1170–1178 (2009).
Nicholas, R. A. Identification of the P2Y(12) receptor: a novel member of the P2Y family of receptors activated by extracellular nucleotides. Mol. Pharmacol. 60, 416–420 (2001).
Gardai, S. J. et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 123, 321–334 (2005).
Gardai, S. J. et al. By binding SIRPalpha or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell 115, 13–23 (2003).
Hildner, K. et al. Batf3 deficiency reveals a critical role for CD8α+ dendritic cells in cytotoxic T cell immunity. Science 322, 1097–1100 (2008).
Sen Santara, S. et al. The NK cell receptor NKp46 recognizes ecto-calreticulin on ER-stressed cells. Nature 616, 348–356 (2023). This provides an elegant demonstration that besides mediating pro-phagocytic effects, CALR can directly bind to NKp46 on the surface of NK cells to promote their cytotoxicity.
Minute, L. et al. Cellular cytotoxicity is a form of immunogenic cell death. J. Immunother. Cancer 8, e000325 (2020).
Jaime-Sanchez, P. et al. Cell death induced by cytotoxic CD8+ T cells is immunogenic and primes caspase-3-dependent spread immunity against endogenous tumor antigens. J. Immunother. Cancer 8, e000528 (2020). With Minute et al. (ref. 47), these articles show that the death of cancer cells as mediated by CD8+ CTLs is a bona fide instance of ICD.
Costa-Mattioli, M. & Walter, P. The integrated stress response: from mechanism to disease. Science 368, e00099-20 (2020).
Lines, C. L., McGrath, M. J., Dorwart, T. & Conn, C. S. The integrated stress response in cancer progression: a force for plasticity and resistance. Front. Oncol. 13, 1206561 (2023).
Humeau, J. et al. Inhibition of transcription by dactinomycin reveals a new characteristic of immunogenic cell stress. EMBO Mol. Med. 12, e11622 (2020).
Senovilla, L. et al. An immunosurveillance mechanism controls cancer cell ploidy. Science 337, 1678–1684 (2012).
Garg, A. D. et al. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. EMBO J. 31, 1062–1079 (2012).
Giglio, P. et al. PKR and GCN2 stress kinases promote an ER stress-independent eIF2α phosphorylation responsible for calreticulin exposure in melanoma cells. Oncoimmunology 7, e1466765 (2018).
Humeau, J. et al. Phosphorylation of eukaryotic initiation factor-2α (eIF2α) in autophagy. Cell Death Dis. 11, 433 (2020).
Sistigu, A. et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat. Med. 20, 1301–1309 (2014).
Wang, Z. et al. cGAS/STING axis mediates a topoisomerase II inhibitor-induced tumor immunogenicity. J. Clin. Invest. 129, 4850–4862 (2019).
Yatim, N. et al. RIPK1 and NF-κB signaling in dying cells determines cross-priming of CD8+ T cells. Science 350, 328–334 (2015).
Zi, M. et al. Improved antitumor immunity of chemotherapy in OSCC treatment by gasdermin-E mediated pyroptosis. Apoptosis 28, 348–361 (2023).
Fridman, W. H. et al. B cells and tertiary lymphoid structures as determinants of tumour immune contexture and clinical outcome. Nat. Rev. Clin. Oncol. 19, 441–457 (2022).
Sarti Kinker, G. & da Silva Medina, T. Tertiary lymphoid structures as hubs of antitumour immunity. Nat. Rev. Cancer 23, 803 (2023).
Michaud, M. et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science 334, 1573–1577 (2011). This paper documents the importance of proficient autophagic responses in cancer cells to support optimal ICD-associated ATP release.
Spisek, R. et al. Bortezomib enhances dendritic cell (DC)-mediated induction of immunity to human myeloma via exposure of cell surface heat shock protein 90 on dying tumor cells: therapeutic implications. Blood 109, 4839–4845 (2007).
Gulla, A. et al. Bortezomib induces anti-multiple myeloma immune response mediated by cGAS/STING pathway activation. Blood Cancer Discov. 2, 468–483 (2021).
Montes de Oca, R. et al. Belantamab mafodotin (GSK2857916) drives immunogenic cell death and immune-mediated antitumor responses in vivo. Mol. Cancer Ther. 20, 1941–1955 (2021).
Liu, P. et al. Crizotinib-induced immunogenic cell death in non-small cell lung cancer. Nat. Commun. 10, 1486 (2019).
Scirocchi, F. et al. Immunogenic cell death and immunomodulatory effects of cabozantinib. Front. Oncol. 11, 755433 (2021).
Choueiri, T. K. et al. Cabozantinib plus nivolumab and Ipilimumab in renal-cell carcinoma. N. Engl. J. Med. 388, 1767–1778 (2023).
Spigel, D. R. et al. Phase 1/2 study of the safety and tolerability of nivolumab plus crizotinib for the first-line treatment of anaplastic lymphoma kinase translocation-positive advanced non-small cell lung cancer (CheckMate 370). J. Thorac. Oncol. 13, 682–688 (2018).
Patel, S. P. et al. Phase Ib study of crizotinib plus pembrolizumab in patients with previously untreated advanced non-small cell lung cancer with ALK translocation. Oncologist 25, 562-e1012 (2020).
Xu, W. et al. Stimuli-responsive nanodelivery systems for amplifying immunogenic cell death in cancer immunotherapy. Immunol. Rev. 321, 181–198 (2023).
Zhang, J. et al. Nanoparticle-based drug delivery systems to enhance cancer immunotherapy in solid tumors. Front. Immunol. 14, 1230893 (2023).
Shalhout, S. Z., Miller, D. M., Emerick, K. S. & Kaufman, H. L. Therapy with oncolytic viruses: progress and challenges. Nat. Rev. Clin. Oncol. 20, 160–177 (2023).
Bommareddy, P. K., Shettigar, M. & Kaufman, H. L. Integrating oncolytic viruses in combination cancer immunotherapy. Nat. Rev. Immunol. 18, 498–513 (2018).
Poh, A. First oncolytic viral therapy for melanoma. Cancer Discov. 6, 6 (2016).
He, B., Gross, M. & Roizman, B. The γ134.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1α to dephosphorylate the α subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc. Natl Acad. Sci. USA 94, 843–848 (1997).
Grigg, C. et al. Talimogene laherparepvec (T-VEC) for the treatment of melanoma and other cancers. Semin. Oncol. 43, 638–646 (2016).
Palanivelu, L., Liu, C. H. & Lin, L. T. Immunogenic cell death: the cornerstone of oncolytic viro-immunotherapy. Front. Immunol. 13, 1038226 (2022).
Pol, J. et al. Trial watch — oncolytic viruses and cancer therapy. Oncoimmunology 5, e1117740 (2016).
Vitale, I. et al. Targeting cancer heterogeneity with immune responses driven by oncolytic peptides. Trends Cancer 7, 557–572 (2021).
Zhou, H. et al. The oncolytic peptide LTX-315 triggers immunogenic cell death. Cell Death Dis. 7, e2134 (2016).
Yamazaki, T. et al. The oncolytic peptide LTX-315 overcomes resistance of cancers to immunotherapy with CTLA4 checkpoint blockade. Cell Death Differ. 23, 1004–1015 (2016).
Yamazaki, T. et al. LTX-315-enabled, radiotherapy-boosted immunotherapeutic control of breast cancer by NK cells. Oncoimmunology 10, 1962592 (2021).
Oslo Børs. Lytix Biopharma’s licensing partner Verrica Pharmaceuticals Inc. reports interim phase II data with LTX-315. News Web. https://newsweb.oslobors.no/message/596578 (2023).
Rodriguez-Ruiz, M. E., Vitale, I., Harrington, K. J., Melero, I. & Galluzzi, L. Immunological impact of cell death signaling driven by radiation on the tumor microenvironment. Nat. Immunol. 21, 120–134 (2020).
Kepp, O., Marabelle, A., Zitvogel, L. & Kroemer, G. Oncolysis without viruses – inducing systemic anticancer immune responses with local therapies. Nat. Rev. Clin. Oncol. 17, 49–64 (2020).
Fucikova, J. et al. High hydrostatic pressure induces immunogenic cell death in human tumor cells. Int. J. Cancer 135, 1165–1177 (2014).
Yu, Z. et al. Treatment of osteosarcoma with microwave thermal ablation to induce immunogenic cell death. Oncotarget 5, 6526–6539 (2014).
Altorki, N. K. et al. Neoadjuvant durvalumab with or without stereotactic body radiotherapy in patients with early-stage non-small-cell lung cancer: a single-centre, randomised phase 2 trial. Lancet Oncol. 22, 824–835 (2021).
Zhou, Q. et al. Sugemalimab versus placebo after concurrent or sequential chemoradiotherapy in patients with locally advanced, unresectable, stage III non-small-cell lung cancer in China (GEMSTONE-301): interim results of a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 23, 209–219 (2022).
Kelly, R. J. et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N. Engl. J. Med. 384, 1191–1203 (2021).
Lim, M. et al. Phase III trial of chemoradiotherapy with temozolomide plus nivolumab or placebo for newly diagnosed glioblastoma with methylated MGMT promoter. Neuro Oncol. 24, 1935–1949 (2022).
Omuro, A. et al. Radiotherapy combined with nivolumab or temozolomide for newly diagnosed glioblastoma with unmethylated MGMT promoter: an international randomized phase III trial. Neuro Oncol. 25, 123–134 (2023).
Lee, N. Y. et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 22, 450–462 (2021).
Galluzzi, L., Aryankalayil, M. J., Coleman, C. N. & Formenti, S. C. Emerging evidence for adapting radiotherapy to immunotherapy. Nat. Rev. Clin. Oncol. 20, 543–557 (2023).
Tatsuno, K. et al. Extracorporeal photochemotherapy induces bona fide immunogenic cell death. Cell Death Dis. 10, 578 (2019).
Ventura, A. et al. Extracorporeal photochemotherapy drives monocyte-to-dendritic cell maturation to induce anticancer immunity. Cancer Res. 78, 4045–4058 (2018).
Li, X., Lovell, J. F., Yoon, J. & Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 17, 657–674 (2020).
Wei, B. M. et al. Extracorporeal photochemotherapy: mechanistic insights driving recent advances and future directions. Yale J. Biol. Med. 93, 145–159 (2020).
Wu, A. et al. The effects of 5-aminolevulinic acid photodynamic therapy on the local immune response of women with cervical intraepithelial neoplasia grade 2. Front. Immunol. 14, 1211114 (2023).
Tsai, Y. C. et al. Boost of innate immunity cytokines as biomarkers of response to extracorporeal photopheresis in patients with leukaemic cutaneous T-cell lymphoma. Br. J. Dermatol. 189, 603–611 (2023).
Menger, L. et al. Cardiac glycosides exert anticancer effects by inducing immunogenic cell death. Sci. Transl. Med. 4, 143ra199 (2012).
Zelenay, S. et al. Cyclooxygenase-dependent tumor growth through evasion of immunity. Cell 162, 1257–1270 (2015).
Huang, Q. et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat. Med. 17, 860–866 (2011).
Hayashi, K. et al. Tipping the immunostimulatory and inhibitory DAMP balance to harness immunogenic cell death. Nat. Commun. 11, 6299 (2020).
Pietrocola, F. et al. Caloric restriction mimetics enhance anticancer immunosurveillance. Cancer Cell 30, 147–160 (2016).
Lévesque, S. et al. A synergistic triad of chemotherapy, immune checkpoint inhibitors, and caloric restriction mimetics eradicates tumors in mice. Oncoimmunology 8, e1657375 (2019).
Castoldi, F. et al. Autophagy-mediated metabolic effects of aspirin. Cell Death Discov. 6, 129 (2020).
Vodnala, S. K. et al. T cell stemness and dysfunction in tumors are triggered by a common mechanism. Science 363, eaau0135 (2019).
Cheng, J. T. et al. Novel transcription regulatory sequences and factors of the immune evasion protein ICP47 (US12) of herpes simplex viruses. Virol. J. 17, 101 (2020).
Keckler, M. S. Dodging the CTL response: viral evasion of Fas and granzyme induced apoptosis. Front. Biosci. 12, 725–732 (2007).
Franz, S. et al. Mumps virus SH protein inhibits NF-κB activation by interacting with tumor necrosis factor receptor 1, interleukin-1 receptor 1, and toll-like receptor 3 complexes. J. Virol. 91, e01037-17 (2017).
Vitale, I., Shema, E., Loi, S. & Galluzzi, L. Intratumoral heterogeneity in cancer progression and response to immunotherapy. Nat. Med. 27, 212–224 (2021).
Solimini, N. L., Luo, J. & Elledge, S. J. Non-oncogene addiction and the stress phenotype of cancer cells. Cell 130, 986–988 (2007).
Nagel, R., Semenova, E. A. & Berns, A. Drugging the addict: non-oncogene addiction as a target for cancer therapy. EMBO Rep. 17, 1516–1531 (2016).
Levine, A. J. p53: 800 million years of evolution and 40 years of discovery. Nat. Rev. Cancer 20, 471–480 (2020).
Wang, M. & Attardi, L. D. A balancing act: p53 activity from tumor suppression to pathology and therapeutic implications. Annu. Rev. Pathol. 17, 205–226 (2022).
Hadian, K. & Stockwell, B. R. The therapeutic potential of targeting regulated non-apoptotic cell death. Nat. Rev. Drug Discov. 22, 723–742 (2023).
Efimova, I. et al. Vaccination with early ferroptotic cancer cells induces efficient antitumor immunity. J. Immunother. Cancer 8, e001369 (2020).
Li, J. et al. Tumor-specific GPX4 degradation enhances ferroptosis-initiated antitumor immune response in mouse models of pancreatic cancer. Sci. Transl. Med. 15, eadg3049 (2023).
Diepstraten, S. T. et al. The manipulation of apoptosis for cancer therapy using BH3-mimetic drugs. Nat. Rev. Cancer 22, 45–64 (2022).
Rongvaux, A. et al. Apoptotic caspases prevent the induction of type I interferons by mitochondrial DNA. Cell 159, 1563–1577 (2014).
White, M. J. et al. Apoptotic caspases suppress mtDNA-induced STING-mediated type I IFN production. Cell 159, 1549–1562 (2014). With Rongvaux et al. (ref. 122), these articles demonstrate that the activation of executioner caspases robustly suppresses type I interferon responses elicited by MOMP.
Rodriguez-Ruiz, M. E. et al. Apoptotic caspases inhibit abscopal responses to radiation and identify a new prognostic biomarker for breast cancer patients. Oncoimmunology 8, e1655964 (2019).
Han, C. et al. Tumor cells suppress radiation-induced immunity by hijacking caspase 9 signaling. Nat. Immunol. 21, 546–554 (2020).
Ning, X. et al. Apoptotic caspases suppress type I interferon production via the cleavage of cGAS, MAVS, and IRF3. Mol. Cell 74, 19–31.e17 (2019).
Suzuki, J., Denning, D. P., Imanishi, E., Horvitz, H. R. & Nagata, S. Xk-related protein 8 and CED-8 promote phosphatidylserine exposure in apoptotic cells. Science 341, 403–406 (2013).
Giampazolias, E. et al. Mitochondrial permeabilization engages NF-κB-dependent anti-tumour activity under caspase deficiency. Nat. Cell Biol. 19, 1116–1129 (2017). This original report provides compelling evidence in support of the notion that caspase-independent cancer cell death is more immunogenic than its caspase-dependent counterpart.
Klionsky, D. J. et al. Autophagy in major human diseases. EMBO J. 40, e108863 (2021).
Wu, Q. et al. IGF1 receptor inhibition amplifies the effects of cancer drugs by autophagy and immune-dependent mechanisms. J. Immunother. Cancer 9, e002722 (2021).
Debnath, J., Gammoh, N. & Ryan, K. M. Autophagy and autophagy-related pathways in cancer. Nat. Rev. Mol. Cell Biol. 24, 560–575 (2023).
Thompson, E. A. & Powell, J. D. Inhibition of the adenosine pathway to potentiate cancer immunotherapy: potential for combinatorial approaches. Annu. Rev. Med. 72, 331–348 (2021).
Azambuja, J. H., Ludwig, N., Braganhol, E. & Whiteside, T. L. Inhibition of the adenosinergic pathway in cancer rejuvenates innate and adaptive immunity. Int. J. Mol. Sci. 20, 5698 (2019).
Chiappori, A. A. et al. Phase I study of taminadenant (PBF509/NIR178), an adenosine 2A receptor antagonist, with or without spartalizumab (PDR001), in patients with advanced non-small cell lung cancer. Clin. Cancer Res. 28, 2313–2320 (2022).
Lim, E. A. et al. Phase Ia/b, open-label, multicenter study of AZD4635 (an adenosine A2A receptor antagonist) as monotherapy or combined with durvalumab, in patients with solid tumors. Clin. Cancer Res. 28, 4871–4884 (2022).
Cascone, T. et al. Neoadjuvant durvalumab alone or combined with novel immuno-oncology agents in resectable lung cancer: the phase II NeoCOAST platform trial. Cancer Discov. 13, 2394–2411 (2023).
Herbst, R. S. et al. COAST: an open-label, phase II, multidrug platform study of durvalumab alone or in combination with oleclumab or monalizumab in patients with unresectable, stage III non-small-cell lung cancer. J. Clin. Oncol. 40, 3383–3393 (2022).
Song, X. et al. Pharmacologic suppression of B7-H4 glycosylation restores antitumor immunity in immune-cold breast cancers. Cancer Discov. 10, 1872–1893 (2020).
Liu, L. et al. Ablation of ERO1A induces lethal endoplasmic reticulum stress responses and immunogenic cell death to activate anti-tumor immunity. Cell Rep. Med. 4, 101206 (2023).
Lin, H. et al. Stanniocalcin 1 is a phagocytosis checkpoint driving tumor immune resistance. Cancer Cell 39, 480–493.e486 (2021).
Feng, M. et al. Programmed cell removal by calreticulin in tissue homeostasis and cancer. Nat. Commun. 9, 3194 (2018).
Liu, P. et al. Immunosuppression by mutated calreticulin released from malignant cells. Mol. Cell 77, 748–760.e749 (2020).
Chao, M. P. et al. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci. Transl. Med. 2, 63ra94 (2010).
Advani, R. et al. CD47 blockade by Hu5F9-G4 and rituximab in non-hodgkin’s lymphoma. N. Engl. J. Med. 379, 1711–1721 (2018).
Ansell, S. M. et al. Phase I study of the CD47 blocker TTI-621 in patients with relapsed or refractory hematologic malignancies. Clin. Cancer Res. 27, 2190–2199 (2021).
Querfeld, C. et al. Intralesional TTI-621, a novel biologic targeting the innate immune checkpoint CD47, in patients with relapsed or refractory mycosis fungoides or Sézary syndrome: a multicentre, phase 1 study. Lancet Haematol. 8, e808–e817 (2021).
van Helden, M. J. et al. BYON4228 is a pan-allelic antagonistic SIRPα antibody that potentiates destruction of antibody-opsonized tumor cells and lacks binding to SIRPγ on T cells. J. Immunother. Cancer 11, e006567 (2023).
Bahri, M. et al. SIRPα-specific monoclonal antibody enables antibody-dependent phagocytosis of neuroblastoma cells. Cancer Immunol. Immunother. 71, 71–83 (2022).
Giampazolias, E. et al. Secreted gelsolin inhibits DNGR-1-dependent cross-presentation and cancer immunity. Cell 184, 4016–4031.e4022 (2021). This article elegantly demonstrates that secreted GSN inhibits the binding of F-actin to DNGR1, hence suppressing antigen cross-presentation by cDC1s.
Kim, Y., Cho, N. Y., Jin, L., Jin, H. Y. & Kang, G. H. Prognostic significance of STING expression in solid tumor: a systematic review and meta-analysis. Front. Oncol. 13, 1244962 (2023).
Chen, C., Wang, J., Dong, C., Lim, D. & Feng, Z. Development of a risk model to predict prognosis in breast cancer based on cGAS-STING-related genes. Front. Genet. 14, 1121018 (2023).
Yamazaki, T. et al. Mitochondrial DNA drives abscopal responses to radiation that are inhibited by autophagy. Nat. Immunol. 21, 1160–1171 (2020). This report connects MOMP-dependent mtDNA-driven cGAS signalling in malignant cells with the ability of radiotherapy to elicit anticancer immune responses.
Harding, S. M. et al. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature 548, 466–470 (2017).
Bartsch, K. et al. Absence of RNase H2 triggers generation of immunogenic micronuclei removed by autophagy. Hum. Mol. Genet. 26, 3960–3972 (2017).
Govindan, R. et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell 150, 1121–1134 (2012).
Levy, J. M. M., Towers, C. G. & Thorburn, A. Targeting autophagy in cancer. Nat. Rev. Cancer 17, 528–542 (2017).
Ladoire, S. et al. The presence of LC3B puncta and HMGB1 expression in malignant cells correlate with the immune infiltrate in breast cancer. Autophagy 12, 864–875 (2016).
Baracco, E. E. et al. Contribution of annexin A1 to anticancer immunosurveillance. Oncoimmunology 8, e1647760 (2019).
Le Naour, J. et al. A TLR3 ligand reestablishes chemotherapeutic responses in the context of FPR1 deficiency. Cancer Discov. 11, 408–423 (2021).
Le Naour, J. et al. Formyl peptide receptor-1 (FPR1) represses intestinal oncogenesis. Oncoimmunology 12, 2237354 (2023).
Carbonnier, V. et al. Rs867228 in FPR1 accelerates the manifestation of luminal B breast cancer. Oncoimmunology 12, 2189823 (2023).
Ghiringhelli, F. et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol. Immunother. 56, 641–648 (2007).
Vincent, J. et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 70, 3052–3061 (2010).
Reijers, I. L. M. et al. Personalized response-directed surgery and adjuvant therapy after neoadjuvant ipilimumab and nivolumab in high-risk stage III melanoma: the PRADO trial. Nat. Med. 28, 1178–1188 (2022).
Chalabi, M. et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat. Med. 26, 566–576 (2020).
Blank, C. U. et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat. Med. 24, 1655–1661 (2018).
Forde, P. M. et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N. Engl. J. Med. 386, 1973–1985 (2022).
Kepp, O. et al. A fluorescent biosensor-based platform for the discovery of immunogenic cancer cell death inducers. Oncoimmunology 8, 1606665 (2019).
Cultrara, C. et al. A biologic-device combination product delivering tumor-derived antigens elicits immunogenic cell death-associated immune responses against glioblastoma. J. Immunother. Cancer 11, e006880 (2023).
Garg, A. D. et al. Dendritic cell vaccines based on immunogenic cell death elicit danger signals and T cell-driven rejection of high-grade glioma. Sci. Transl. Med. 8, 328ra327 (2016).
Vedunova, M. et al. DC vaccines loaded with glioma cells killed by photodynamic therapy induce Th17 anti-tumor immunity and provide a four-gene signature for glioma prognosis. Cell Death Dis. 13, 1062 (2022).
Petroni, G., Formenti, S. C., Chen-Kiang, S. & Galluzzi, L. Immunomodulation by anticancer cell cycle inhibitors. Nat. Rev. Immunol. 20, 669–679 (2020).
Chuprin, J. et al. Humanized mouse models for immuno-oncology research. Nat. Rev. Clin. Oncol. 20, 192–206 (2023).
Wculek, S. K. et al. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 20, 7–24 (2020).
Richter, C. et al. Generation of inducible immortalized dendritic cells with proper immune function in vitro and in vivo. PLoS ONE 8, e62621 (2013).
Zhao, L. et al. A genotype–phenotype screening system using conditionally immortalized immature dendritic cells. STAR Protoc. 2, 100732 (2021).
Zhang, S. et al. Anticancer effects of ikarugamycin and astemizole identified in a screen for stimulators of cellular immune responses. J. Immunother. Cancer 11, e006785 (2023).
Zhao, L. et al. BCL2 inhibition reveals a dendritic cell-specific immune checkpoint that controls tumor immunosurveillance. Cancer Discov. 13, 2448–2469 (2023). This article provides abundant evidence to support the notion that BCL-2 inhibits antigen cross-presentation by cDC1s by preventing mtDNA-driven cGAS signalling.
Theisen, D. J. et al. WDFY4 is required for cross-presentation in response to viral and tumor antigens. Science 362, 694–699 (2018).
Li, L. et al. Lurbinectedin for the treatment of small cell lung cancer. Drugs Today 57, 377–385 (2021).
Baines, A. C. et al. FDA approval summary: belantamab mafodotin for patients with relapsed or refractory multiple myeloma. Clin. Cancer Res. 28, 4629–4633 (2022).
Pozzi, C. et al. The EGFR-specific antibody cetuximab combined with chemotherapy triggers immunogenic cell death. Nat. Med. 22, 624–631 (2016).
DeSelm, C. et al. Low-dose radiation conditioning enables CAR T cells to mitigate antigen escape. Mol. Ther. 26, 2542–2552 (2018).
Sugita, M. et al. Radiation therapy improves CAR T cell activity in acute lymphoblastic leukemia. Cell Death Dis. 14, 305 (2023).
Yamamoto, K. et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature 581, 100–105 (2020). This paper elegantly demonstrates that efficient autophagic responses in malignant cells limit anticancer immunity by degrading MHC class I molecules.
Vanpouille-Box, C. et al. TGFβ is a master regulator of radiation therapy-induced antitumor immunity. Cancer Res. 75, 2232–2242 (2015).
Cappell, K. M. & Kochenderfer, J. N. Long-term outcomes following CAR T cell therapy: what we know so far. Nat. Rev. Clin. Oncol. 20, 359–371 (2023).
Larson, R. C. & Maus, M. V. Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat. Rev. Cancer 21, 145–161 (2021).
Yang, J., Chen, Y., Jing, Y., Green, M. R. & Han, L. Advancing CAR T cell therapy through the use of multidimensional omics data. Nat. Rev. Clin. Oncol. 20, 211–228 (2023).
Pahl, J. H. et al. Anti-EGFR antibody cetuximab enhances the cytolytic activity of natural killer cells toward osteosarcoma. Clin. Cancer Res. 18, 432–441 (2012).
Hinterberger, M. et al. Intratumoral virotherapy with 4-1BBL armed modified vaccinia Ankara eradicates solid tumors and promotes protective immune memory. J. Immunother. Cancer 9, e001586 (2021).
Charpentier, M., Formenti, S. & Demaria, S. CD40 agonism improves anti-tumor T cell priming induced by the combination of radiation therapy plus CTLA4 inhibition and enhances tumor response. Oncoimmunology 12, 2258011 (2023).
Huang, F. Y. et al. A recombinant oncolytic Newcastle virus expressing MIP-3α promotes systemic antitumor immunity. J. Immunother. Cancer 8, e000330 (2020).
Lu, J. et al. Nano-enabled pancreas cancer immunotherapy using immunogenic cell death and reversing immunosuppression. Nat. Commun. 8, 1811 (2017).
Bausart, M. et al. Combination of local immunogenic cell death-inducing chemotherapy and DNA vaccine increases the survival of glioblastoma-bearing mice. Nanomedicine 50, 102681 (2023).
Kroemer, G., McQuade, J. L., Merad, M., Andre, F. & Zitvogel, L. Bodywide ecological interventions on cancer. Nat. Med. 29, 59–74 (2023).
Blake, S. J., Wolf, Y., Boursi, B. & Lynn, D. J. Role of the microbiota in response to and recovery from cancer therapy. Nat. Rev. Immunol. https://doi.org/10.1038/s41577-023-00951-0 (2023).
Dai, J. et al. Acetylation blocks cGAS activity and inhibits self-DNA-induced autoimmunity. Cell 176, 1447–1460.e1414 (2019).
Obradovic, M. M. S. et al. Glucocorticoids promote breast cancer metastasis. Nature 567, 540–544 (2019).
Yang, H. et al. Stress-glucocorticoid-TSC22D3 axis compromises therapy-induced antitumor immunity. Nat. Med. 25, 1428–1441 (2019).
Nunez-Ruiz, A., Sanchez-Brena, F., Lopez-Pacheco, C., Acevedo-Dominguez, N. A. & Soldevila, G. Obesity modulates the immune macroenvironment associated with breast cancer development. PLoS ONE 17, e0266827 (2022).
Dyck, L. et al. Suppressive effects of the obese tumor microenvironment on CD8 T cell infiltration and effector function. J. Exp. Med. 219, e20210042 (2022).
Boi, S. K. et al. Obesity diminishes response to PD-1-based immunotherapies in renal cancer. J. Immunother. Cancer 8, e000725 (2020).
Gomes-Santos, I. L. et al. Exercise training improves tumor control by increasing CD8+ T-cell infiltration via CXCR3 signaling and sensitizes breast cancer to immune checkpoint blockade. Cancer Immunol. Res. 9, 765–778 (2021).
Acknowledgements
Among other sources, L.G. is or has been supported (as a PI unless otherwise indicated) by one R01 grant from the NIH/NCI (grant no, CA271915), by two Breakthrough Level 2 grants from the US DoD BCRP (grant nos. BC180476P1 and BC210945), by a grant from the STARR Cancer Consortium (grant no. I16-0064), by a Transformative Breast Cancer Consortium grant from the US DoD BCRP (grant no. W81XWH2120034, PI: Formenti), by a U54 grant from NIH/NCI (grant no. CA274291, PI: Deasy, Formenti, Weichselbaum), by the 2019 Laura Ziskin Prize in Translational Research (grant no. ZP-6177, PI: Formenti) from the Stand Up to Cancer (SU2C), by a Mantle Cell Lymphoma Research Initiative (MCL-RI, PI: Chen-Kiang) grant from the Leukaemia and Lymphoma Society (LLS) and by a Rapid Response Grant from the Functional Genomics Initiative (New York, USA). Among other sources, G.K. is supported by the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR) — Projets blancs; AMMICa US23/CNRS UMS3655; Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; Fondation pour la Recherche Médicale (FRM); a donation by Elior; Equipex Onco-Pheno-Screen; European Joint Programme on Rare Diseases (EJPRD); European Research Council Advanced Investigator Award (ERC-2021-ADG, ICD-Cancer, grant no. 101052444), as well as by European Union Horizon 2020 Projects Oncobiome, Prevalung (grant no. 101095604) and Crimson. Views and opinions expressed are those of the authors only and do not necessarily reflect those of the European Union, the European Research Council or any other granting authority. Neither the European Union nor any other granting authority can be held responsible for them.
Author information
Authors and Affiliations
Contributions
L.G., D.S., G.K. and F.M.M. wrote the first version of the article, with constructive input from E.G. E.G. built display items under direct supervision from L.G. All authors approve the submitted version of the article.
Corresponding authors
Ethics declarations
Competing interests
L.G. holds or has held research contracts with Lytix Biopharma, Promontory and Onxeo, has received consulting or advisory honoraria from Boehringer-Ingelheim, AstraZeneca, OmniSEQ, Onxeo, The Longevity Labs, Inzen, Imvax, Sotio, Promontory, Noxopharm, EduCom and the Luke Heller TECPR2 Foundation, and holds Promontory stock options. G.K. has held research contracts with Daiichi Sankyo, Eleor, Kaleido, Lytix Pharma, PharmaMar, Osasuna Therapeutics, Samsara Therapeutics, Sanofi, Tollys and Vascage. G.K. is on the Board of Directors of the Bristol Myers Squibb Foundation France. D.S. is a full-time employee of Violet Therapeutics. G.K. is a scientific co-founder of everImmune, Osasuna Therapeutics, Samsara Therapeutics and Therafast Bio. G.K. is in the scientific advisory boards of Hevolution, Institut Servier, Longevity Vision Funds and Rejuveron Life Sciences. G.K. is the inventor of patents covering therapeutic targeting of ageing, cancer, cystic fibrosis and metabolic disorders. G.K.’s wife, Laurence Zitvogel, has held research contracts with Glaxo Smyth Kline, Incyte, Lytix, Kaleido, Innovate Pharma, Daiichi Sankyo, Pilege, Merus, Transgene, 9m, Tusk and Roche, was on the Board of Directors of Transgene, is a co-founder of everImmune and holds patents covering the treatment of cancer and the therapeutic manipulation of the microbiota. G.K.’s brother, Romano Kroemer, was an employee of Sanofi and now consults for Boehringer-Ingelheim. The funders had no role in the design of the study, in the writing of the manuscript or in the decision to publish the results. F.M.M. is a full-time employee of Sonata Therapeutics.
Peer review
Peer review information
Nature Reviews Drug Discovery thanks Dmitri Krysko, Emily Cheng and the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ClinicalTrials.gov: https://clinicaltrials.gov
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Galluzzi, L., Guilbaud, E., Schmidt, D. et al. Targeting immunogenic cell stress and death for cancer therapy. Nat Rev Drug Discov (2024). https://doi.org/10.1038/s41573-024-00920-9
Accepted:
Published:
DOI: https://doi.org/10.1038/s41573-024-00920-9