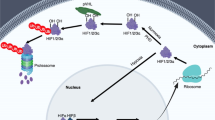
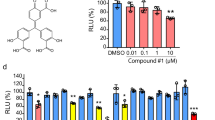
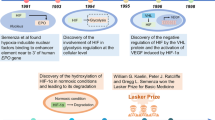
Abstract
Hypoxia-inducible factors (HIFs) are highly conserved transcription factors that are crucial for adaptation of metazoans to limited oxygen availability. Recently, HIF activation and inhibition have emerged as therapeutic targets in various human diseases. Pharmacologically desirable effects of HIF activation include erythropoiesis stimulation, cellular metabolism optimization during hypoxia and adaptive responses during ischaemia and inflammation. By contrast, HIF inhibition has been explored as a therapy for various cancers, retinal neovascularization and pulmonary hypertension. This Review discusses the biochemical mechanisms that control HIF stabilization and the molecular strategies that can be exploited pharmacologically to activate or inhibit HIFs. In addition, we examine medical conditions that benefit from targeting HIFs, the potential side effects of HIF activation or inhibition and future challenges in this field.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Taylor, C. T. & McElwain, J. C. Ancient atmospheres and the evolution of oxygen sensing via the hypoxia-inducible factor in metazoans. Physiology 25, 272–279 (2010).
Mills, D. B. et al. The last common ancestor of animals lacked the HIF pathway and respired in low-oxygen environments. eLife 7, e31176 (2018).
Semenza, G. L., Roth, P. H., Fang, H. M. & Wang, G. L. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J. Biol. Chem. 269, 23757–23763 (1994).
Liu, Y., Cox, S. R., Morita, T. & Kourembanas, S. Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5′ enhancer. Circ. Res. 77, 638–643 (1995).
Poth, J. M., Brodsky, K., Ehrentraut, H., Grenz, A. & Eltzschig, H. K. Transcriptional control of adenosine signaling by hypoxia-inducible transcription factors during ischemic or inflammatory disease. J. Mol. Med. 91, 183–193 (2013).
Eltzschig, H. K., Sitkovsky, M. V. & Robson, S. C. Purinergic signaling during inflammation. N. Engl. J. Med. 367, 2322–2333 (2012).
Prabhakar, N. R. & Semenza, G. L. Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol. Rev. 92, 967–1003 (2012).
Semenza, G. L. Oxygen sensing, homeostasis, and disease. N. Engl. J. Med. 365, 537–547 (2011).
Eckle, T. et al. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat. Med. 18, 774–782 (2012). This study reports that adenosine-dependent Per2 stabilization facilitates HIF-elicited cardiac metabolic adaptation and ischaemia tolerance.
Eltzschig, H. K. & Eckle, T. Ischemia and reperfusion — from mechanism to translation. Nat. Med. 17, 1391–1401 (2011).
Lee, P., Chandel, N. S. & Simon, M. C. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell Biol. 21, 268–283 (2020).
Smith, T. G., Robbins, P. A. & Ratcliffe, P. J. The human side of hypoxia-inducible factor. Br. J. Haematol. 141, 325–334 (2008).
Manalo, D. J. et al. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood 105, 659–669 (2005).
Neudecker, V., Yuan, X., Bowser, J. L. & Eltzschig, H. K. MicroRNAs in mucosal inflammation. J. Mol. Med. 95, 935–949 (2017).
Neudecker, V., Brodsky, K. S., Kreth, S., Ginde, A. A. & Eltzschig, H. K. Emerging roles for microRNAs in perioperative medicine. Anesthesiology 124, 489–506 (2016).
Ju, C. et al. Hypoxia-inducible factor-1alpha-dependent induction of miR122 enhances hepatic ischemia tolerance. J. Clin. Invest. 131, e140300 (2021). This paper demonstrates that miRNAs can function in feed-forward loops by repressing PHDs, thereby enhancing their transcriptional induction through HIFs and providing tissue protection during liver ischemia–reperfusion injury.
Wang, G. L. & Semenza, G. L. Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J. Biol. Chem. 268, 21513–21518 (1993).
Semenza, G. L., Nejfelt, M. K., Chi, S. M. & Antonarakis, S. E. Hypoxia-inducible nuclear factors bind to an enhancer element located 3′ to the human erythropoietin gene. Proc. Natl Acad. Sci. USA 88, 5680–5684 (1991). This study is among the first reports from the Nobel laureate Gregg Semenza to describe a novel protein as a HIF that functions as a transcriptional enhancer of EPO.
Semenza, G. L., Koury, S. T., Nejfelt, M. K., Gearhart, J. D. & Antonarakis, S. E. Cell-type-specific and hypoxia-inducible expression of the human erythropoietin gene in transgenic mice. Proc. Natl Acad. Sci. USA 88, 8725–8729 (1991).
Fandrey, J., Schodel, J., Eckardt, K. U., Katschinski, D. M. & Wenger, R. H. Now a nobel gas: oxygen. Pflug. Arch. 471, 1343–1358 (2019).
Ivan, M. et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292, 464–468 (2001). This study reports the identification of VHL tumour suppressor protein as a key molecule in the oxygen-dependent degradation of HIFα.
Jaakkola, P. et al. Targeting of HIF-alpha to the von Hippel–Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292, 468–472 (2001).
Epstein, A. C. et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107, 43–54 (2001). This study describes EGL9 as a key dioxygenase for oxygen-dependent HIF prolyl hydroxylation in C. elegans and defined isoforms of conserved HIF-PHDs in mammalian cells.
Eltzschig, H. K. et al. HIF-1-dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J. Exp. Med. 202, 1493–1505 (2005).
Eltzschig, H. K. et al. Endogenous adenosine produced during hypoxia attenuates neutrophil accumulation: coordination by extracellular nucleotide metabolism. Blood 104, 3986–3992 (2004).
Thompson, L. F. et al. Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J. Exp. Med. 200, 1395–1405 (2004).
Eltzschig, H. K. et al. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J. Exp. Med. 198, 783–796 (2003).
Wicks, E. E. & Semenza, G. L. Hypoxia-inducible factors: cancer progression and clinical translation. J. Clin. Invest. 132, e159839 (2022).
Eltzschig, H. K. & Carmeliet, P. Hypoxia and inflammation. N. Engl. J. Med. 364, 656–665 (2011).
Bowser, J. L., Lee, J. W., Yuan, X. & Eltzschig, H. K. The hypoxia–adenosine link during inflammation. J. Appl. Physiol. 123, 1303–1320 (2017).
Eckle, T. et al. HIF1A reduces acute lung injury by optimizing carbohydrate metabolism in the alveolar epithelium. PLoS Biol. 11, e1001665 (2013). This study demonstrates that HIF1α is stabilized in the lungs during ARDS, and functions in endogenous lung protection by optimizing glucose metabolism in alveolar epithelial cells.
Bartels, K., Grenz, A. & Eltzschig, H. K. Hypoxia and inflammation are two sides of the same coin. Proc. Natl Acad. Sci. USA 110, 18351–18352 (2013).
Karhausen, J. et al. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J. Clin. Invest. 114, 1098–1106 (2004).
Clambey, E. T. et al. Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc. Natl Acad. Sci. USA 109, E2784–E2793 (2012).
Campbell, E. L. et al. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity 40, 66–77 (2014). This study shows that infiltrating neutrophils deplete local oxygen to stabilize HIF and promote intestinal inflammation resolution in murine colitis models.
Eltzschig, H. K., Bratton, D. L. & Colgan, S. P. Targeting hypoxia signalling for the treatment of ischaemic and inflammatory diseases. Nat. Rev. Drug. Discov. 13, 852–869 (2014).
Hatfield, S. M. et al. Immunological mechanisms of the antitumor effects of supplemental oxygenation. Sci. Transl. Med. 7, 277ra230 (2015).
Lee, J. W., Ko, J., Ju, C. & Eltzschig, H. K. Hypoxia signaling in human diseases and therapeutic targets. Exp. Mol. Med. 51, 1–13 (2019).
Ong, S. G. et al. HIF-1 reduces ischaemia-reperfusion injury in the heart by targeting the mitochondrial permeability transition pore. Cardiovasc. Res. 104, 24–36 (2014).
Guo, Y. et al. Systematic review with meta-analysis: HIF-1alpha attenuates liver ischemia-reperfusion injury. Transpl. Rev. 29, 127–134 (2015).
Gao, R. Y. et al. Hypoxia-inducible factor-2alpha reprograms liver macrophages to protect against acute liver injury through the production of interleukin-6. Hepatology 71, 2105–2117 (2020).
Kapitsinou, P. P. et al. Endothelial HIF-2 mediates protection and recovery from ischemic kidney injury. J. Clin. Invest. 124, 2396–2409 (2014).
Hill, P. et al. Inhibition of hypoxia inducible factor hydroxylases protects against renal ischemia-reperfusion injury. J. Am. Soc. Nephrol. 19, 39–46 (2008).
Woods, P. S. et al. HIF-1alpha induces glycolytic reprograming in tissue-resident alveolar macrophages to promote cell survival during acute lung injury. eLife 11, e77457 (2022).
Kim, Y. I. et al. Local stabilization of hypoxia-inducible factor-1alpha controls intestinal inflammation via enhanced gut barrier function and immune regulation. Front. Immunol. 11, 609689 (2020).
Dowdell, A. S. et al. The HIF target ATG9A is essential for epithelial barrier function and tight junction biogenesis. Mol. Biol. Cell 31, 2249–2258 (2020).
Lin, A. E. et al. Role of hypoxia inducible factor-1alpha (HIF-1alpha) in innate defense against uropathogenic escherichia coli infection. PLoS Pathog. 11, e1004818 (2015).
Bhandari, T. & Nizet, V. Hypoxia-inducible factor (HIF) as a pharmacological target for prevention and treatment of infectious diseases. Infect. Dis. Ther. 3, 159–174 (2014).
Suhara, T. et al. Inhibition of the oxygen sensor PHD2 in the liver improves survival in lactic acidosis by activating the Cori cycle. Proc. Natl Acad. Sci. USA 112, 11642–11647 (2015). This study shows that PHD2 is a novel therapeutic target for lactic acidosis and indicates that pharmacological enhancement of PHD2 might benefit infectious and ischaemic diseases.
Sanghani, N. S. & Haase, V. H. Hypoxia-inducible factor activators in renal anemia: current clinical experience. Adv. Chronic Kidney Dis. 26, 253–266 (2019).
Eckardt, K. U. et al. Safety and efficacy of vadadustat for anemia in patients undergoing dialysis. N. Engl. J. Med. 384, 1601–1612 (2021). This study reports two randomized, phase III clinical trials that showed that HIF-PHDi vadadustat was non-inferior to darbepoetin alfa in cardiovascular safety and efficacy in correcting anaemia in patients with CKD undergoing dialysis.
Chertow, G. M. et al. Vadadustat in patients with anemia and non-dialysis-dependent CKD. N. Engl. J. Med. 384, 1589–1600 (2021).
Chen, N. et al. Roxadustat for anemia in patients with kidney disease not receiving dialysis. N. Engl. J. Med. 381, 1001–1010 (2019).
Chen, N. et al. Roxadustat treatment for anemia in patients undergoing long-term dialysis. N. Engl. J. Med. 381, 1011–1022 (2019). This study reports the results from a randomized and multicentre phase III clinical trial that showed that HIF-PHDi roxadustat was noninferior to parenteral epoetin alfa in correcting anaemia in Chinese patients undergoing dialysis.
Zhou, J. & Gong, K. Belzutifan: a novel therapy for von Hippel-Lindau disease. Nat. Rev. Nephrol. 18, 205–206 (2022).
Jonasch, E. et al. Belzutifan for renal cell carcinoma in von Hippel-Lindau disease. N. Engl. J. Med. 385, 2036–2046 (2021). This study reports the results from a randomized and multicentre phase III clinical trial that showed the safety and efficacy of HIF2α inhibitor belzutifan as a treatment for RCC and non-RCC neoplasms in patients with VHL disease.
Kamihara, J. et al. Belzutifan, a potent HIF2alpha inhibitor, in the pacak-zhuang syndrome. N. Engl. J. Med. 385, 2059–2065 (2021).
Choueiri, T. K. & Kaelin, W. G. Jr. Targeting the HIF2-VEGF axis in renal cell carcinoma. Nat. Med. 26, 1519–1530 (2020).
Salman, S. et al. HIF inhibitor 32-134D eradicates murine hepatocellular carcinoma in combination with anti-PD1 therapy. J. Clin. Invest. 132, e156774 (2022).
Pullamsetti, S. S., Mamazhakypov, A., Weissmann, N., Seeger, W. & Savai, R. Hypoxia-inducible factor signaling in pulmonary hypertension. J. Clin. Invest. 130, 5638–5651 (2020).
Zhang, J. et al. HIF-1alpha and HIF-2alpha redundantly promote retinal neovascularization in patients with ischemic retinal disease. J. Clin. Invest. 131, e139202 (2021). This article provides evidence that targeting both HIF1α and HIF2α is necessary to prevent retinal neovascularization in patients with sickle cell retinopathy.
Dengler, V. L., Galbraith, M. & Espinosa, J. M. Transcriptional regulation by hypoxia inducible factors. Crit. Rev. Biochem. Mol. Biol. 49, 1–15 (2014).
Semenza, G. L. Pharmacologic targeting of hypoxia-inducible factors. Annu. Rev. Pharmacol. Toxicol. 59, 379–403 (2019).
Diao, X. et al. Identification of oleoylethanolamide as an endogenous ligand for HIF-3alpha. Nat. Commun. 13, 2529 (2022).
Wu, D., Potluri, N., Lu, J., Kim, Y. & Rastinejad, F. Structural integration in hypoxia-inducible factors. Nature 524, 303–308 (2015).
Semenza, G. L. & Wang, G. L. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell Biol. 12, 5447–5454 (1992).
Tian, H., McKnight, S. L. & Russell, D. W. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 11, 72–82 (1997). This study reports the identification of EPAS1/HIF2α as an important player in vascularization.
Mastrogiannaki, M., Matak, P. & Peyssonnaux, C. The gut in iron homeostasis: role of HIF-2 under normal and pathological conditions. Blood 122, 885–892 (2013).
Knutson, A. K., Williams, A. L., Boisvert, W. A. & Shohet, R. V. HIF in the heart: development, metabolism, ischemia, and atherosclerosis. J. Clin. Invest. 131, e137557 (2021).
Hu, C. J., Wang, L. Y., Chodosh, L. A., Keith, B. & Simon, M. C. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol. Cell Biol. 23, 9361–9374 (2003).
Takeda, N. et al. Differential activation and antagonistic function of HIF-alpha isoforms in macrophages are essential for NO homeostasis. Genes Dev. 24, 491–501 (2010).
Branco-Price, C. et al. Endothelial cell HIF-1alpha and HIF-2alpha differentially regulate metastatic success. Cancer Cell 21, 52–65 (2012).
Makino, Y. et al. Inhibitory PAS domain protein is a negative regulator of hypoxia-inducible gene expression. Nature 414, 550–554 (2001).
Makino, Y., Kanopka, A., Wilson, W. J., Tanaka, H. & Poellinger, L. Inhibitory PAS domain protein (IPAS) is a hypoxia-inducible splicing variant of the hypoxia-inducible factor-3alpha locus. J. Biol. Chem. 277, 32405–32408 (2002).
Semenza, G. L. The genomics and genetics of oxygen homeostasis. Annu. Rev. Genomics Hum. Genet. 21, 183–204 (2020).
McNeill, L. A. et al. Hypoxia-inducible factor asparaginyl hydroxylase (FIH-1) catalyses hydroxylation at the beta-carbon of asparagine-803. Biochem. J. 367, 571–575 (2002).
Freedman, S. J. et al. Structural basis for recruitment of CBP/p300 by hypoxia-inducible factor-1 alpha. Proc. Natl Acad. Sci. USA 99, 5367–5372 (2002). This report provides the solution structure as a mechanism of the specific recognition of CBP–p300 by HIF1α.
Zhang, N. et al. The asparaginyl hydroxylase factor inhibiting HIF-1alpha is an essential regulator of metabolism. Cell Metab. 11, 364–378 (2010).
Hirota, K. HIF-alpha prolyl hydroxylase inhibitors and their implications for biomedicine: a comprehensive review. Biomedicines 9, 468 (2021).
Maxwell, P. H. et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399, 271–275 (1999). This study shows that VHL tumour suppressor gene product pVHL is important for oxygen-dependent degradation of HIFα.
Iwai, K. et al. Identification of the von Hippel–Lindau tumor-suppressor protein as part of an active E3 ubiquitin ligase complex. Proc. Natl Acad. Sci. USA 96, 12436–12441 (1999).
Schofield, C. J. & Ratcliffe, P. J. Signalling hypoxia by HIF hydroxylases. Biochem. Biophys. Res. Commun. 338, 617–626 (2005).
Kasper, L. H. et al. Two transactivation mechanisms cooperate for the bulk of HIF-1-responsive gene expression. EMBO J. 24, 3846–3858 (2005).
Wenger, R. H., Stiehl, D. P. & Camenisch, G. Integration of oxygen signaling at the consensus HRE. Sci. STKE 2005, re12 (2005).
Hartmann, H. et al. Hypoxia-independent activation of HIF-1 by Enterobacteriaceae and their siderophores. Gastroenterology 134, 756–767 (2008).
Appelhoff, R. J. et al. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J. Biol. Chem. 279, 38458–38465 (2004).
Yeh, T. L. et al. Molecular and cellular mechanisms of HIF prolyl hydroxylase inhibitors in clinical trials. Chem. Sci. 8, 7651–7668 (2017).
Markolovic, S., Wilkins, S. E. & Schofield, C. J. Protein hydroxylation catalyzed by 2-oxoglutarate-dependent oxygenases. J. Biol. Chem. 290, 20712–20722 (2015).
Hodges, V. M., Rainey, S., Lappin, T. R. & Maxwell, A. P. Pathophysiology of anemia and erythrocytosis. Crit. Rev. Oncol. Hematol. 64, 139–158 (2007).
Dame, C. et al. Erythropoietin mRNA expression in human fetal and neonatal tissue. Blood 92, 3218–3225 (1998).
Zhang, Y. et al. Erythropoietin action in stress response, tissue maintenance and metabolism. Int. J. Mol. Sci. 15, 10296–10333 (2014).
Obara, N. et al. Repression via the GATA box is essential for tissue-specific erythropoietin gene expression. Blood 111, 5223–5232 (2008).
Souma, T., Suzuki, N. & Yamamoto, M. Renal erythropoietin-producing cells in health and disease. Front. Physiol. 6, 167 (2015).
Haase, V. H. Hypoxia-inducible factor-prolyl hydroxylase inhibitors in the treatment of anemia of chronic kidney disease. Kidney Int. Suppl. 11, 8–25 (2021).
Macdougall, I. C. et al. Antibody-mediated pure red cell aplasia in chronic kidney disease patients receiving erythropoiesis-stimulating agents: new insights. Kidney Int. 81, 727–732 (2012).
Warnecke, C. et al. Differentiating the functional role of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha (EPAS-1) by the use of RNA interference: erythropoietin is a HIF-2alpha target gene in Hep3B and Kelly cells. FASEB J. 18, 1462–1464 (2004).
Flamme, I. et al. Mimicking hypoxia to treat anemia: HIF-stabilizer BAY 85-3934 (molidustat) stimulates erythropoietin production without hypertensive effects. PLoS ONE 9, e111838 (2014).
Kapitsinou, P. P. et al. Hepatic HIF-2 regulates erythropoietic responses to hypoxia in renal anemia. Blood 116, 3039–3048 (2010).
Minamishima, Y. A. & Kaelin, W. G. Jr. Reactivation of hepatic EPO synthesis in mice after PHD loss. Science 329, 407 (2010).
Peyssonnaux, C. et al. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs). J. Clin. Invest. 117, 1926–1932 (2007).
Qian, Z. M. et al. Divalent metal transporter 1 is a hypoxia-inducible gene. J. Cell Physiol. 226, 1596–1603 (2011).
Taylor, M. et al. Hypoxia-inducible factor-2alpha mediates the adaptive increase of intestinal ferroportin during iron deficiency in mice. Gastroenterology 140, 2044–2055 (2011).
Singh, A. K. et al. Daprodustat for the treatment of anemia in patients not undergoing dialysis. N. Engl. J. Med. 385, 2313–2324 (2021).
Singh, A. K. et al. Daprodustat for the treatment of anemia in patients undergoing dialysis. N. Engl. J. Med. 385, 2325–2335 (2021). This study reports the results from a randomized phase III clinical trial that demonstrated that HIF-PHDi daprodustat was noninferior to ESAs in cardiovascular safety and efficacy in correcting anaemia in patients with CKD undergoing dialysis.
Koeppen, M. et al. Hypoxia-inducible factor 2-alpha-dependent induction of amphiregulin dampens myocardial ischemia-reperfusion injury. Nat. Commun. 9, 816 (2018).
Eltzschig, H. K., Bonney, S. K. & Eckle, T. Attenuating myocardial ischemia by targeting A2B adenosine receptors. Trends Mol. Med. 19, 345–354 (2013).
Bowser, J. L., Phan, L. H. & Eltzschig, H. K. The hypoxia-adenosine link during Intestinal Inflammation. J. Immunol. 200, 897–907 (2018).
Vohwinkel, C. U., Hoegl, S. & Eltzschig, H. K. Hypoxia signaling during acute lung injury. J. Appl. Physiol. 119, 1157–1163 (2015).
Eckle, T. et al. Identification of hypoxia-inducible factor HIF-1A as transcriptional regulator of the A2B adenosine receptor during acute lung injury. J. Immunol. 192, 1249–1256 (2014).
Vohwinkel, C. U. et al. HIF1A-dependent induction of alveolar epithelial PFKFB3 dampens acute lung injury. JCI Insight 7, e157855 (2022).
Zhao, C. et al. Deficiency of HIF-1alpha enhances influenza A virus replication by promoting autophagy in alveolar type II epithelial cells. Emerg. Microbes Infect. 9, 691–706 (2020).
McClendon, J. et al. Hypoxia-inducible factor 1alpha signaling promotes repair of the alveolar epithelium after acute lung injury. Am. J. Pathol. 187, 1772–1786 (2017).
Gong, H. et al. HIF2alpha signaling inhibits adherens junctional disruption in acute lung injury. J. Clin. Invest. 125, 652–664 (2015).
Jiang, X. et al. Endothelial hypoxia-inducible factor-2alpha is required for the maintenance of airway microvasculature. Circulation 139, 502–517 (2019).
Colgan, S. P., Furuta, G. T. & Taylor, C. T. Hypoxia and innate immunity: keeping up with the HIFsters. Annu. Rev. Immunol. 38, 341–363 (2020). This is an authoritative review on the importance of HIF in the regulation of innate immunity.
Palazon, A., Goldrath, A. W., Nizet, V. & Johnson, R. S. HIF transcription factors, inflammation, and immunity. Immunity 41, 518–528 (2014).
Taylor, C. T. & Scholz, C. C. The effect of HIF on metabolism and immunity. Nat. Rev. Nephrol. 18, 573–587 (2022).
McGettrick, A. F. & O’Neill, L. A. J. The role of HIF in immunity and inflammation. Cell Metab. 32, 524–536 (2020).
Berg, N. K. et al. Hypoxia-inducible factor-dependent induction of myeloid-derived netrin-1 attenuates natural killer cell infiltration during endotoxin-induced lung injury. FASEB J. 35, e21334 (2021).
Wu, G. et al. Hypoxia exacerbates inflammatory acute lung injury via the Toll-like receptor 4 signaling pathway. Front. Immunol. 9, 1667 (2018).
Wing, P. A. C. et al. Hypoxic and pharmacological activation of HIF inhibits SARS-CoV-2 infection of lung epithelial cells. Cell Rep. 35, 109020 (2021). This is the first study to show the potential benefit of HIF-PHDi during SARS-CoV-2 infection.
Wing, P. A. C. et al. Hypoxia inducible factors regulate infectious SARS-CoV-2, epithelial damage and respiratory symptoms in a hamster COVID-19 model. PLoS Pathog. 18, e1010807 (2022).
Nakamura, M., Imamura, T., Sobajima, M. & Kinugawa, K. Initial experience of hypoxia-inducible factor prolyl hydroxylase inhibitors in patients with heart failure and renal anemia. Heart Vessels 38, 284–290 (2023).
Wen, T., Zhang, X., Wang, Z. & Zhou, R. Hypoxia-inducible factor prolyl hydroxylase inhibitors in patients with renal anemia: a meta-analysis of randomized trials. Nephron 144, 572–582 (2020).
Bartels, K., Karhausen, J., Clambey, E. T., Grenz, A. & Eltzschig, H. K. Perioperative organ injury. Anesthesiology 119, 1474–1489 (2013).
Williams, G. W., Berg, N. K., Reskallah, A., Yuan, X. & Eltzschig, H. K. Acute respiratory distress syndrome: contemporary management and novel approaches during COVID-19. Anesthesiology 134, 270–282 (2021).
Gumbert, S. D. et al. Perioperative acute kidney injury. Anesthesiology 132, 180–204 (2020).
Macdougall, I. C. New anemia therapies: translating novel strategies from bench to bedside. Am. J. Kidney Dis. 59, 444–451 (2012).
Agrawal, D. et al. Desidustat in anemia due to non-dialysis-dependent chronic kidney disease: a phase 3 study (DREAM-ND). Am. J. Nephrol. 53, 352–360 (2022).
Maxwell, P. H. & Eckardt, K. U. HIF prolyl hydroxylase inhibitors for the treatment of renal anaemia and beyond. Nat. Rev. Nephrol. 12, 157–168 (2016). This is an authoritative review that discusses the advantages and challenges of using HIF-PHDis as treatment for renal anaemia.
Percy, M. J. et al. Novel exon 12 mutations in the HIF2A gene associated with erythrocytosis. Blood 111, 5400–5402 (2008).
Percy, M. J. et al. A gain-of-function mutation in the HIF2A gene in familial erythrocytosis. N. Engl. J. Med. 358, 162–168 (2008).
Percy, M. J. et al. A family with erythrocytosis establishes a role for prolyl hydroxylase domain protein 2 in oxygen homeostasis. Proc. Natl Acad. Sci. USA 103, 654–659 (2006).
Beck, J., Henschel, C., Chou, J., Lin, A. & Del Balzo, U. Evaluation of the carcinogenic potential of roxadustat (FG-4592), a small molecule inhibitor of hypoxia-inducible factor prolyl hydroxylase in CD-1 mice and Sprague Dawley rats. Int. J. Toxicol. 36, 427–439 (2017).
Provenzano, R. et al. Efficacy and cardiovascular safety of roxadustat for treatment of anemia in patients with non-dialysis-dependent CKD: pooled results of three randomized clinical trials. Clin. J. Am. Soc. Nephrol. 16, 1190–1200 (2021).
Kiriakidis, S. et al. Complement C1q is hydroxylated by collagen prolyl 4 hydroxylase and is sensitive to off-target inhibition by prolyl hydroxylase domain inhibitors that stabilize hypoxia-inducible factor. Kidney Int. 92, 900–908 (2017).
Schejbel, L. et al. Molecular basis of hereditary C1q deficiency–revisited: identification of several novel disease-causing mutations. Genes Immun. 12, 626–634 (2011).
Fallah, J. & Rini, B. I. HIF inhibitors: status of current clinical development. Curr. Oncol. Rep. 21, 6 (2019).
Aquino-Galvez, A. et al. Effects of 2-methoxyestradiol on apoptosis and HIF-1alpha and HIF-2alpha expression in lung cancer cells under normoxia and hypoxia. Oncol. Rep. 35, 577–583 (2016).
Gheorghiade, M., van Veldhuisen, D. J. & Colucci, W. S. Contemporary use of digoxin in the management of cardiovascular disorders. Circulation 113, 2556–2564 (2006).
Zhang, H. et al. Digoxin and other cardiac glycosides inhibit HIF-1alpha synthesis and block tumor growth. Proc. Natl Acad. Sci. USA 105, 19579–19586 (2008).
Currie, G. M., Wheat, J. M. & Kiat, H. Pharmacokinetic considerations for digoxin in older people. Open Cardiovasc. Med. J. 5, 130–135 (2011).
Ren, X., Diao, X., Zhuang, J. & Wu, D. Structural basis for the allosteric inhibition of hypoxia-inducible factor (HIF)-2 by belzutifan. Mol. Pharmacol. 102, 240–247 (2022).
Rapisarda, A. et al. Identification of small molecule inhibitors of hypoxia-inducible factor 1 transcriptional activation pathway. Cancer Res. 62, 4316–4324 (2002).
Rapisarda, A. et al. Topoisomerase I-mediated inhibition of hypoxia-inducible factor 1: mechanism and therapeutic implications. Cancer Res. 64, 1475–1482 (2004).
Welsh, S., Williams, R., Kirkpatrick, L., Paine-Murrieta, G. & Powis, G. Antitumor activity and pharmacodynamic properties of PX-478, an inhibitor of hypoxia-inducible factor-1alpha. Mol. Cancer Ther. 3, 233–244 (2004).
Jacoby, J. J. et al. Treatment with HIF-1alpha antagonist PX-478 inhibits progression and spread of orthotopic human small cell lung cancer and lung adenocarcinoma in mice. J. Thorac. Oncol. 5, 940–949 (2010).
Koh, M. Y. et al. Molecular mechanisms for the activity of PX-478, an antitumor inhibitor of the hypoxia-inducible factor-1alpha. Mol. Cancer Ther. 7, 90–100 (2008).
Miranda, E. et al. A cyclic peptide inhibitor of HIF-1 heterodimerization that inhibits hypoxia signaling in cancer cells. J. Am. Chem. Soc. 135, 10418–10425 (2013).
Burslem, G. M., Kyle, H. F., Nelson, A., Edwards, T. A. & Wilson, A. J. Hypoxia inducible factor (HIF) as a model for studying inhibition of protein-protein interactions. Chem. Sci. 8, 4188–4202 (2017).
Greenberger, L. M. et al. A RNA antagonist of hypoxia-inducible factor-1alpha, EZN-2968, inhibits tumor cell growth. Mol. Cancer Ther. 7, 3598–3608 (2008).
Zimmer, M. et al. Small-molecule inhibitors of HIF-2a translation link its 5′UTR iron-responsive element to oxygen sensing. Mol. Cell 32, 838–848 (2008).
Zhang, D. L., Ghosh, M. C. & Rouault, T. A. The physiological functions of iron regulatory proteins in iron homeostasis - an update. Front. Pharmacol. 5, 124 (2014).
Dai, Z. et al. Therapeutic targeting of vascular remodeling and right heart failure in pulmonary arterial hypertension with a HIF-2alpha inhibitor. Am. J. Respir. Crit. Care Med. 198, 1423–1434 (2018). This study reports the beneficial therapeutic effect of pharmacological HIF2α inhibitor C76 in rodent models of pulmonary hypertension.
Scheuermann, T. H. et al. Artificial ligand binding within the HIF2alpha PAS-B domain of the HIF2 transcription factor. Proc. Natl Acad. Sci. USA 106, 450–455 (2009).
Scheuermann, T. H. et al. Allosteric inhibition of hypoxia inducible factor-2 with small molecules. Nat. Chem. Biol. 9, 271–276 (2013).
Wehn, P. M. et al. Design and activity of specific hypoxia-inducible factor-2alpha (HIF-2alpha) inhibitors for the treatment of clear cell renal cell carcinoma: discovery of clinical candidate (S)-3-((2,2-difluoro-1-hydroxy-7-(methylsulfonyl)-2,3-dihydro-1 H-inden-4-yl)oxy)-5-fluorobenzonitrile (PT2385). J. Med. Chem. 61, 9691–9721 (2018).
Courtney, K. D. et al. HIF-2 complex dissociation, target inhibition, and acquired resistance with PT2385, a first-in-class HIF-2 inhibitor, in patients with clear cell renal cell carcinoma. Clin. Cancer Res. 26, 793–803 (2020).
Courtney, K. D. et al. Phase I dose-escalation trial of PT2385, a first-in-class hypoxia-inducible factor-2alpha antagonist in patients with previously treated advanced clear cell renal cell carcinoma. J. Clin. Oncol. 36, 867–874 (2018). This study reports the results from a first-in-human phase I clinical trial indicating a favourable safety profile and activity of HIF2α inhibitor PT2385 in patients with heavily pretreated ccRCC.
Xu, R. et al. 3-[(1S,2S,3R)-2,3-Difluoro-1-hydroxy-7-methylsulfonylindan-4-yl]oxy-5-fluorobenzonitrile (PT2977), a hypoxia-inducible factor 2α (HIF-2α) inhibitor for the treatment of clear cell renal cell carcinoma. J. Med. Chem. 62, 6876–6893 (2019).
Choueiri, T. K. et al. Inhibition of hypoxia-inducible factor-2alpha in renal cell carcinoma with belzutifan: a phase 1 trial and biomarker analysis. Nat. Med. 27, 802–805 (2021).
No authors listed. FDA OK’s HIF2α inhibitor belzutifan. Cancer Discov. 11, 2360–2361 (2021).
Wong, S. C. et al. HIF2alpha-targeted RNAi therapeutic inhibits clear cell renal cell carcinoma. Mol. Cancer Ther. 17, 140–149 (2018).
Ma, Y. et al. HIF2 inactivation and tumor suppression with a tumor-directed RNA-silencing drug in mice and humans. Clin. Cancer Res. 28, 5405–5418 (2022).
Muz, B., de la Puente, P., Azab, F. & Azab, A. K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 3, 83–92 (2015).
Semenza, G. L. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol. Sci. 33, 207–214 (2012).
Rapisarda, A. et al. Schedule-dependent inhibition of hypoxia-inducible factor-1alpha protein accumulation, angiogenesis, and tumor growth by topotecan in U251-HRE glioblastoma xenografts. Cancer Res. 64, 6845–6848 (2004).
Ardizzoni, A. et al. Topotecan, a new active drug in the second-line treatment of small-cell lung cancer: a phase II study in patients with refractory and sensitive disease. The European Organization for Research and Treatment of Cancer Early Clinical Studies Group and New Drug Development Office, and the Lung Cancer Cooperative Group. J. Clin. Oncol. 15, 2090–2096 (1997).
Tibes, R. et al. Results from a phase I, dose-escalation study of PX-478, an orally available inhibitor of HIF-1α. J. Clin. Oncol. 28, 3076–3076 (2010).
Jeong, W. et al. Pilot trial of EZN-2968, an antisense oligonucleotide inhibitor of hypoxia-inducible factor-1 alpha (HIF-1alpha), in patients with refractory solid tumors. Cancer Chemother. Pharmacol. 73, 343–348 (2014).
Wu, J. et al. Evaluation of a locked nucleic acid form of antisense oligo targeting HIF-1alpha in advanced hepatocellular carcinoma. World J. Clin. Oncol. 10, 149–160 (2019).
Cancer Genome Atlas Research Network. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 499, 43–49 (2013).
Kasherman, L., Siu, D. H. W., Woodford, R. & Harris, C. A. Angiogenesis inhibitors and immunomodulation in renal cell cancers: the past, present, and future. Cancers 14, 1406 (2022).
Pandey, A. K. et al. Mechanisms of VEGF (vascular endothelial growth factor) inhibitor-associated hypertension and vascular disease. Hypertension 71, e1–e8 (2018).
Kamba, T. & McDonald, D. M. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br. J. Cancer 96, 1788–1795 (2007).
Ganner, A. et al. VHL suppresses RAPTOR and inhibits mTORC1 signaling in clear cell renal cell carcinoma. Sci. Rep. 11, 14827 (2021).
Battelli, C. & Cho, D. C. mTOR inhibitors in renal cell carcinoma. Therapy 8, 359–367 (2011).
Toschi, A., Lee, E., Gadir, N., Ohh, M. & Foster, D. A. Differential dependence of hypoxia-inducible factors 1 alpha and 2 alpha on mTORC1 and mTORC2. J. Biol. Chem. 283, 34495–34499 (2008).
Motzer, R. J. et al. Nivolumab versus everolimus in patients with advanced renal cell carcinoma: updated results with long-term follow-up of the randomized, open-label, phase 3 CheckMate 025 trial. Cancer 126, 4156–4167 (2020).
Raval, R. R. et al. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol. Cell Biol. 25, 5675–5686 (2005).
Chen, W. et al. Targeting renal cell carcinoma with a HIF-2 antagonist. Nature 539, 112–117 (2016).
Pelosci, A. LITESPARK-005 trial reveals PFS benefit of belzutifan in advanced RCC. Cancer Network, Home of the Journal ONCOLOGY (23 August 2023).
Stransky, L. A. et al. Sensitivity of VHL mutant kidney cancers to HIF2 inhibitors does not require an intact p53 pathway. Proc. Natl Acad. Sci. USA 119, e2120403119 (2022).
Davis, L. et al. Targeting HIF-2alpha in the tumor microenvironment: redefining the role of HIF-2alpha for solid cancer therapy. Cancers 14, 1259 (2022).
Feldman, D. R. et al. Phase I trial of bevacizumab plus escalated doses of sunitinib in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 27, 1432–1439 (2009).
Redlich, A. et al. Pseudohypoxic pheochromocytomas and paragangliomas dominate in children. Pediatr. Blood Cancer 68, e28981 (2021).
Yang, C. et al. Germ-line PHD1 and PHD2 mutations detected in patients with pheochromocytoma/paraganglioma-polycythemia. J. Mol. Med. 93, 93–104 (2015).
Zhuang, Z. et al. Somatic HIF2A gain-of-function mutations in paraganglioma with polycythemia. N. Engl. J. Med. 367, 922–930 (2012).
Pacak, K. et al. New syndrome of paraganglioma and somatostatinoma associated with polycythemia. J. Clin. Oncol. 31, 1690–1698 (2013).
Yang, C. et al. Novel HIF2A mutations disrupt oxygen sensing, leading to polycythemia, paragangliomas, and somatostatinomas. Blood 121, 2563–2566 (2013).
Wang, H. et al. A transgenic mouse model of Pacak–Zhuang syndrome with an EPAS1 gain-of-function mutation. Cancers 11, 667 (2019).
Maron, B. A. Revised definition of pulmonary hypertension and approach to management: a clinical primer. J. Am. Heart Assoc. 12, e029024 (2023).
He, M. et al. Hypoxia induces the dysfunction of human endothelial colony-forming cells via HIF-1alpha signaling. Respir. Physiol. Neurobiol. 247, 87–95 (2018).
Shan, F., Li, J. & Huang, Q. Y. HIF-1 alpha-induced up-regulation of miR-9 contributes to phenotypic modulation in pulmonary artery smooth muscle cells during hypoxia. J. Cell Physiol. 229, 1511–1520 (2014).
Zeng, Y. et al. Hypoxia inducible factor-1 mediates expression of miR-322: potential role in proliferation and migration of pulmonary arterial smooth muscle cells. Sci. Rep. 5, 12098 (2015).
Luo, Y. et al. CD146-HIF-1alpha hypoxic reprogramming drives vascular remodeling and pulmonary arterial hypertension. Nat. Commun. 10, 3551 (2019).
Tang, H. et al. Endothelial HIF-2alpha contributes to severe pulmonary hypertension due to endothelial-to-mesenchymal transition. Am. J. Physiol. Lung Cell Mol. Physiol. 314, L256–L275 (2018).
Hu, C. J. et al. Suppression of HIF2 signalling attenuates the initiation of hypoxia-induced pulmonary hypertension. Eur. Respir. J. 54, 1900378 (2019).
Kim, Y. M. et al. Hypoxia-inducible factor-1alpha in pulmonary artery smooth muscle cells lowers vascular tone by decreasing myosin light chain phosphorylation. Circ. Res. 112, 1230–1233 (2013).
Kojima, H. et al. Hypoxia-inducible factor-1 alpha deletion in myeloid lineage attenuates hypoxia-induced pulmonary hypertension. Physiol. Rep. 7, e14025 (2019).
Yu, Y. A. et al. Nonclassical monocytes sense hypoxia, regulate pulmonary vascular remodeling, and promote pulmonary hypertension. J. Immunol. 204, 1474–1485 (2020).
Cowburn, A. S. et al. HIF2alpha–arginase axis is essential for the development of pulmonary hypertension. Proc. Natl Acad. Sci. USA 113, 8801–8806 (2016).
Labrousse-Arias, D. et al. HIF-2alpha-mediated induction of pulmonary thrombospondin-1 contributes to hypoxia-driven vascular remodelling and vasoconstriction. Cardiovasc. Res. 109, 115–130 (2016).
Wang, S. et al. EPAS1 (endothelial PAS domain protein 1) orchestrates transactivation of endothelial ICAM1 (intercellular adhesion molecule 1) by small nucleolar RNA host gene 5 (SNHG5) to promote hypoxic pulmonary hypertension. Hypertension 78, 1080–1091 (2021).
Tan, Q. et al. Erythrocytosis and pulmonary hypertension in a mouse model of human HIF2A gain of function mutation. J. Biol. Chem. 288, 17134–17144 (2013).
Jiang, Y. et al. Topotecan prevents hypoxia-induced pulmonary arterial hypertension and inhibits hypoxia-inducible factor-1alpha and TRPC channels. Int. J. Biochem. Cell Biol. 104, 161–170 (2018).
Ghosh, M. C. et al. Therapeutic inhibition of HIF-2alpha reverses polycythemia and pulmonary hypertension in murine models of human diseases. Blood 137, 2509–2519 (2021). This study shows the beneficial therapeutic effect of pharmacological HIF2α inhibitor belzutifan in a murine model of polycythaemia, pulmonary hypertension, pulmonary fibrosis and complications due to gain-of-function mutation of HIF2A.
Campochiaro, P. A. Ocular neovascularization. J. Mol. Med. 91, 311–321 (2013).
Ozaki, H. et al. Hypoxia inducible factor-1alpha is increased in ischemic retina: temporal and spatial correlation with VEGF expression. Invest. Ophthalmol. Vis. Sci. 40, 182–189 (1999).
Kelly, B. D. et al. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ. Res. 93, 1074–1081 (2003).
Barben, M., Schori, C., Samardzija, M. & Grimm, C. Targeting Hif1a rescues cone degeneration and prevents subretinal neovascularization in a model of chronic hypoxia. Mol. Neurodegener. 13, 12 (2018).
Dioum, E. M., Clarke, S. L., Ding, K., Repa, J. J. & Garcia, J. A. HIF-2alpha-haploinsufficient mice have blunted retinal neovascularization due to impaired expression of a proangiogenic gene battery. Invest. Ophthalmol. Vis. Sci. 49, 2714–2720 (2008).
Qin, Y. et al. PAI-1 is a vascular cell-specific HIF-2-dependent angiogenic factor that promotes retinal neovascularization in diabetic patients. Sci. Adv. 8, eabm1896 (2022).
Zeng, M. et al. The HIF-1 antagonist acriflavine: visualization in retina and suppression of ocular neovascularization. J. Mol. Med. 95, 417–429 (2017).
Usui-Ouchi, A. et al. An allosteric peptide inhibitor of HIF-1alpha regulates hypoxia-induced retinal neovascularization. Proc. Natl Acad. Sci. USA 117, 28297–28306 (2020).
Miwa, Y. et al. Pharmacological HIF inhibition prevents retinal neovascularization with improved visual function in a murine oxygen-induced retinopathy model. Neurochem. Int. 128, 21–31 (2019).
Pan, X. & Lv, Y. Effects and mechanism of action of PX-478 in oxygen-induced retinopathy in mice. Ophthalmic Res. 63, 182–193 (2020).
Cheng, X. et al. Marked and rapid effects of pharmacological HIF-2alpha antagonism on hypoxic ventilatory control. J. Clin. Invest. 130, 2237–2251 (2020).
Macias, D. et al. HIF-2alpha is essential for carotid body development and function. eLife 7, e34681 (2018).
Cho, H. et al. On-target efficacy of a HIF-2alpha antagonist in preclinical kidney cancer models. Nature 539, 107–111 (2016).
Watts, E. R. & Walmsley, S. R. Inflammation and hypoxia: HIF and PHD isoform selectivity. Trends Mol. Med. 25, 33–46 (2019).
Lee, J. W. et al. Transcription-independent induction of ERBB1 through hypoxia-inducible factor 2A provides cardioprotection during ischemia and reperfusion. Anesthesiology 132, 763–780 (2020).
Zuk, A. et al. Preclinical characterization of vadadustat (AKB-6548), an oral small molecule hypoxia-inducible factor prolyl-4-hydroxylase inhibitor, for the potential treatment of renal anemia. J. Pharmacol. Exp. Ther. 383, 11–24 (2022).
Li, J. et al. PMN-derived netrin-1 attenuates cardiac ischemia-reperfusion injury via myeloid ADORA2B signaling. J. Exp. Med. 218, e20210008 (2021).
Ruan, W. et al. The hypoxia-adenosine link during myocardial ischemia-reperfusion injury. Biomedicines 10, 1939 (2022).
Conrad, C. & Eltzschig, H. K. Disease mechanisms of perioperative organ injury. Anesth. Analg. 131, 1730–1750 (2020).
Hara, K., Takahashi, N., Wakamatsu, A. & Caltabiano, S. Pharmacokinetics, pharmacodynamics and safety of single, oral doses of GSK1278863, a novel HIF-prolyl hydroxylase inhibitor, in healthy Japanese and Caucasian subjects. Drug. Metab. Pharmacokinet. 30, 410–418 (2015).
Kansagra, K. A. et al. Phase I clinical study of ZYAN1, a novel prolyl-hydroxylase (PHD) inhibitor to evaluate the safety, tolerability, and pharmacokinetics following oral administration in healthy volunteers. Clin. Pharmacokinet. 57, 87–102 (2018).
Parmar, D. V. et al. Outcomes of desidustat treatment in people with anemia and chronic kidney disease: a phase 2 study. Am. J. Nephrol. 49, 470–478 (2019).
Bottcher, M. et al. First-in-man-proof of concept study with molidustat: a novel selective oral HIF-prolyl hydroxylase inhibitor for the treatment of renal anaemia. Br. J. Clin. Pharmacol. 84, 1557–1565 (2018).
Beck, H. et al. Discovery of molidustat (BAY 85-3934): a small-molecule oral HIF-prolyl hydroxylase (HIF-PH) inhibitor for the treatment of renal anemia. ChemMedChem 13, 988–1003 (2018).
Czock, D. & Keller, F. Clinical pharmacokinetics and pharmacodynamics of roxadustat. Clin. Pharmacokinet. 61, 347–362 (2022).
Provenzano, R. et al. Oral hypoxia–inducible factor prolyl hydroxylase inhibitor roxadustat (FG-4592) for the treatment of anemia in patients with CKD. Clin. J. Am. Soc. Nephrol. 11, 982–991 (2016).
Shibata, T. et al. Evaluation of food and spherical carbon adsorbent effects on the pharmacokinetics of roxadustat in healthy nonelderly adult male japanese subjects. Clin. Pharmacol. Drug. Dev. 8, 304–313 (2019).
Dhillon, S. Roxadustat: first global approval. Drugs 79, 563–572 (2019).
Provenzano, R. et al. Roxadustat (FG-4592) versus epoetin alfa for anemia in patients receiving maintenance hemodialysis: a phase 2, randomized, 6-to 19-week, open-label, active-comparator, dose-ranging, safety and exploratory efficacy study. Am. J. Kidney Dis. 67, 912–924 (2016).
Chen, N., Hao, C. & Liu, B. A phase 3, randomized, open-label, active-controlled study of efficacy and safety of roxadustat for treatment of anemia in subjects with CKD on dialysis. J. Am. Soc. Nephrol. 29, B5 (2018).
Besarab, A. et al. Roxadustat (FG-4592): correction of anemia in incident dialysis patients. J. Am. Soc. Nephrol. 27, 1225–1233 (2016).
Besarab, A. et al. Randomized placebo-controlled dose-ranging and pharmacodynamics study of roxadustat (FG-4592) to treat anemia in nondialysis-dependent chronic kidney disease (NDD-CKD) patients. Nephrol. Dial. Transplant. 30, 1665–1673 (2015).
Chavan, A. et al. Effect of moderate hepatic impairment on the pharmacokinetics of vadadustat, an oral hypoxia-inducible factor prolyl hydroxylase inhibitor. Clin. Pharmacol. Drug. Dev. 10, 950–958 (2021).
Nwogu, J. I. et al. Inhibition of collagen synthesis with prolyl 4-hydroxylase inhibitor improves left ventricular function and alters the pattern of left ventricular dilatation after myocardial infarction. Circulation 104, 2216–2221 (2001).
Xi, L., Taher, M., Yin, C., Salloum, F. & Kukreja, R. C. Cobalt chloride induces delayed cardiac preconditioning in mice through selective activation of HIF-1α and AP-1 and iNOS signaling. Am. J. Physiol. Heart Circ. Physiol. 287, H2369–H2375 (2004).
Bao, W. et al. Chronic inhibition of hypoxia-inducible factor prolyl 4-hydroxylase improves ventricular performance, remodeling, and vascularity after myocardial infarction in the rat. J. Cardiovasc. Pharmacol. 56, 147–155 (2010).
Signore, P. E. et al. A small-molecule inhibitor of hypoxia-inducible factor prolyl hydroxylase improves obesity, nephropathy and cardiomyopathy in obese ZSF1 rats. PLoS ONE 16, e0255022 (2021).
Philipp, S. et al. Stabilization of hypoxia inducible factor rather than modulation of collagen metabolism improves cardiac function after acute myocardial infarction in rats. Eur. J. Heart Fail. 8, 347–354 (2006).
Vogler, M. et al. Pre-and post-conditional inhibition of prolyl-4-hydroxylase domain enzymes protects the heart from an ischemic insult. Pflügers Arch. 467, 2141–2149 (2015).
Keränen, M. et al. Differential effects of pharmacological HIF preconditioning of donors versus recipients in rat cardiac allografts. Am. J. Transplant. 13, 600–610 (2013).
Eckle, T., Köhler, D., Lehmann, R., El Kasmi, K. C. & Eltzschig, H. K. Hypoxia-inducible factor-1 is central to cardioprotection: a new paradigm for ischemic preconditioning. Circulation 118, 166–175 (2008).
Natarajan, R., Salloum, F. N., Fisher, B. J., Kukreja, R. C. & Fowler, A. A. III Hypoxia inducible factor-1 activation by prolyl 4-hydroxylase-2 gene silencing attenuates myocardial ischemia reperfusion injury. Circ. Res. 98, 133–140 (2006).
Natarajan, R. et al. Activation of hypoxia-inducible factor-1 via prolyl-4 hydoxylase-2 gene silencing attenuates acute inflammatory responses in postischemic myocardium. Am. J. Physiol. Heart Circ. Physiol. 293, H1571–H1580 (2007).
Ockaili, R. et al. HIF-1 activation attenuates postischemic myocardial injury: role for heme oxygenase-1 in modulating microvascular chemokine generation. Am. J. Physiol. Heart Circ. Physiol. 289, H542–H548 (2005).
Zhao, H.-X. et al. Attenuation of myocardial injury by postconditioning: role of hypoxia inducible factor-1α. Basic Res. Cardiol. 105, 109–118 (2010).
Huang, M. et al. Short hairpin RNA interference therapy for ischemic heart disease. Circulation 118, S226–S233 (2008).
Hyvärinen, J. et al. Hearts of hypoxia-inducible factor prolyl 4-hydroxylase-2 hypomorphic mice show protection against acute ischemia–reperfusion injury. J. Biol. Chem. 285, 13646–13657 (2010).
Kerkelä, R. et al. Activation of hypoxia response in endothelial cells contributes to ischemic cardioprotection. Mol. Cell. Biol. 33, 3321–3329 (2013).
Hölscher, M. et al. Cardiomyocyte-specific prolyl-4-hydroxylase domain 2 knock out protects from acute myocardial ischemic injury. J. Biol. Chem. 286, 11185–11194 (2011).
Adluri, R. S. et al. Disruption of hypoxia-inducible transcription factor-prolyl hydroxylase domain-1 (PHD-1−/−) attenuates ex vivo myocardial ischemia/reperfusion injury through hypoxia-inducible factor-1α transcription factor and its target genes in mice. Antioxid. Redox Signal. 15, 1789–1797 (2011).
Oriowo, B. et al. Targeted gene deletion of prolyl hydroxylase domain protein 3 triggers angiogenesis and preserves cardiac function by stabilizing hypoxia inducible factor 1 alpha following myocardial infarction. Curr. Pharm. Des. 20, 1305–1310 (2014).
Xie, L. et al. Depletion of PHD3 protects heart from ischemia/reperfusion injury by inhibiting cardiomyocyte apoptosis. J. Mol. Cell. Cardiol. 80, 156–165 (2015).
Deguchi, H. et al. Roxadustat markedly reduces myocardial ischemia reperfusion injury in mice. Circ. J. 84, 1028–1033 (2020).
Zhong, Z. et al. Activation of the oxygen-sensing signal cascade prevents mitochondrial injury after mouse liver ischemia-reperfusion. Am. J. Physiol. Gastrointest. Liver Physiol. 295, G823–G832 (2008).
Harnoss, J. M. et al. Prolyl hydroxylase inhibition mitigates allograft injury during liver transplant. Transplantation 106, e430–e440 (2022).
Harnoss, J. M. et al. Prolyl hydroxylase inhibition enhances liver regeneration without induction of tumor growth. Ann. Surg. 265, 782–791 (2017).
Schneider, M. et al. Loss or silencing of the PHD1 prolyl hydroxylase protects livers of mice against ischemia/reperfusion injury. Gastroenterology 138, 1143–1154 (2010). e1141–1142.
Liu, J. et al. Double knockdown of PHD1 and Keap1 attenuated hypoxia-induced injuries in hepatocytes. Front. Physiol. 8, 291 (2017).
Mollenhauer, M. et al. Deficiency of the oxygen sensor PHD1 augments liver regeneration after partial hepatectomy. Langenbecks Arch. Surg. 397, 1313–1322 (2012).
Matsumoto, M. et al. Induction of renoprotective gene expression by cobalt ameliorates ischemic injury of the kidney in rats. J. Am. Soc. Nephrol. 14, 1825–1832 (2003).
Bernhardt, W. M. et al. Preconditional activation of hypoxia-inducible factors ameliorates ischemic acute renal failure. J. Am. Soc. Nephrol. 17, 1970–1978 (2006).
Xie, D. et al. Kidney-targeted delivery of prolyl hydroxylase domain protein 2 small interfering RNA with nanoparticles alleviated renal ischemia/reperfusion injury. J. Pharmacol. Exp. Ther. 378, 235–243 (2021).
Jamadarkhana, P. et al. Treatment with a novel hypoxia-inducible factor hydroxylase inhibitor (TRC160334) ameliorates ischemic acute kidney injury. Am. J. Nephrol. 36, 208–218 (2012).
Yang, Y. et al. Hypoxia-inducible factor prolyl hydroxylase inhibitor roxadustat (FG-4592) protects against cisplatin-induced acute kidney injury. Clin. Sci. 132, 825–838 (2018).
Ito, M. et al. Prolyl hydroxylase inhibition protects the kidneys from ischemia via upregulation of glycogen storage. Kidney Int. 97, 687–701 (2020).
Bernhardt, W. et al. Donor treatment with a PHD-inhibitor activating HIFs prevents graft injury and prolongs survival in an allogenic kidney transplant model. Proc. Natl Acad. Sci. USA 106, 21276–21281 (2009).
Tambuwala, M. M. et al. Loss of prolyl hydroxylase-1 protects against colitis through reduced epithelial cell apoptosis and increased barrier function. Gastroenterology 139, 2093–2101 (2010).
Cummins, E. P. et al. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology 134, 156–165.e151 (2008).
Tambuwala, M. M. et al. Targeted delivery of the hydroxylase inhibitor DMOG provides enhanced efficacy with reduced systemic exposure in a murine model of colitis. J. Control. Release 217, 221–227 (2015).
Manresa, M. C. et al. Hydroxylase inhibition regulates inflammation-induced intestinal fibrosis through the suppression of ERK-mediated TGF-β1 signaling. Am. J. Physiol. Gastrointest. Liver Physiol. 311, G1076–G1090 (2016).
Halligan, D. N. et al. Hypoxia-inducible factor hydroxylase inhibition enhances the protective effects of cyclosporine in colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 317, G90–G97 (2019).
Robinson, A. et al. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology 134, 145–155 (2008).
Marks, E. et al. Oral delivery of prolyl hydroxylase inhibitor: AKB-4924 promotes localized mucosal healing in a mouse model of colitis. Inflamm. Bowel Dis. 21, 267–275 (2015).
Keely, S. et al. Contribution of epithelial innate immunity to systemic protection afforded by prolyl hydroxylase inhibition in murine colitis. Mucosal Immunol. 7, 114–123 (2014).
Van Welden, S. et al. Haematopoietic prolyl hydroxylase‐1 deficiency promotes M2 macrophage polarization and is both necessary and sufficient to protect against experimental colitis. J. Pathol. 241, 547–558 (2017).
Okumura, C. Y. et al. A new pharmacological agent (AKB-4924) stabilizes hypoxia inducible factor-1 (HIF-1) and increases skin innate defenses against bacterial infection. J. Mol. Med. 90, 1079–1089 (2012).
Zinkernagel, A. S., Peyssonnaux, C., Johnson, R. S. & Nizet, V. Pharmacologic augmentation of hypoxia-inducible factor—1α with mimosine boosts the bactericidal capacity of phagocytes. J. Infect. Dis. 197, 214–217 (2008).
Hirota, S. A. et al. Hypoxia-inducible factor signaling provides protection in Clostridium difficile-induced intestinal injury. Gastroenterology 139, 259–269.e253 (2010).
Schaible, B. et al. Hypoxia modulates infection of epithelial cells by Pseudomonas aeruginosa. PLoS ONE 8, e56491 (2013).
Hirai, K., Furusho, H., Hirota, K. & Sasaki, H. Activation of hypoxia-inducible factor 1 attenuates periapical inflammation and bone loss. Int. J. Oral Sci. 10, 12 (2018).
Wood, J. G. et al. Heme oxygenase‐1 upregulation following prolyl hydroxylase inhibition attenuates hypoxia‐induced microvascular inflammation. FASEB J. 23, 762.719 (2009).
Howard, J. M. et al. Upregulation of HIF-1 attenuates hemorrhagic shock/resuscitation-induced leukocyte adherence via an iNOS-dependent pathway. J. Am. Coll. Surg. 207, S39 (2008).
Figg, W. D. Jr. et al. Structural basis of prolyl hydroxylase domain inhibition by molidustat. ChemMedChem 16, 2082–2088 (2021).
Wu, D. et al. Bidirectional modulation of HIF-2 activity through chemical ligands. Nat. Chem. Biol. 15, 367–376 (2019).
Wallace, E. M. et al. A small-molecule antagonist of HIF2alpha is efficacious in preclinical models of renal cell carcinoma. Cancer Res. 76, 5491–5500 (2016).
Haase, V. H. HIF-prolyl hydroxylases as therapeutic targets in erythropoiesis and iron metabolism. Hemodial. Int. 21, S110–S124 (2017).
Eckle, T. et al. Crosstalk between the equilibrative nucleoside transporter ENT2 and alveolar Adora2b adenosine receptors dampens acute lung injury. FASEB J. 27, 3078–3089 (2013).
Aherne, C. M. et al. Coordination of ENT2-dependent adenosine transport and signaling dampens mucosal inflammation. JCI Insight 3, e121521 (2018).
Ruan, W. et al. Targeting myocardial equilibrative nucleoside transporter ENT1 provides cardioprotection by enhancing myeloid Adora2b signaling. JCI Insight 8, e166011 (2023).
Wang, G. L. & Semenza, G. L. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc. Natl Acad. Sci. USA 90, 4304–4308 (1993).
Fraisl, P., Aragones, J. & Carmeliet, P. Inhibition of oxygen sensors as a therapeutic strategy for ischaemic and inflammatory disease. Nat. Rev. Drug. Discov. 8, 139–152 (2009).
Aragones, J. et al. Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat. Genet. 40, 170–180 (2008).
Ruan, W., Yuan, X. & Eltzschig, H. K. Circadian rhythm as a therapeutic target. Nat. Rev. Drug. Discov. 20, 287–307 (2021).
Semenza, G. L. HIF-1 mediates the Warburg effect in clear cell renal carcinoma. J. Bioenerg. Biomembr. 39, 231–234 (2007).
Carmeliet, P. et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 394, 485–490 (1998).
Forsythe, J. A. et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell Biol. 16, 4604–4613 (1996).
Eltzschig, H. K., Weissmuller, T., Mager, A. & Eckle, T. Nucleotide metabolism and cell-cell interactions. Methods Mol. Biol. 341, 73–87 (2006).
Synnestvedt, K. et al. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J. Clin. Invest. 110, 993–1002 (2002).
Ahmad, A. et al. Adenosine A2A receptor is a unique angiogenic target of HIF-2alpha in pulmonary endothelial cells. Proc. Natl Acad. Sci. USA 106, 10684–10689 (2009).
Ohta, A. & Sitkovsky, M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature 414, 916–920 (2001).
Morote-Garcia, J. C., Rosenberger, P., Kuhlicke, J. & Eltzschig, H. K. HIF-1-dependent repression of adenosine kinase attenuates hypoxia-induced vascular leak. Blood 111, 5571–5580 (2008).
Yuan, X. et al. Alternative adenosine receptor activation: the netrin-Adora2b link. Front. Pharmacol. 13, 944994 (2022).
Heck-Swain, K. L. et al. Myeloid hypoxia-inducible factor HIF1A provides cardio-protection during ischemia and reperfusion via induction of netrin-1. Front. Cardiovasc. Med. 9, 970415 (2022).
Ohta, A. A metabolic immune checkpoint: adenosine in tumor microenvironment. Front. Immunol. 7, 109 (2016).
Choudhry, H. & Harris, A. L. Advances in hypoxia-inducible factor biology. Cell Metab. 27, 281–298 (2018).
Acknowledgements
H.K.E. is supported by National Institute of Health Grants R01HL154720, R01HL154720-03S1, R01HL165748, R01HL169519, R01DK122796, T32GM135118, Department of Defense Grant W81XWH2110032, and sponsored contract through Akebia Therapeutics. X.Y. is supported by National Institute of Health Grants R01HL155950, R01HL155950-02S1, R01HL169519, Parker B. Francis Fellowship, and sponsored contract through Akebia Therapeutics. W.R. is supported by Natural Science Foundation of Hunan Province Grant 018JJ3736, Young Talent Foundation of Hunan Province Grant 2021RC3034, and 2022 International Anesthesia Research Society Mentored Research Award.
Author information
Authors and Affiliations
Contributions
X.Y. and W.R. equally researched data for the article, made substantial contribution to discussion of content, wrote the article and reviewed and edited the manuscript before submission. B.B. and P.C. reviewed and edited the article before submission. H.K.E. contributed substantially to conceptualization of the content of the article and reviewed and edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
H.K.E., B.B. and X.Y. received research funding through a contract between Akebia Therapeutics and UTHealth to support a clinical trial on the effect of vadadustat in hospitalized patients with COVID-19 (NCT04478071). Akebia Therapeutics is not involved in conceptualization, design, data collection, analysis, decision to publish or preparation of the manuscript. W.R. and P.C. declare no competing interests.
Peer review
Peer review information
Nature Reviews Drug Discovery thanks Kevin Gardner and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
The Human Protein Atlas: https://www.proteinatlas.org/
Worldwide Protein Data Bank: https://www.wwpdb.org
Glossary
- ADORA2B receptor
-
A G-protein-coupled receptor that is activated by the signalling molecule adenosine. It has a role in regulating various physiological processes, including inflammation, angiogenesis and immune cell function.
- Angiopoietin 1 (ANGPT1) and ANGPT2
-
Two growth factors that have crucial roles in the regulation of angiogenesis, blood vessel maturation and vascular stability. Whereas ANGPT1 generally promotes vessel stabilization and maturation, ANGPT2 is often associated with vascular destabilization and remodelling, facilitating the actions of other angiogenic factors such as vascular endothelial growth factor.
- C-terminal transactivation domain
-
(C-TAD). A region found at the C terminus of certain transcription factors, including hypoxia-inducible factors, which is responsible for activating target gene transcription by recruiting coactivators and other components of the transcription machinery to the target gene promoter.
- Erythropoiesis-stimulating agents
-
(ESAs). A group of medications that mimic the effects of erythropoietin, promoting the production of red blood cells in the bone marrow and used to treat conditions such as anaemia.
- Erythropoietin
-
(EPO). A glycoprotein cytokine primarily produced by the kidneys in response to cellular hypoxia. Its main biological function is to stimulate erythropoiesis in the bone marrow.
- Factor inhibiting HIF
-
(FIH). Also known as asparaginyl hydroxylase; hydroxylates a specific asparagine residue in the C-terminal transactivation domain of hypoxia-inducible factors (HIFs). This modification inhibits the interaction between HIFs and the transcriptional coactivators histone acetyltransferase p300 and cyclic adenosine monophosphate response element binding protein (CREB) binding protein (CBP), thereby reducing HIF-mediated gene transcription.
- Hepcidin
-
Hepcidin is a liver-produced peptide hormone that regulates iron metabolism by inhibiting iron release from cells and decreasing dietary iron absorption, thereby maintaining iron homeostasis in the body.
- Hypoxia-responsive elements
-
(HREs). Short DNA sequences found in the regulatory regions of hypoxia-inducible genes, which allow for their transcriptional activation by hypoxia-inducible factors when cellular oxygen levels are low, thereby promoting cellular adaptation to hypoxic conditions.
- Lipopolysaccharide (LPS) treatment
-
The experimental administration of LPSs, molecules from the outer membrane of Gram-negative bacteria, to induce an inflammatory response in the lungs, simulating conditions such as acute respiratory distress syndrome for research purposes.
- Locked nucleic acid
-
A modified RNA nucleotide in which the ribose ring is ‘locked’ by a methylene bridge connecting the 2′-O atom with the 4′-C atom, which enhances its thermal stability and its affinity for complementary RNA and DNA strands, making it a valuable tool in molecular biology and medical research, particularly in the fields of genomics and therapeutics.
- Pacak–Zhuang syndrome
-
A rare condition characterized by the presence of paragangliomas, often in the head and neck region, and associated with somatic mutations in the HIF2A gene, which encodes hypoxia-inducible factor 2α. This syndrome may also be accompanied by polycythaemia and, in some cases, duodenal somatostatinomas.
- Polycythaemia
-
A condition characterized by an increased number of red blood cells in the bloodstream, which can thicken the blood, slow its flow and increase the risk of clotting. It can be a primary disease owing to mutations in the bone marrow cells, referred to as polycythaemia vera, or secondary, often as a response to hypoxia or certain tumours.
- Respiratory bursts
-
Rapid increases in cellular oxygen consumption, primarily in immune cells such as neutrophils and macrophages, that lead to the production of reactive oxygen species to combat pathogens during an immune response.
- Von Hippel–Lindau protein
-
(pVHL). A tumour suppressor protein involved in regulation of cellular responses to oxygen levels by targeting hypoxia-inducible factors for degradation under normal oxygen conditions.
Rights and permissions
About this article
Cite this article
Yuan, X., Ruan, W., Bobrow, B. et al. Targeting hypoxia-inducible factors: therapeutic opportunities and challenges. Nat Rev Drug Discov 23, 175–200 (2024). https://doi.org/10.1038/s41573-023-00848-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41573-023-00848-6
This article is cited by
-
Unraveling the role of HIF-1α in sepsis: from pathophysiology to potential therapeutics—a narrative review
Critical Care (2024)
-
PHD1-3 oxygen sensors in vivo—lessons learned from gene deletions
Pflügers Archiv - European Journal of Physiology (2024)