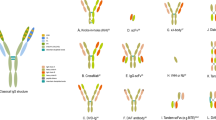
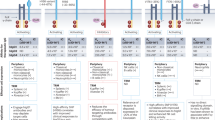
Abstract
Bispecific antibodies (bsAbs) enable novel mechanisms of action and/or therapeutic applications that cannot be achieved using conventional IgG-based antibodies. Consequently, development of these molecules has garnered substantial interest in the past decade and, as of the end of 2023, 14 bsAbs have been approved: 11 for the treatment of cancer and 3 for non-oncology indications. bsAbs are available in different formats, address different targets and mediate anticancer function via different molecular mechanisms. Here, we provide an overview of recent developments in the field of bsAbs for cancer therapy. We focus on bsAbs that are approved or in clinical development, including bsAb-mediated dual modulators of signalling pathways, tumour-targeted receptor agonists, bsAb–drug conjugates, bispecific T cell, natural killer cell and innate immune cell engagers, and bispecific checkpoint inhibitors and co-stimulators. Finally, we provide an outlook into next-generation bsAbs in earlier stages of development, including trispecifics, bsAb prodrugs, bsAbs that induce degradation of tumour targets and bsAbs acting as cytokine mimetics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Brinkmann, U. & Kontermann, R. E. Bispecific antibodies. Science 372, 916–917 (2021).
Labrijn, A. F., Janmaat, M. L., Reichert, J. M. & Parren, P. Bispecific antibodies: a mechanistic review of the pipeline. Nat. Rev. Drug. Discov. 18, 585–608 (2019). This review introduces a mechanistic categorization of bsAbs.
Yu, Y. J. et al. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci. Transl. Med. 3, 84ra44 (2011).
Niewoehner, J. et al. Increased brain penetration and potency of a therapeutic antibody using a monovalent molecular shuttle. Neuron 81, 49–60 (2014).
Weber, F. et al. Brain shuttle antibody for Alzheimer’s disease with attenuated peripheral effector function due to an inverted binding mode. Cell Rep. 22, 149–162 (2018).
Zhao, P., Zhang, N. & An, Z. Engineering antibody and protein therapeutics to cross the blood–brain barrier. Antib. Ther. 5, 311–331 (2022).
Nie, S. et al. Biology drives the discovery of bispecific antibodies as innovative therapeutics. Antib. Ther. 3, 18–62 (2020).
Galvez-Cancino, F. et al. Fcγ receptors and immunomodulatory antibodies in cancer. Nat. Rev. Cancer 24, 51–71 (2024). This review provides an overview of the role of Fcγ receptors in the MoA of antibodies.
Schlothauer, T. et al. Novel human IgG1 and IgG4 Fc-engineered antibodies with completely abolished immune effector functions. Protein Eng. Des. Sel. 29, 457–466 (2016).
Pyzik, M., Kozicky, L. K., Gandhi, A. K. & Blumberg, R. S. The therapeutic age of the neonatal Fc receptor. Nat. Rev. Immunol. 23, 415–432 (2023).
Brinkmann, U. & Kontermann, R. E. The making of bispecific antibodies. mAbs 9, 182–212 (2017).
Kontermann, R. E. & Brinkmann, U. Bispecific antibodies. Drug. Discov. Today 20, 838–847 (2015).
Elshiaty, M., Schindler, H. & Christopoulos, P. Principles and current clinical landscape of multispecific antibodies against cancer. Int. J. Mol. Sci. 22, 5632 (2021).
Wang, S. et al. The state of the art of bispecific antibodies for treating human malignancies. EMBO Mol. Med. 13, e14291 (2021).
Antonarelli, G. et al. Research and clinical landscape of bispecific antibodies for the treatment of solid malignancies. Pharmaceuticals 14, 884 (2021).
Neijssen, J. et al. Discovery of amivantamab (JNJ-61186372), a bispecific antibody targeting EGFR and MET. J. Biol. Chem. 296, 100641 (2021). This article describes the discovery of amivantamab, the first approved dual RTK-targeting bsAb.
Syed, Y. Y. Amivantamab: first approval. Drugs 81, 1349–1353 (2021).
Zhou, C. et al. Amivantamab plus chemotherapy in NSCLC with EGFR exon 20 insertions. N. Engl. J. Med. 389, 2039–2051 (2023).
Herpers, B. et al. Functional patient-derived organoid screenings identify MCLA-158 as a therapeutic EGFR × LGR5 bispecific antibody with efficacy in epithelial tumors. Nat. Cancer 3, 418–436 (2022).
Schram, A. M. et al. Zenocutuzumab, a HER2 × HER3 bispecific antibody, is effective therapy for tumors driven by NRG1 gene rearrangements. Cancer Discov. 12, 1233–1247 (2022).
Xue, J. et al. Prediction of human pharmacokinetics and clinical effective dose of SI-B001, an EGFR/HER3 bi-specific monoclonal antibody. J. Pharm. Sci. 109, 3172–3180 (2020).
Kast, F. et al. Engineering an anti-HER2 biparatopic antibody with a multimodal mechanism of action. Nat. Commun. 12, 3790 (2021).
Oganesyan, V. et al. Structural insights into the mechanism of action of a biparatopic anti-HER2 antibody. J. Biol. Chem. 293, 8439–8448 (2018).
Weisser, N. E. et al. An anti-HER2 biparatopic antibody that induces unique HER2 clustering and complement-dependent cytotoxicity. Nat. Commun. 14, 1394 (2023).
Scheuer, W. et al. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 69, 9330–9336 (2009).
Meric-Bernstam, F. et al. Zanidatamab, a novel bispecific antibody, for the treatment of locally advanced or metastatic HER2-expressing or HER2-amplified cancers: a phase 1, dose-escalation and expansion study. Lancet Oncol. 23, 1558–1570 (2022).
Huang, S. et al. Structural and functional characterization of MBS301, an afucosylated bispecific anti-HER2 antibody. mAbs 10, 864–875 (2018).
Zhang, J. et al. First-in-human HER2-targeted bispecific antibody KN026 for the treatment of patients with HER2-positive metastatic breast cancer: results from a phase I study. Clin. Cancer Res. 28, 618–628 (2022).
Kontermann, R. E. Dual targeting strategies with bispecific antibodies. mAbs 4, 182–197 (2012).
Binz, H. K. et al. Design and characterization of MP0250, a tri-specific anti-HGF/anti-VEGF DARPin® drug candidate. mAbs 9, 1262–1269 (2017).
Baird, R. D. et al. First-in-human phase I study of MP0250, a first-in-class DARPin drug candidate targeting VEGF and HGF, in patients with advanced solid tumors. J. Clin. Oncol. 39, 145–154 (2021).
Hober, S., Lindbo, S. & Nilvebrant, J. Bispecific applications of non-immunoglobulin scaffold binders. Methods 154, 143–152 (2019).
Kovalchuk, B. et al. Nintedanib and a bi-specific anti-VEGF/Ang2 nanobody selectively prevent brain metastases of lung adenocarcinoma cells. Clin. Exp. Metastasis 37, 637–648 (2020).
Hofmann, I. et al. Pharmacodynamic and antitumor activity of BI 836880, a dual VEGF and angiopoietin 2 inhibitor, alone and combined with programmed cell death protein-1 inhibition. J. Pharmacol. Exp. Ther. 384, 331–342 (2022).
Kienast, Y. et al. Ang-2-VEGF-A CrossMab, a novel bispecific human IgG1 antibody blocking VEGF-A and Ang-2 functions simultaneously, mediates potent antitumor, antiangiogenic, and antimetastatic efficacy. Clin. Cancer Res. 19, 6730–6740 (2013).
Bendell, J. C. et al. The McCAVE Trial: vanucizumab plus mFOLFOX-6 versus bevacizumab plus mFOLFOX-6 in patients with previously untreated metastatic colorectal carcinoma (mCRC). Oncologist 25, e451–e459 (2020).
Li, J. L. & Harris, A. L. Crosstalk of VEGF and Notch pathways in tumour angiogenesis: therapeutic implications. Front. Biosci. 14, 3094–3110 (2009).
Fu, S. et al. Phase Ib study of navicixizumab plus paclitaxel in patients with platinum-resistant ovarian, primary peritoneal, or fallopian tube cancer. J. Clin. Oncol. 40, 2568–2577 (2022).
Strickler, J. H. et al. Efficacy and safety of dilpacimab (ABT-165) versus bevacizumab plus FOLFIRI in metastatic colorectal cancer: a phase II study. Future Oncol. 18, 3011–3020 (2022).
Hack, S. P., Zhu, A. X. & Wang, Y. Augmenting anticancer immunity through combined targeting of angiogenic and PD-1/PD-L1 pathways: challenges and opportunities. Front. Immunol. 11, 598877 (2020).
Cui, X. et al. A novel bispecific antibody targeting PD-L1 and VEGF with combined anti-tumor activities. Front. Immunol. 12, 778978 (2021).
Silver, A. B., Leonard, E. K., Gould, J. R. & Spangler, J. B. Engineered antibody fusion proteins for targeted disease therapy. Trends Pharmacol. Sci. 42, 1064–1081 (2021).
Kontermann, R. E. Antibody–cytokine fusion proteins. Arch. Biochem. Biophys. 526, 194–205 (2012).
Runbeck, E., Crescioli, S., Karagiannis, S. N. & Papa, S. Utilizing immunocytokines for cancer therapy. Antibodies 10, 10 (2021).
Holland, P. M. Death receptor agonist therapies for cancer, which is the right TRAIL? Cytokine Growth F. R. 25, 185–193 (2014).
Dubuisson, A. & Micheau, O. Antibodies and derivatives targeting DR4 and DR5 for cancer therapy. Antibodies 6, 16 (2017).
Melero, I. et al. CD137 (4-1BB)-based cancer immunotherapy on its 25th anniversary. Cancer Discov. 13, 552–569 (2023).
Claus, C., Ferrara-Koller, C. & Klein, C. The emerging landscape of novel 4-1BB (CD137) agonistic drugs for cancer immunotherapy. mAbs 15, 2167189 (2023).
Wilson, N. S. et al. An Fcγ receptor-dependent mechanism drives antibody-mediated target-receptor signaling in cancer cells. Cancer Cell 19, 101–113 (2011).
Garcia-Martinez, J. M. et al. Selective tumor cell apoptosis and tumor regression in CDH17-positive colorectal cancer models using BI 905711, a novel liver-sparing TRAILR2 agonist. Mol. Cancer Ther. 20, 96–108 (2021).
Brunker, P. et al. RG7386, a novel tetravalent FAP-DR5 antibody, effectively triggers FAP-dependent, avidity-driven DR5 hyperclustering and tumor cell apoptosis. Mol. Cancer Ther. 15, 946–957 (2016).
Shivange, G. et al. A single-agent dual-specificity targeting of FOLR1 and DR5 as an effective strategy for ovarian cancer. Cancer Cell 34, 331–345.e11 (2018).
Cheal, S. M., Chung, S. K., Vaughn, B. A., Cheung, N. V. & Larson, S. M. Pretargeting: a path forward for radioimmunotherapy. J. Nucl. Med. 63, 1302–1315 (2022).
Bodet-Milin, C. et al. Clinical results in medullary thyroid carcinoma suggest high potential of pretargeted immuno-PET for tumor imaging and theranostic approaches. Front. Med. 6, 124 (2019).
Chung, S. K. et al. Efficacy of HER2-targeted intraperitoneal 225Ac α-pretargeted radioimmunotherapy for small-volume ovarian peritoneal carcinomatosis. J. Nucl. Med. 64, 1439–1445 (2023).
Santich, B. H. et al. A self-assembling and disassembling (SADA) bispecific antibody (bsAb) platform for curative two-step pretargeted radioimmunotherapy. Clin. Cancer Res. 27, 532–541 (2021).
Harding, J. J. et al. Zanidatamab for HER2-amplified, unresectable, locally advanced or metastatic biliary tract cancer (HERIZON-BTC-01): a multicentre, single-arm, phase 2b study. Lancet Oncol. 24, 772–782 (2023).
Perez Bay, A. E. et al. A bispecific MET × MET antibody–drug conjugate with cleavable linker is processed in recycling and late endosomes. Mol. Cancer Ther. 22, 357–370 (2023).
Sharma, P. et al. Immune checkpoint therapy — current perspectives and future directions. Cell 186, 1652–1669 (2023).
Zhang, T., Lin, Y. & Gao, Q. Bispecific antibodies targeting immunomodulatory checkpoints for cancer therapy. Cancer Biol. Med. 20, 181–195 (2023).
Koopmans, I. et al. A novel bispecific antibody for EGFR-directed blockade of the PD-1/PD-L1 immune checkpoint. Oncoimmunology 7, e1466016 (2018).
Mittal, D. et al. Blockade of ErbB2 and PD-L1 using a bispecific antibody to improve targeted anti-ErbB2 therapy. Oncoimmunology 8, e1648171 (2019).
Chen, Y. L. et al. A bispecific antibody targeting HER2 and PD-L1 inhibits tumor growth with superior efficacy. J. Biol. Chem. 297, 101420 (2021).
Koopmans, I. et al. Bispecific antibody approach for improved melanoma-selective PD-L1 immune checkpoint blockade. J. Invest. Dermatol. 139, 2343–2351.e3 (2019).
Zhang, Y. et al. A tumor-targeted immune checkpoint blocker. Proc. Natl Acad. Sci. USA 116, 15889–15894 (2019).
Wan, C. et al. Enhanced efficacy of simultaneous PD-1 and PD-L1 immune checkpoint blockade in high-grade serous ovarian cancer. Cancer Res. 81, 158–173 (2021).
Kotanides, H. et al. Bispecific targeting of PD-1 and PD-L1 enhances T-cell activation and antitumor immunity. Cancer Immunol. Res. 8, 1300–1310 (2020).
Pang, X. et al. Cadonilimab, a tetravalent PD-1/CTLA-4 bispecific antibody with trans-binding and enhanced target binding avidity. mAbs 15, 2180794 (2023). This article describes the preclinical properties of the first approved dual checkpoint-targeting bsAb, cadonilimab.
Zhao, Y. et al. A multicenter, open-label phase Ib/II study of cadonilimab (anti PD-1 and CTLA-4 bispecific antibody) monotherapy in previously treated advanced non-small-cell lung cancer (AK104-202 study). Lung Cancer 184, 107355 (2023).
Gao, X. et al. Safety and antitumour activity of cadonilimab, an anti-PD-1/CTLA-4 bispecific antibody, for patients with advanced solid tumours (COMPASSION-03): a multicentre, open-label, phase 1b/2 trial. Lancet Oncol. 24, 1134–1146 (2023).
Frentzas, S. et al. A phase 1a/1b first-in-human study (COMPASSION-01) evaluating cadonilimab in patients with advanced solid tumors. Cell Rep. Med. 4, 101242 (2023).
Keam, S. J. Cadonilimab: first approval. Drugs 82, 1333–1339 (2022).
Berezhnoy, A. et al. Development and preliminary clinical activity of PD-1-guided CTLA-4 blocking bispecific DART molecule. Cell Rep. Med. 1, 100163 (2020).
Mazor, Y. et al. Improving target cell specificity using a novel monovalent bispecific IgG design. mAbs 7, 377–389 (2015).
Dovedi, S. J. et al. Design and efficacy of a monovalent bispecific PD-1/CTLA4 antibody that enhances CTLA4 blockade on PD-1+ activated T cells. Cancer Discov. 11, 1100–1117 (2021).
Jiang, H. et al. PD-L1/LAG-3 bispecific antibody enhances tumor-specific immunity. Oncoimmunology 10, 1943180 (2021).
Kraman, M. et al. FS118, a bispecific antibody targeting LAG-3 and PD-L1, enhances T-cell activation resulting in potent antitumor activity. Clin. Cancer Res. 26, 3333–3344 (2020).
Yap, T. A. et al. A phase 1 first-in-human study of FS118, a tetravalent bispecific antibody targeting LAG-3 and PD-L1 in patients with advanced cancer and PD-L1 resistance. Clin. Cancer Res. 29, 888–898 (2023).
Hellmann, M. D. et al. Safety and immunogenicity of LY3415244, a bispecific antibody against TIM-3 and PD-L1, in patients with advanced solid tumors. Clin. Cancer Res. 27, 2773–2781 (2021).
Luke, J. J. et al. The PD-1- and LAG-3-targeting bispecific molecule tebotelimab in solid tumors and hematologic cancers: a phase 1 trial. Nat. Med. 29, 2814–2824 (2023).
Gulley, J. L. et al. Dual inhibition of TGF-β and PD-L1: a novel approach to cancer treatment. Mol. Oncol. 16, 2117–2134 (2022).
Lan, Y. et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-β. Sci. Transl. Med. 10, eaan5488 (2018).
Lind, H. et al. Dual targeting of TGF-β and PD-L1 via a bifunctional anti-PD-L1/TGF-βRII agent: status of preclinical and clinical advances. J. Immunother. Cancer 8, e000433 (2020).
Feng, J. et al. SHR-1701, a bifunctional fusion protein targeting PD-L1 and TGFβ, for recurrent or metastatic cervical cancer: a clinical expansion cohort of a phase I study. Clin. Cancer Res. 28, 5297–5305 (2022).
Yi, M. et al. The construction, expression, and enhanced anti-tumor activity of YM101: a bispecific antibody simultaneously targeting TGF-β and PD-L1. J. Hematol. Oncol. 14, 27 (2021).
Yi, M. et al. TGF-β: a novel predictor and target for anti-PD-1/PD-L1 therapy. Front. Immunol. 13, 1061394 (2022).
Li, S. et al. Cancer immunotherapy via targeted TGF-β signalling blockade in TH cells. Nature 587, 121–125 (2020).
Fernandes, R. A. et al. Immune receptor inhibition through enforced phosphatase recruitment. Nature 586, 779–784 (2020).
Ren, J. et al. Induced CD45 proximity potentiates natural killer cell receptor antagonism. ACS Synth. Biol. 11, 3426–3439 (2022).
Gammelgaard, O. L. et al. Targeting two distinct epitopes on human CD73 with a bispecific antibody improves anticancer activity. J. Immunother. Cancer 10, e004554 (2022).
Xu, Y. et al. An engineered IL15 cytokine mutein fused to an anti-PD1 improves intratumoral T-cell function and antitumor immunity. Cancer Immunol. Res. 9, 1141–1157 (2021).
Shen, S. et al. Engineered IL-21 cytokine muteins fused to anti-PD-1 antibodies can improve CD8+ T cell function and anti-tumor immunity. Front. Immunol. 11, 832 (2020).
Li, Y. et al. Targeting IL-21 to tumor-reactive T cells enhances memory T cell responses and anti-PD-1 antibody therapy. Nat. Commun. 12, 951 (2021).
Ren, Z. et al. Selective delivery of low-affinity IL-2 to PD-1+ T cells rejuvenates antitumor immunity with reduced toxicity. J. Clin. Invest. 132, e153604 (2022).
Codarri Deak, L. et al. PD-1-cis IL-2R agonism yields better effectors from stem-like CD8+ T cells. Nature 610, 161–172 (2022). This article describes how PD1 targeting of IL-2 results in the generation of a unique T cell subset.
Shen, J. et al. An engineered concealed IL-15-R elicits tumor-specific CD8+ T cell responses through PD-1-cis delivery. J. Exp. Med. 219, e20220745 (2022).
Sulzmaier, F. J. et al. INBRX-120, a CD8α-targeted detuned IL-2 that selectively expands and activates tumoricidal effector cells for safe and durable in vivo responses. J. Immunother. Cancer 11, e006116 (2023).
Wu, W., Chia, T., Lu, J. et al. IL-2Rα-biased agonist enhances antitumor immunity by invigorating tumor-infiltrating CD25+CD8+ T cells. Nat. Cancer 4, 1309–1325 (2023).
Goebeler, M. E. & Bargou, R. C. T cell-engaging therapies—BiTEs and beyond. Nat. Rev. Clin. Oncol. 17, 418–434 (2020).
van de Donk, N. & Zweegman, S. T-cell-engaging bispecific antibodies in cancer. Lancet 402, 142–158 (2023).
Seimetz, D., Lindhofer, H. & Bokemeyer, C. Development and approval of the trifunctional antibody catumaxomab (anti-EpCAM × anti-CD3) as a targeted cancer immunotherapy. Cancer Treat. Rev. 36, 458–467 (2010). This review describes the development of catumaxomab, the first approved bsAb.
Bargou, R. et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science 321, 974–977 (2008). This article presents first clinical proof-of-concept data for blinatumomab, the second approved bsAb.
Einsele, H. et al. The BiTE (bispecific T-cell engager) platform: development and future potential of a targeted immuno-oncology therapy across tumor types. Cancer 126, 3192–3201 (2020).
Bacac, M. et al. CD20-TCB with obinutuzumab pretreatment as next-generation treatment of hematologic malignancies. Clin. Cancer Res. 24, 4785–4797 (2018).
Bacac, M. et al. A novel carcinoembryonic antigen T-cell bispecific antibody (CEA TCB) for the treatment of solid tumors. Clin. Cancer Res. 22, 3286–3297 (2016).
Budde, L. E. et al. Safety and efficacy of mosunetuzumab, a bispecific antibody, in patients with relapsed or refractory follicular lymphoma: a single-arm, multicentre, phase 2 study. Lancet Oncol. 23, 1055–1065 (2022).
Hindie, E. Teclistamab in relapsed or refractory multiple myeloma. N. Engl. J. Med. 387, 1721 (2022). This article describes the pivotal clinical data resulting in the approval of teclistamab.
Dickinson, M. J. et al. Glofitamab for relapsed or refractory diffuse large B-cell lymphoma. N. Engl. J. Med. 387, 2220–2231 (2022). This article describes the pivotal clinical data resulting in the approval of glofitamab.
Thieblemont, C. et al. Epcoritamab, a novel, subcutaneous CD3 × CD20 bispecific T-cell-engaging antibody, in relapsed or refractory large B-cell lymphoma: dose expansion in a phase I/II trial. J. Clin. Oncol. 41, 2238–2247 (2022). This article describes the pivotal clinical data resulting in the approval of epcoritamab.
Chari, A. et al. Talquetamab, a T-cell-redirecting GPRC5D bispecific antibody for multiple myeloma. N. Engl. J. Med. 387, 2232–2244 (2022). This article describes the pivotal clinical data resulting in the approval of talquetamab.
Lesokhin, A. M. et al. Elranatamab in relapsed or refractory multiple myeloma: phase 2 MagnetisMM-3 trial results. Nat. Med. https://doi.org/10.1038/s41591-023-02528-9 (2023). This article describes the pivotal clinical data resulting in the approval of elrantamab.
Bannerji, R. et al. Odronextamab, a human CD20 × CD3 bispecific antibody in patients with CD20-positive B-cell malignancies (ELM-1): results from the relapsed or refractory non-Hodgkin lymphoma cohort in a single-arm, multicentre, phase 1 trial. Lancet Haematol. 9, e327–e339 (2022).
Weinstock, M. et al. Complete responses to odronextamab in two patients with diffuse large B-cell lymphoma refractory to chimeric antigen receptor T-cell therapy. Br. J. Haematol. 199, 366–370 (2022).
Rentsch, V. et al. Glofitamab treatment in relapsed or refractory DLBCL after CAR T-cell therapy. Cancers 14, 2516 (2022).
Engelberts, P. J. et al. DuoBody-CD3 × CD20 induces potent T-cell-mediated killing of malignant B cells in preclinical models and provides opportunities for subcutaneous dosing. eBioMedicine 52, 102625 (2020).
Gurumurthi, A., Westin, J. R. & Subklewe, M. The race is on: bispecifics vs CAR T-cells in B-cell lymphoma. Blood Adv. 7, 5713–5716 (2023).
Liddy, N. et al. Monoclonal TCR-redirected tumor cell killing. Nat. Med. 18, 980–987 (2012).
Nathan, P. et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N. Engl. J. Med. 385, 1196–1206 (2021). This article describes the pivotal clinical data resulting in the approval of tebentafusp.
Berman, D. M. & Bell, J. I. Redirecting polyclonal T cells against cancer with soluble T cell receptors. Clin. Cancer Res. 29, 697–704 (2022).
Kingwell, K. T cell receptor therapeutics hit the immuno-oncology stage. Nat. Rev. Drug. Discov. 21, 321–323 (2022).
Augsberger, C. et al. Targeting intracellular WT1 in AML with a novel RMF–peptide–MHC-specific T-cell bispecific antibody. Blood 138, 2655–2669 (2021).
Douglass, J. et al. Bispecific antibodies targeting mutant RAS neoantigens. Sci. Immunol. 6, eabd5515 (2021).
Hsiue, E. H. et al. Targeting a neoantigen derived from a common TP53 mutation. Science 371, eabc8697 (2021). This article describes a BiTE targeting a p53 mutant-derived MHC-presented neoantigen.
Moritz, A. et al. High-throughput peptide–MHC complex generation and kinetic screenings of TCRs with peptide-receptive HLA-A*02:01 molecules. Sci. Immunol. 4, eaav0860 (2019).
Yang, X. et al. Facile repurposing of peptide–MHC-restricted antibodies for cancer immunotherapy. Nat. Biotechnol. 41, 932–943 (2023).
Hattori, T. et al. Creating MHC-restricted neoantigens with covalent inhibitors that can be targeted by immune therapy. Cancer Discov. 13, 132–145 (2023).
Zhang, Z. et al. A covalent inhibitor of K-RasG12C induces MHC class I presentation of haptenated peptide neoepitopes targetable by immunotherapy. Cancer Cell 40, 1060–1069.e7 (2022). Together with Hattori et al. (2023), this article shows how covalent K-Ras inhibitors can be recognized by a BiTE.
Leclercq, G. et al. Novel strategies for the mitigation of cytokine release syndrome induced by T cell engaging therapies with a focus on the use of kinase inhibitors. Oncoimmunology 11, 2083479 (2022).
Leclercq-Cohen, G. et al. Dissecting the mechanisms underlying the cytokine release syndrome (CRS) mediated by T-cell bispecific antibodies. Clin. Cancer Res. 29, 4449–4463 (2023).
Mandikian, D. et al. Relative target affinities of T-cell-dependent bispecific antibodies determine biodistribution in a solid tumor mouse model. Mol. Cancer Ther. 17, 776–785 (2018).
Staflin, K. et al. Target arm affinities determine preclinical efficacy and safety of anti-HER2/CD3 bispecific antibody. JCI Insight 5, e133757 (2020).
Zuch de Zafra, C. L. et al. Targeting multiple myeloma with AMG 424, a novel anti-CD38/CD3 bispecific T-cell-recruiting antibody optimized for cytotoxicity and cytokine release. Clin. Cancer Res. 25, 3921–3933 (2019).
Trinklein, N. D. et al. Efficient tumor killing and minimal cytokine release with novel T-cell agonist bispecific antibodies. mAbs 11, 639–652 (2019). This article illustrates the advantage of applying low-affinity CD3 antibodies in TCEs.
Dang, K. et al. Attenuating CD3 affinity in a PSMA × CD3 bispecific antibody enables killing of prostate tumor cells with reduced cytokine release. J. Immunother. Cancer 9, e002488 (2021).
Liu, C. Y. et al. Structure-based engineering of a novel CD3ε-targeting antibody for reduced polyreactivity. mAbs 15, 2189974 (2023).
Pouleau, B. et al. Pre-clinical characterization of ISB 1342, a CD38 × CD3 T-cell engager for relapsed/refractory multiple myeloma. Blood 142, 260–273 (2023).
Hinrichs, C. S. & Restifo, N. P. Reassessing target antigens for adoptive T-cell therapy. Nat. Biotechnol. 31, 999–1008 (2013). This review outlines the challenges in identifying suitable target antigens for T cell-based therapies.
Arvedson, T., Bailis, J. M., Urbig, T. & Stevens, J. L. Considerations for design, manufacture, and delivery for effective and safe T-cell engager therapies. Curr. Opin. Biotechnol. 78, 102799 (2022).
Wang, J. et al. Characterization of anti-CD79b/CD3 bispecific antibody, a potential therapy for B cell malignancies. Cancer Immunol. Immunother. 72, 493–507 (2023).
Seckinger, A. et al. Target expression, generation, preclinical activity, and pharmacokinetics of the BCMA-T cell bispecific antibody EM801 for multiple myeloma treatment. Cancer Cell 31, 396–410 (2017).
Hipp, S. et al. A novel BCMA/CD3 bispecific T-cell engager for the treatment of multiple myeloma induces selective lysis in vitro and in vivo. Leukemia 31, 1743–1751 (2017).
Li, J. et al. Membrane-proximal epitope facilitates efficient T cell synapse formation by anti-FcRH5/CD3 and is a requirement for myeloma cell killing. Cancer Cell 31, 383–395 (2017).
Parker, K. R. et al. Single-cell analyses identify brain mural cells expressing CD19 as potential off-tumor targets for CAR-T immunotherapies. Cell 183, 126–142.e17 (2020).
Nair-Gupta, P. et al. A novel C2 domain binding CD33 × CD3 bispecific antibody with potent T-cell redirection activity against acute myeloid leukemia. Blood Adv. 4, 906–919 (2020).
Krupka, C. et al. CD33 target validation and sustained depletion of AML blasts in long-term cultures by the bispecific T-cell-engaging antibody AMG 330. Blood 123, 356–365 (2014).
Al-Hussaini, M. et al. Targeting CD123 in acute myeloid leukemia using a T-cell-directed dual-affinity retargeting platform. Blood 127, 122–131 (2016).
Chichili, G. R. et al. A CD3 × CD123 bispecific DART for redirecting host T cells to myelogenous leukemia: preclinical activity and safety in nonhuman primates. Sci. Transl. Med. 7, 289ra282 (2015).
Ravandi, F. et al. Phase 1 study of vibecotamab identifies an optimized dose for treatment of relapsed/refractory acute myeloid leukemia. Blood Adv. 7, 6492–6505 (2023).
Arvedson, T. et al. Targeting solid tumors with bispecific T cell engager immune therapy. Annu. Rev. Canc Biol. 6, 17–34 (2022). This review summarizes the challenges faced when developing TCEs for treatment of solid tumours.
Drago, J. Z., Modi, S. & Chandarlapaty, S. Unlocking the potential of antibody–drug conjugates for cancer therapy. Nat. Rev. Clin. Oncol. 18, 327–344 (2021).
Rius Ruiz, I. et al. p95HER2-T cell bispecific antibody for breast cancer treatment. Sci. Transl. Med. 10, eaat1445 (2018).
Choi, B. D. et al. Systemic administration of a bispecific antibody targeting EGFRvIII successfully treats intracerebral glioma. Proc. Natl Acad. Sci. USA 110, 270–275 (2013).
Iurlaro, R. et al. A novel EGFRvIII T-cell bispecific antibody for the treatment of glioblastoma. Mol. Cancer Ther. 21, 1499–1509 (2022).
Sternjak, A. et al. Preclinical assessment of AMG 596, a bispecific T-cell engager (BiTE) immunotherapy targeting the tumor-specific antigen EGFRvIII. Mol. Cancer Ther. 20, 925–933 (2021).
Crawford, A. et al. A mucin 16 bispecific T cell-engaging antibody for the treatment of ovarian cancer. Sci. Transl. Med. 11, eaau7534 (2019).
Zhu, G. et al. Targeting CLDN18.2 by CD3 bispecific and ADC modalities for the treatments of gastric and pancreatic cancer. Sci. Rep. 9, 8420 (2019).
Paz-Ares, L. et al. Tarlatamab, a first-in-class DLL3-targeted bispecific T cell engager, in recurrent small-cell lung cancer: an open-label, phase 1 study. J. Clin. Oncol. 41, 2893–2903 (2023).
Lin, T. Y., Park, J. A., Long, A., Guo, H. F. & Cheung, N. V. Novel potent anti-STEAP1 bispecific antibody to redirect T cells for cancer immunotherapy. J. Immunother. Cancer 9, e003114 (2021).
Ahn, M. J. et al. Tarlatamab for patients with previously treated small-cell lung cancer. N. Engl. J. Med. 389, 2063–2075 (2023). This article provides compelling clinical evidence for the anti-tumour efficacy of TCEs in solid tumour indications.
Kelly, W. K. et al. Xaluritamig, a STEAP1 × CD3 XmAb 2 + 1 immune therapy for metastatic castration-resistant prostate cancer: results from dose exploration in a first-in-human study. Cancer Discov. 14, 76–89 (2023).
Nolan-Stevaux, O. et al. AMG 509 (Xaluritamig), an anti-STEAP1 XmAb 2 + 1 T-cell redirecting immune therapy with avidity-dependent activity against prostate cancer. Cancer Discov. 14, 90–103 (2023).
Slaga, D. et al. Avidity-based binding to HER2 results in selective killing of HER2-overexpressing cells by anti-HER2/CD3. Sci. Transl. Med. 10, eaat5775 (2018). This article illustrates the benefit of making use of avidity-mediated selectivity gain for TCEs.
Avanzino, B. C. et al. A T-cell engaging bispecific antibody with a tumor-selective bivalent folate receptor α binding arm for the treatment of ovarian cancer. Oncoimmunology 11, 2113697 (2022).
Dicara, D. M. et al. Development of T-cell engagers selective for cells co-expressing two antigens. mAbs 14, 2115213 (2022).
Tapia-Galisteo, A. et al. Trispecific T-cell engagers for dual tumor-targeting of colorectal cancer. Oncoimmunology 11, 2034355 (2022).
Braciak, T. A. et al. Dual-targeting triplebody 33-16-123 (SPM-2) mediates effective redirected lysis of primary blasts from patients with a broad range of AML subtypes in combination with natural killer cells. Oncoimmunology 7, e1472195 (2018).
Roskopf, C. C. et al. Dual-targeting triplebody 33-3-19 mediates selective lysis of biphenotypic CD19+CD33+ leukemia cells. Oncotarget 7, 22579–22589 (2016).
Zhao, L. et al. A novel CD19/CD22/CD3 trispecific antibody enhances therapeutic efficacy and overcomes immune escape against B-ALL. Blood 140, 1790–1802 (2022).
Mensurado, S., Blanco-Dominguez, R. & Silva-Santos, B. The emerging roles of γδ T cells in cancer immunotherapy. Nat. Rev. Clin. Oncol. 20, 178–191 (2023).
Saura-Esteller, J. et al. γδ T-cell based cancer immunotherapy: past–present–future. Front. Immunol. 13, 915837 (2022).
Lai, A. Y. et al. Cutting edge: bispecific γδ T cell engager containing heterodimeric BTN2A1 and BTN3A1 promotes targeted activation of Vγ9Vδ2+ T cells in the presence of costimulation by CD28 or NKG2D. J. Immunol. 209, 1475–1480 (2022).
van Diest, E. et al. TCR anti-CD3 bispecific molecules (GABs) as novel immunotherapeutic compounds. J. Immunother. Cancer 9, e003850 (2021).
Ganesan, R. et al. Selective recruitment of γδ T cells by a bispecific antibody for the treatment of acute myeloid leukemia. Leukemia 35, 2274–2284 (2021).
Oberg, H. H. et al. Novel bispecific antibodies increase γδ T-cell cytotoxicity against pancreatic cancer cells. Cancer Res. 74, 1349–1360 (2014).
de Weerdt, I. et al. A bispecific single-domain antibody boosts autologous Vγ9Vδ2-T cell responses toward CD1d in chronic lymphocytic leukemia. Clin. Cancer Res. 27, 1744–1755 (2021).
Schmittnaegel, M. et al. A new class of bifunctional major histocompatibility class I antibody fusion molecules to redirect CD8 T cells. Mol. Cancer Ther. 15, 2130–2142 (2016).
Millar, D. G. et al. Antibody-mediated delivery of viral epitopes to tumors harnesses CMV-specific T cells for cancer therapy. Nat. Biotechnol. 38, 420–425 (2020).
Sefrin, J. P. et al. Sensitization of tumors for attack by virus-specific CD8+ T-cells through antibody-mediated delivery of immunogenic T-cell epitopes. Front. Immunol. 10, 1962 (2019).
van der Wulp, W. et al. Antibody-mediated delivery of viral epitopes to redirect EBV-specific CD8+ T-cell immunity towards cancer cells. Cancer Gene Ther. 31, 58–68 (2023).
Cruz, J. W. et al. A novel bispecific antibody platform to direct complement activity for efficient lysis of target cells. Sci. Rep. 9, 12031 (2019).
Oostindie, S. C. et al. Logic-gated antibody pairs that selectively act on cells co-expressing two antigens. Nat. Biotechnol. 40, 1509–1519 (2022).
Peng, H. et al. ROR1-targeting switchable CAR-T cells for cancer therapy. Oncogene 41, 4104–4114 (2022).
Wermke, M. et al. Proof of concept for a rapidly switchable universal CAR-T platform with UniCAR-T-CD123 in relapsed/refractory AML. Blood 137, 3145–3148 (2021).
Bachmann, M. The UniCAR system: a modular CAR T cell approach to improve the safety of CAR T cells. Immunol. Lett. 211, 13–22 (2019).
Karches, C. H. et al. Bispecific antibodies enable synthetic agonistic receptor-transduced T cells for tumor immunotherapy. Clin. Cancer Res. 25, 5890–5900 (2019). This article shows the use of bsAbs to engage adoptively transferred CAR-T cells.
Cho, J. H., Collins, J. J. & Wong, W. W. Universal chimeric antigen receptors for multiplexed and logical control of T cell responses. Cell 173, 1426–1438.e11 (2018).
Lee, Y. G. et al. Use of a single CAR T cell and several bispecific adapters facilitates eradication of multiple antigenically different solid tumors. Cancer Res. 79, 387–396 (2019).
Park, J. A. & Cheung, N. V. Overcoming tumor heterogeneity by ex vivo arming of T cells using multiple bispecific antibodies. J. Immunother. Cancer 10, e003771 (2022).
Demaria, O. et al. Harnessing innate immunity in cancer therapy. Nature 574, 45–56 (2019).
Delidakis, G., Kim, J. E., George, K. & Georgiou, G. Improving antibody therapeutics by manipulating the Fc domain: immunological and structural considerations. Annu. Rev. Biomed. Eng. 24, 249–274 (2022).
Shimasaki, N., Jain, A. & Campana, D. NK cells for cancer immunotherapy. Nat. Rev. Drug. Discov. 19, 200–218 (2020).
Whalen, K. A. et al. Engaging natural killer cells for cancer therapy via NKG2D, CD16A and other receptors. mAbs 15, 2208697 (2023).
Reusch, U. et al. A novel tetravalent bispecific TandAb (CD30/CD16A) efficiently recruits NK cells for the lysis of CD30+ tumor cells. mAbs 6, 728–739 (2014).
Bartlett, N. L. et al. A phase 1b study of AFM13 in combination with pembrolizumab in patients with relapsed or refractory Hodgkin lymphoma. Blood 136, 2401–2409 (2020).
Kerbauy, L. N. et al. Combining AFM13, a bispecific CD30/CD16 antibody, with cytokine-activated blood and cord blood-derived NK cells facilitates CAR-like responses against CD30+ malignancies. Clin. Cancer Res. 27, 3744–3756 (2021).
Ellwanger, K. et al. Redirected optimized cell killing (ROCK®): a highly versatile multispecific fit-for-purpose antibody platform for engaging innate immunity. mAbs 11, 899–918 (2019).
Wingert, S. et al. Preclinical evaluation of AFM24, a novel CD16A-specific innate immune cell engager targeting EGFR-positive tumors. mAbs 13, 1950264 (2021).
Kakiuchi-Kiyota, S. et al. A BCMA/CD16A bispecific innate cell engager for the treatment of multiple myeloma. Leukemia 36, 1006–1014 (2022).
Gantke, T. et al. Trispecific antibodies for CD16A-directed NK cell engagement and dual-targeting of tumor cells. Protein Eng. Des. Sel. 30, 673–684 (2017).
Harwardt, J. et al. Generation of a symmetrical trispecific NK cell engager based on a two-in-one antibody. Front. Immunol. 14, 1170042 (2023).
Kellner, C. et al. Tumor cell lysis and synergistically enhanced antibody-dependent cell-mediated cytotoxicity by NKG2D engagement with a bispecific immunoligand targeting the HER2 antigen. Biol. Chem. 403, 545–556 (2022).
Gauthier, L. et al. Multifunctional natural killer cell engagers targeting NKp46 trigger protective tumor immunity. Cell 177, 1701–1713.e16 (2019). This article introduces a novel NKp46-based class of natural killer cell-engaging bsAbs.
Pekar, L. et al. Affinity maturation of B7-H6 translates into enhanced NK cell-mediated tumor cell lysis and improved proinflammatory cytokine release of bispecific immunoligands via NKp30 engagement. J. Immunol. 206, 225–236 (2021).
Klausz, K. et al. Multifunctional NK cell-engaging antibodies targeting EGFR and NKp30 elicit efficient tumor cell killing and proinflammatory cytokine release. J. Immunol. 209, 1724–1735 (2022).
Demaria, O. et al. Antitumor immunity induced by antibody-based natural killer cell engager therapeutics armed with not-α IL-2 variant. Cell Rep. Med. 3, 100783 (2022).
Li, B. et al. CD89-mediated recruitment of macrophages via a bispecific antibody enhances anti-tumor efficacy. Oncoimmunology 7, e1380142 (2017).
Heemskerk, N. et al. Augmented antibody-based anticancer therapeutics boost neutrophil cytotoxicity. J. Clin. Invest. 131, e134680 (2021).
Vukovic, N. et al. A human IgE bispecific antibody shows potent cytotoxic capacity mediated by monocytes. J. Biol. Chem. 298, 102153 (2022).
Maute, R., Xu, J. & Weissman, I. L. CD47–SIRPα-targeted therapeutics: status and prospects. Immunooncol. Technol. 13, 100070 (2022).
Dheilly, E. et al. Selective blockade of the ubiquitous checkpoint receptor CD47 is enabled by dual-targeting bispecific antibodies. Mol. Ther. 25, 523–533 (2017).
Buatois, V. et al. Preclinical development of a bispecific antibody that safely and effectively targets CD19 and CD47 for the treatment of B-cell lymphoma and leukemia. Mol. Cancer Ther. 17, 1739–1751 (2018).
Hatterer, E. et al. Co-engaging CD47 and CD19 with a bispecific antibody abrogates B-cell receptor/CD19 association leading to impaired B-cell proliferation. mAbs 11, 322–334 (2019).
Liu, B. et al. Elimination of tumor by CD47/PD-L1 dual-targeting fusion protein that engages innate and adaptive immune responses. mAbs 10, 315–324 (2018).
Chen, S. H. et al. Dual checkpoint blockade of CD47 and PD-L1 using an affinity-tuned bispecific antibody maximizes antitumor immunity. J. Immunother. Cancer 9, e003464 (2021).
Tahk, S. et al. SIRPα–αCD123 fusion antibodies targeting CD123 in conjunction with CD47 blockade enhance the clearance of AML-initiating cells. J. Hematol. Oncol. 14, 155 (2021).
Mayes, P. A., Hance, K. W. & Hoos, A. The promise and challenges of immune agonist antibody development in cancer. Nat. Rev. Drug. Discov. 17, 509–527 (2018).
Kraehenbuehl, L., Weng, C. H., Eghbali, S., Wolchok, J. D. & Merghoub, T. Enhancing immunotherapy in cancer by targeting emerging immunomodulatory pathways. Nat. Rev. Clin. Oncol. 19, 37–50 (2022).
Yu, X. et al. Reducing affinity as a strategy to boost immunomodulatory antibody agonism. Nature 614, 539–547 (2023).
Segal, N. H. et al. Results from an integrated safety analysis of urelumab, an agonist anti-CD137 monoclonal antibody. Clin. Cancer Res. 23, 1929–1936 (2017).
Muller, D. Targeting co-stimulatory receptors of the TNF superfamily for cancer immunotherapy. BioDrugs 37, 21–33 (2023).
Hinner, M. J. et al. Tumor-localized costimulatory T-cell engagement by the 4-1BB/HER2 bispecific antibody-anticalin fusion PRS-343. Clin. Cancer Res. 25, 5878–5889 (2019).
Claus, C. et al. Tumor-targeted 4-1BB agonists for combination with T cell bispecific antibodies as off-the-shelf therapy. Sci. Transl. Med. 11, eaav5989 (2019). Together with Hinner et al. (2019), this article provides examples of potent 4-1BB × CD137 targeting co-stimulatory bsAbs for combination immunotherapy.
Melero, I. et al. A first-in-human study of the fibroblast activation protein-targeted, 4-1BB agonist RO7122290 in patients with advanced solid tumors. Sci. Transl. Med. 15, eabp9229 (2023).
Esensten, J. H., Bluestone, J. A. & Lim, W. A. Engineering therapeutic T cells: from synthetic biology to clinical trials. Annu. Rev. Pathol. 12, 305–330 (2017).
Hunig, T. The rise and fall of the CD28 superagonist TGN1412 and its return as TAB08: a personal account. FEBS J. 283, 3325–3334 (2016).
Otz, T., Grosse-Hovest, L., Hofmann, M., Rammensee, H. G. & Jung, G. A bispecific single-chain antibody that mediates target cell-restricted, supra-agonistic CD28 stimulation and killing of lymphoma cells. Leukemia 23, 71–77 (2009).
Skokos, D. et al. A class of costimulatory CD28-bispecific antibodies that enhance the antitumor activity of CD3-bispecific antibodies. Sci. Transl. Med. 12, eaaw7888 (2020). This article provides an example of a potent CD28-targeting co-stimulatory bsAb for combination immunotherapy.
Wei, J. et al. CD22-targeted CD28 bispecific antibody enhances antitumor efficacy of odronextamab in refractory diffuse large B cell lymphoma models. Sci. Transl. Med. 14, eabn1082 (2022).
Waite, J. C. et al. Tumor-targeted CD28 bispecific antibodies enhance the antitumor efficacy of PD-1 immunotherapy. Sci. Transl. Med. 12, eaba2325 (2020).
Enell Smith, K., Deronic, A., Hagerbrand, K., Norlen, P. & Ellmark, P. Rationale and clinical development of CD40 agonistic antibodies for cancer immunotherapy. Expert. Opin. Biol. Ther. 21, 1635–1646 (2021).
Ma, D. Y. & Clark, E. A. The role of CD40 and CD154/CD40L in dendritic cells. Semin. Immunol. 21, 265–272 (2009).
Hagerbrand, K. et al. Bispecific antibodies targeting CD40 and tumor-associated antigens promote cross-priming of T cells resulting in an antitumor response superior to monospecific antibodies. J. Immunother. Cancer 10, e005018 (2022).
Luke, J. J. et al. Phase I study of ABBV-428, a mesothelin-CD40 bispecific, in patients with advanced solid tumors. J. Immunother. Cancer 9, e002015 (2021).
Ye, S. et al. A bispecific molecule targeting CD40 and tumor antigen mesothelin enhances tumor-specific immunity. Cancer Immunol. Res. 7, 1864–1875 (2019).
Sum, E. et al. Fibroblast activation protein α-targeted CD40 agonism abrogates systemic toxicity and enables administration of high doses to induce effective antitumor immunity. Clin. Cancer Res. 27, 4036–4053 (2021).
Rigamonti, N. et al. A multispecific anti-CD40 DARPin construct induces tumor-selective CD40 activation and tumor regression. Cancer Immunol. Res. 10, 626–640 (2022).
Salomon, R. et al. Bispecific antibodies increase the therapeutic window of CD40 agonists through selective dendritic cell targeting. Nat. Cancer 3, 287–302 (2022).
Gaspar, M. et al. CD137/OX40 bispecific antibody induces potent antitumor activity that is dependent on target coengagement. Cancer Immunol. Res. 8, 781–793 (2020).
Muik, A. et al. DuoBody-CD40 × 4-1BB induces dendritic-cell maturation and enhances T-cell activation through conditional CD40 and 4-1BB agonist activity. J. Immunother. Cancer 10, e004322 (2022).
Peper-Gabriel, J. K. et al. The PD-L1/4-1BB bispecific antibody-anticalin fusion protein PRS-344/S095012 elicits strong T-cell stimulation in a tumor-localized manner. Clin. Cancer Res. 28, 3387–3399 (2022).
Muik, A. et al. An Fc-inert PD-L1 × 4-1BB bispecific antibody mediates potent anti-tumor immunity in mice by combining checkpoint inhibition and conditional 4-1BB co-stimulation. Oncoimmunology 11, 2030135 (2022).
Warmuth, S. et al. Engineering of a trispecific tumor-targeted immunotherapy incorporating 4-1BB co-stimulation and PD-L1 blockade. Oncoimmunology 10, 2004661 (2021).
Jeong, S. et al. Novel anti-4-1BB × PD-L1 bispecific antibody augments anti-tumor immunity through tumor-directed T-cell activation and checkpoint blockade. J. Immunother. Cancer 9, e002428 (2021).
Lakins, M. A. et al. FS222, a CD137/PD-L1 tetravalent bispecific antibody, exhibits low toxicity and antitumor activity in colorectal cancer models. Clin. Cancer Res. 26, 4154–4167 (2020).
Geuijen, C. et al. A human CD137 × PD-L1 bispecific antibody promotes anti-tumor immunity via context-dependent T cell costimulation and checkpoint blockade. Nat. Commun. 12, 4445 (2021).
Ramaswamy, M. et al. Immunomodulation of T- and NK-cell responses by a bispecific antibody targeting CD28 homolog and PD-L1. Cancer Immunol. Res. 10, 200–214 (2022).
Kvarnhammar, A. M. et al. The CTLA-4 × OX40 bispecific antibody ATOR-1015 induces anti-tumor effects through tumor-directed immune activation. J. Immunother. Cancer 7, 103 (2019).
Vitale, L. A. et al. Development of CDX-527: a bispecific antibody combining PD-1 blockade and CD27 costimulation for cancer immunotherapy. Cancer Immunol. Immunother. 69, 2125–2137 (2020).
Qiao, Y. et al. Cancer immune therapy with PD-1-dependent CD137 co-stimulation provides localized tumour killing without systemic toxicity. Nat. Commun. 12, 6360 (2021).
Chan, S. et al. An anti-PD-1-GITR-L bispecific agonist induces GITR clustering-mediated T cell activation for cancer immunotherapy. Nat. Cancer 3, 337–354 (2022).
Chiu, D. et al. A PSMA-targeting CD3 bispecific antibody induces antitumor responses that are enhanced by 4-1BB costimulation. Cancer Immunol. Res. 8, 596–608 (2020).
Yao, Y., Hu, Y. & Wang, F. Trispecific antibodies for cancer immunotherapy. Immunology 169, 389–399 (2023).
Tapia-Galisteo, A., Compte, M., Alvarez-Vallina, L. & Sanz, L. When three is not a crowd: trispecific antibodies for enhanced cancer immunotherapy. Theranostics 13, 1028–1041 (2023).
Wu, L. et al. Trispecific antibodies enhance the therapeutic efficacy of tumor-directed T cells through T cell receptor co-stimulation. Nat. Cancer 1, 86–98 (2020). This article provides an example of a trispecific TCE with integrated co-stimulation.
Seung, E. et al. A trispecific antibody targeting HER2 and T cells inhibits breast cancer growth via CD4 cells. Nature 603, 328–334 (2022).
Lucchi, R., Bentanachs, J. & Oller-Salvia, B. The masking game: design of activatable antibodies and mimetics for selective therapeutics and cell control. ACS Cent. Sci. 7, 724–738 (2021).
Metz, S. et al. Bispecific antibody derivatives with restricted binding functionalities that are activated by proteolytic processing. Protein Eng. Des. Sel. 25, 571–580 (2012).
Autio, K. A., Boni, V., Humphrey, R. W. & Naing, A. Probody therapeutics: an emerging class of therapies designed to enhance on-target effects with reduced off-tumor toxicity for use in immuno-oncology. Clin. Cancer Res. 26, 984–989 (2020).
Panchal, A. et al. COBRA: a highly potent conditionally active T cell engager engineered for the treatment of solid tumors. mAbs 12, 1792130 (2020).
Dettling, D. E. et al. Regression of EGFR positive established solid tumors in mice with the conditionally active T cell engager TAK-186. J. Immunother. Cancer 10, e004336 (2022).
Geiger, M. et al. Protease-activation using anti-idiotypic masks enables tumor specificity of a folate receptor 1-T cell bispecific antibody. Nat. Commun. 11, 3196 (2020). This article provides an example of a protease-activated TCE.
Boustany, L. M. et al. A probody T cell-engaging bispecific antibody targeting EGFR and CD3 inhibits colon cancer growth with limited toxicity. Cancer Res. 82, 4288–4298 (2022).
Cattaruzza, F. et al. Precision-activated T-cell engagers targeting HER2 or EGFR and CD3 mitigate on-target, off-tumor toxicity for immunotherapy in solid tumors. Nat. Cancer 4, 485–501 (2023).
Banaszek, A. et al. On-target restoration of a split T cell-engaging antibody for precision immunotherapy. Nat. Commun. 10, 5387 (2019). This article introduces the concept of on-target assembly of split T cell-engaging antibodies.
Dickopf, S. et al. Prodrug-activating chain exchange (PACE) converts targeted prodrug derivatives to functional bi- or multispecific antibodies. Biol. Chem. 403, 495–508 (2022).
Vasic, V. et al. Targeted chain-exchange-mediated reconstitution of a split type-I cytokine for conditional immunotherapy. mAbs 15, 2245111 (2023).
Kamata-Sakurai, M. et al. Antibody to CD137 activated by extracellular adenosine triphosphate is tumor selective and broadly effective in vivo without systemic immune activation. Cancer Discov. 11, 158–175 (2021). This article shows how extracellular ATP can serve to activate antibody binding and activity in the TME.
Wells, J. A. & Kumru, K. Extracellular targeted protein degradation: an emerging modality for drug discovery. Nat. Rev. Drug. Discov. https://doi.org/10.1038/s41573-023-00833-z (2023). This Review provides an overview about the emerging area of antibody-based protein degradation approaches.
Pance, K. et al. Modular cytokine receptor-targeting chimeras for targeted degradation of cell surface and extracellular proteins. Nat. Biotechnol. 41, 273–281 (2023).
Siepe, D. H., Picton, L. K. & Garcia, K. C. Receptor elimination by E3 ubiquitin ligase recruitment (REULR): a targeted protein degradation toolbox. ACS Synth. Biol. 12, 1081–1093 (2023).
Marei, H. et al. Antibody targeting of E3 ubiquitin ligases for receptor degradation. Nature 610, 182–189 (2022).
Zhao, P. et al. Enhanced anti-angiogenetic effect of transferrin receptor-mediated delivery of VEGF-trap in a glioblastoma mouse model. mAbs 14, 2057269 (2022).
Gramespacher, J. A., Cotton, A. D., Burroughs, P. W. W., Seiple, I. B. & Wells, J. A. Roadmap for optimizing and broadening antibody-based PROTACs for degradation of cell surface proteins. ACS Chem. Biol. 17, 1259–1268 (2022).
Cotton, A. D., Nguyen, D. P., Gramespacher, J. A., Seiple, I. B. & Wells, J. A. Development of antibody-based PROTACs for the degradation of the cell-surface immune checkpoint protein PD-L1. J. Am. Chem. Soc. 143, 593–598 (2021).
Stadler, C. R. et al. Elimination of large tumors in mice by mRNA-encoded bispecific antibodies. Nat. Med. 23, 815–817 (2017).
Huang, C. et al. Lipid nanoparticle delivery system for mRNA encoding B7H3-redirected bispecific antibody displays potent antitumor effects on malignant tumors. Adv. Sci. 10, e2205532 (2023).
Heidbuechel, J. P. W. & Engeland, C. E. Oncolytic viruses encoding bispecific T cell engagers: a blueprint for emerging immunovirotherapies. J. Hematol. Oncol. 14, 63 (2021).
Fajardo, C. A. et al. Oncolytic adenoviral delivery of an EGFR-targeting T-cell engager improves antitumor efficacy. Cancer Res. 77, 2052–2063 (2017).
Speck, T. et al. Targeted BiTE expression by an oncolytic vector augments therapeutic efficacy against solid tumors. Clin. Cancer Res. 24, 2128–2137 (2018).
Wing, A. et al. Improving CART-cell therapy of solid tumors with oncolytic virus-driven production of a bispecific T-cell engager. Cancer Immunol. Res. 6, 605–616 (2018).
Yin, Y. et al. Locally secreted BiTEs complement CAR T cells by enhancing killing of antigen heterogeneous solid tumors. Mol. Ther. 30, 2537–2553 (2022).
Choi, B. D. et al. CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat. Biotechnol. 37, 1049–1058 (2019).
Liu, X. et al. Improved anti-leukemia activities of adoptively transferred T cells expressing bispecific T-cell engager in mice. Blood Cancer J. 6, e430 (2016).
Harris, K. E. et al. A bispecific antibody agonist of the IL-2 heterodimeric receptor preferentially promotes in vivo expansion of CD8 and NK cells. Sci. Rep. 11, 10592 (2021).
Yen, M. et al. Facile discovery of surrogate cytokine agonists. Cell 185, 1414–1430.e19 (2022). This article shows how single-domain antibodies can serve for the generation of cytokine mimetics.
Lipinski, B. et al. Generation and engineering of potent single domain antibody-based bispecific IL-18 mimetics resistant to IL-18BP decoy receptor inhibition. mAbs 15, 2236265 (2023).
Quijano-Rubio, A. et al. A split, conditionally active mimetic of IL-2 reduces the toxicity of systemic cytokine therapy. Nat. Biotechnol. 41, 532–540 (2023).
Silva, D. A. et al. De novo design of potent and selective mimics of IL-2 and IL-15. Nature 565, 186–191 (2019).
Acknowledgements
The authors thank all contributors, team members and collaborators involved in the field of bispecific antibodies (bsAbs) over the past decade. In the context of bsAbs, C.K. thanks P. Umana, Roche Innovation Center Zurich and all his colleagues at the Roche Innovation Centers in Zurich, Munich and Basel and Wolfgang Schaefer, Mannheim. The authors thank I. Wiesner, Roche Diagnostics GmbH, Penzberg, for help with antibody databases. The authors apologize to those authors whose work was overseen by error or could not be cited due to space limitation and the breadth of the field, or due to the unavailability of peer-reviewed manuscripts.
Author information
Authors and Affiliations
Contributions
C.K., U.B., J.M.R. and R.E.K. researched data for the article and wrote the article. All authors contributed substantially to discussion of the content and edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
C.K. and U.B. declare employment, stock ownership and patents/royalties with Roche. J.M.R. is employed by The Antibody Society, a non-profit trade association funded by corporate sponsors that develops antibody therapeutics or provides services to companies that develop antibody therapeutics, and is Editor-in-Chief of mAbs, a biomedical journal focused on topics relevant to antibody therapeutic development. R.E.K. is a consultant for Immatics, Roche, SunRock and Oncomatryx.
Peer review
Peer review information
Nature Reviews Drug Discovery thanks John R. Desjarlais and the other, anonymous, reviewer for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Klein, C., Brinkmann, U., Reichert, J.M. et al. The present and future of bispecific antibodies for cancer therapy. Nat Rev Drug Discov 23, 301–319 (2024). https://doi.org/10.1038/s41573-024-00896-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41573-024-00896-6