Abstract
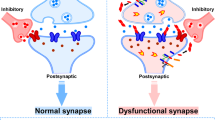
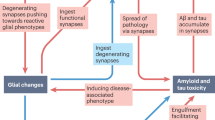
Synapse dysfunction and loss are hallmarks of neurodegenerative diseases that correlate with cognitive decline. However, the mechanisms and therapeutic strategies to prevent or reverse synaptic damage remain elusive. In this Review, we discuss recent advances in understanding the molecular and cellular pathways that impair synapses in neurodegenerative diseases, including the effects of protein aggregation and neuroinflammation. We also highlight emerging therapeutic approaches that aim to restore synaptic function and integrity, such as enhancing synaptic plasticity, preventing synaptotoxicity, modulating neuronal network activity and targeting immune signalling. We discuss the preclinical and clinical evidence for each strategy, as well as the challenges and opportunities for developing effective synapse-targeting therapeutics for neurodegenerative diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Wilson, D. M. et al. Hallmarks of neurodegenerative diseases. Cell 186, 693–714 (2023).
Terry, R. D. et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 30, 572–580 (1991).
DeKosky, S. T. & Scheff, S. W. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann. Neurol. 27, 457–464 (1990).
Blennow, K., Bogdanovic, N., Alafuzoff, I., Ekman, R. & Davidsson, P. Synaptic pathology in Alzheimer’s disease: relation to severity of dementia, but not to senile plaques, neurofibrillary tangles, or the ApoE4 allele. J. Neural Transm. 103, 603–618 (1996).
Scheff, S. W., Price, D. A., Schmitt, F. A., DeKosky, S. T. & Mufson, E. J. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology 68, 1501–1508 (2007).
Südhof, T. C. Towards an understanding of synapse formation. Neuron 100, 276–293 (2018).
Sheng, M. & Hoogenraad, C. C. The postsynaptic architecture of excitatory synapses: a more quantitative view. Annu. Rev. Biochem. 76, 823–847 (2007).
Dejanovic, B. et al. Palmitoylation of gephyrin controls receptor clustering and plasticity of GABAergic synapses. PLoS Biol. 12, e1001908 (2014).
Avery, M. C. & Krichmar, J. L. Neuromodulatory systems and their interactions: a review of models, theories, and experiments. Front. Neural Circuits 11, 108 (2017).
Ferreira-Vieira, T. H., Guimaraes, I. M., Silva, F. R. & Ribeiro, F. M. Alzheimer’s disease: targeting the cholinergic system. Curr. Neuropharmacol. 14, 101–115 (2016).
Allen, N. J. & Eroglu, C. Cell biology of astrocyte-synapse interactions. Neuron 96, 697–708 (2017).
Christopherson, K. S. et al. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 120, 421–433 (2005).
Stevens, B. et al. The classical complement cascade mediates CNS synapse elimination. Cell 131, 1164–1178 (2007).
Paolicelli, R. C. et al. Synaptic pruning by microglia is necessary for normal brain development. Science 333, 1456–1458 (2011).
Chung, W.-S. et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 504, 394–400 (2013).
Lee, J.-H. et al. Astrocytes phagocytose adult hippocampal synapses for circuit homeostasis. Nature 590, 612–617 (2021).
Hammond, T. R., Marsh, S. E. & Stevens, B. Immune signaling in neurodegeneration. Immunity 50, 955–974 (2019).
Bohlen, C. J., Friedman, B. A., Dejanovic, B. & Sheng, M. Microglia in brain development, homeostasis, and neurodegeneration. Annu. Rev. Genet. 53, 263–288 (2019).
Dejanovic, B. et al. Changes in the synaptic proteome in tauopathy and rescue of tau-induced synapse loss by C1q antibodies. Neuron 100, 1322–1336.e7 (2018).
Dejanovic, B. et al. Complement C1q-dependent excitatory and inhibitory synapse elimination by astrocytes and microglia in Alzheimer’s disease mouse models. Nat. Aging 2, 837–850 (2022).
Wu, T. et al. Complement C3 is activated in human AD brain and is required for neurodegeneration in mouse models of amyloidosis and tauopathy. Cell Rep. 28, 2111–2123.e6 (2019).
Zhang, J. et al. Neurotoxic microglia promote TDP-43 proteinopathy in progranulin deficiency. Nature 588, 459–465 (2020).
Lui, H. et al. Progranulin deficiency promotes circuit-specific synaptic pruning by microglia via complement activation. Cell 165, 921–935 (2016).
Vasek, M. J. et al. A complement–microglial axis drives synapse loss during virus-induced memory impairment. Nature 534, 538–543 (2016).
Hong, S. et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352, aad8373 (2016).
Werneburg, S. et al. Targeted complement inhibition at synapses prevents microglial synaptic engulfment and synapse loss in demyelinating disease. Immunity 52, 167–182.e7 (2020).
Comer, A. L. et al. Increased expression of schizophrenia-associated gene C4 leads to hypoconnectivity of prefrontal cortex and reduced social interaction. PLoS Biol. 18, e3000604 (2020).
Wilton, D. K. et al. Microglia and complement mediate early corticostriatal synapse loss and cognitive dysfunction in Huntington’s disease. Nat. Med. https://doi.org/10.1038/s41591-023-02566-3 (2023).
Yilmaz, M. et al. Overexpression of schizophrenia susceptibility factor human complement C4A promotes excessive synaptic loss and behavioral changes in mice. Nat. Neurosci. 24, 214–224 (2021).
Zhou, J. et al. The neuronal pentraxin Nptx2 regulates complement activity and restrains microglia-mediated synapse loss in neurodegeneration. Sci. Transl Med. 15, eadf0141 (2023).
Hansen, D. V., Hanson, J. E. & Sheng, M. Microglia in Alzheimer’s disease. J. Cell Biol. 217, 459–472 (2018).
Srinivasan, K. et al. Alzheimer’s patient microglia exhibit enhanced aging and unique transcriptional activation. Cell Rep. 31, 107843 (2020).
Smajić, S. et al. Single-cell sequencing of human midbrain reveals glial activation and a Parkinson-specific neuronal state. Brain 145, awab446 (2021).
Menden, K. et al. Integrated multi-omics analysis reveals common and distinct dysregulated pathways for genetic subtypes of frontotemporal dementia. Preprint at bioRxiv https://doi.org/10.21203/rs.3.rs-153135/v1 (2021).
Limone, F. et al. Single-nucleus sequencing reveals enriched expression of genetic risk factors sensitises motor neurons to degeneration in ALS. Preprint at bioRxiv https://doi.org/10.1101/2021.07.12.452054 (2021).
Wilton, D. K., Dissing-Olesen, L. & Stevens, B. Neuron-glia signaling in synapse elimination. Annu. Rev. Neurosci. 42, 107–127 (2019).
Liddelow, S. A. et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 (2017).
Yun, S. P. et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat. Med. 24, 931–938 (2018).
Liddelow, S. A. & Barres, B. A. Reactive astrocytes: production, function, and therapeutic potential. Immunity 46, 957–967 (2017).
Millecamps, S. & Julien, J.-P. Axonal transport deficits and neurodegenerative diseases. Nat. Rev. Neurosci. 14, 161–176 (2013).
Geden, M. J. & Deshmukh, M. Axon degeneration: context defines distinct pathways. Curr. Opin. Neurobiol. 39, 108–115 (2016).
Telias, M. & Segal, M. Editorial: pathological hyperactivity and hyperexcitability in the central nervous system. Front. Mol. Neurosci. 15, 955542 (2022).
Palop, J. J. & Mucke, L. Network abnormalities and interneuron dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 17, 777–792 (2016).
Do-Ha, D., Buskila, Y. & Ooi, L. Impairments in motor neurons, interneurons and astrocytes contribute to hyperexcitability in ALS: underlying mechanisms and paths to therapy. Mol. Neurobiol. 55, 1410–1418 (2018).
Pilotto, F. et al. Early molecular layer interneuron hyperactivity triggers Purkinje neuron degeneration in SCA1. Neuron 111, 2523–2543 (2023).
Dong, X., Wang, Y. & Qin, Z. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol. Sin. 30, 379–387 (2009).
Soto, C. & Pritzkow, S. Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases. Nat. Neurosci. 21, 1332–1340 (2018).
Hesse, R. et al. Comparative profiling of the synaptic proteome from Alzheimer’s disease patients with focus on the APOE genotype. Acta Neuropathol. Commun. 7, 214 (2019).
Aryal, S. et al. Deep proteomics identifies shared molecular pathway alterations in synapses of patients with schizophrenia and bipolar disorder and mouse model. Cell Rep. 42, 112497 (2023).
Martínez-Serra, R., Alonso-Nanclares, L., Cho, K. & Giese, K. P. Emerging insights into synapse dysregulation in Alzheimer’s disease. Brain Commun. 4, fcac083 (2022).
Largo-Barrientos, P. et al. Lowering synaptogyrin-3 expression rescues tau-induced memory defects and synaptic loss in the presence of microglial activation. Neuron 109, 767–777 (2021).
Zhou, L. et al. Tau association with synaptic vesicles causes presynaptic dysfunction. Nat. Commun. 8, 15295 (2017).
Zhao, X. et al. Caspase-2 cleavage of tau reversibly impairs memory. Nat. Med. 22, 1268–1276 (2016).
Hoover, B. R. et al. Mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron 68, 1067–1081 (2010).
Ittner, L. M. et al. Dendritic function of tau mediates amyloid-β toxicity in Alzheimer’s disease mouse models. Cell 142, 387–397 (2009).
Nieweg, K., Andreyeva, A., Stegen, B., van, Tanriöver, G. & Gottmann, K. Alzheimer’s disease-related amyloid-β induces synaptotoxicity in human iPS cell-derived neurons. Cell Death Dis. 6, e1709 (2015).
Sharma, M. & Burré, J. α-Synuclein in synaptic function and dysfunction. Trends Neurosci. 46, 153–166 (2023).
Sengupta, U. & Kayed, R. Amyloid β, tau, and α-synuclein aggregates in the pathogenesis, prognosis, and therapeutics for neurodegenerative diseases. Prog. Neurobiol. 214, 102270 (2022).
Hackos, D. H. & Hanson, J. E. Diverse modes of NMDA receptor positive allosteric modulation: mechanisms and consequences. Neuropharmacology 112, 34–45 (2017).
Salpietro, V. et al. AMPA receptor GluA2 subunit defects are a cause of neurodevelopmental disorders. Nat. Commun. 10, 3094 (2019).
Singh, T. et al. Rare coding variants in ten genes confer substantial risk for schizophrenia. Nature 604, 509–516 (2022).
Lauterborn, J. C., Lynch, G., Vanderklish, P., Arai, A. & Gall, C. M. Positive modulation of AMPA receptors increases neurotrophin expression by hippocampal and cortical neurons. J. Neurosci. 20, 8–21 (2000).
Lynch, G. & Gall, C. M. Ampakines and the threefold path to cognitive enhancement. Trends Neurosci. 29, 554–562 (2006).
Rex, C. S. et al. Restoration of long-term potentiation in middle-aged hippocampus after induction of brain-derived neurotrophic factor. J. Neurophysiol. 96, 677–685 (2006).
Jourdi, H. et al. Positive AMPA receptor modulation rapidly stimulates BDNF release and increases dendritic mRNA translation. J. Neurosci. 29, 8688–8697 (2009).
Baudry, M. et al. Ampakines promote spine actin polymerization, long-term potentiation, and learning in a mouse model of Angelman syndrome. Neurobiol. Dis. 47, 210–215 (2012).
Hampson, R. E., Rogers, G., Lynch, G. & Deadwyler, S. A. Facilitative effects of the ampakine cx516 on short-term memory in rats: enhancement of delayed-nonmatch-to-sample performance. J. Neurosci. 18, 2740–2747 (1998).
Black, M. D. Therapeutic potential of positive AMPA modulators and their relationship to AMPA receptor subunits. A review of preclinical data. Psychopharmacology 179, 154–163 (2005).
Goff, D. C. et al. A placebo-controlled add-on trial of the ampakine, CX516, for cognitive deficits in schizophrenia. Neuropsychopharmacology 33, 465–472 (2008).
Partin, K. M. AMPA receptor potentiators: from drug design to cognitive enhancement. Curr. Opin. Pharmacol. 20, 46–53 (2015).
Ward, S. E., Bax, B. D. & Harries, M. Challenges for and current status of research into positive modulators of AMPA receptors. Brit. J. Pharmacol. 160, 181–190 (2010).
Shaffer, C. L. et al. Positive allosteric modulation of AMPA receptors from efficacy to toxicity: the interspecies exposure-response continuum of the novel potentiator PF-4778574. J. Pharmacol. Exp. Ther. 347, 212–224 (2013).
Chappell, A. S. et al. AMPA potentiator treatment of cognitive deficits in Alzheimer disease. Neurology 68, 1008–1012 (2007).
Bernard, K. et al. A 24-week double-blind placebo-controlled study of the efficacy and safety of the AMPA modulator S47445 in patients with mild to moderate Alzheimer’s disease and depressive symptoms. Alzheimer’s Dement. 5, 231–240 (2019).
Ranganathan, M. et al. Attenuation of ketamine-induced impairment in verbal learning and memory in healthy volunteers by the AMPA receptor potentiator PF-04958242. Mol. Psychiatry 22, 1633–1640 (2017).
Hansen, K. B. et al. Structure, function, and allosteric modulation of NMDA receptors. J. Gen. Physiol. 150, 1081–1105 (2018).
Lin, C.-H., Huang, Y.-J., Lin, C.-J., Lane, H.-Y. & Tsai, G. E. NMDA neurotransmission dysfunction in mild cognitive impairment and Alzheimer’s disease. Curr. Pharm. Des. 20, 5169–5179 (2013).
Javitt, D. C. & Zukin, S. R. Recent advances in the phencyclidine model of schizophrenia. Am. J. Psychiatry 148, 1301–1308 (1991).
Krystal, J. H. et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans: psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch. Gen. Psychiatry 51, 199–214 (1994).
Lahti, A. C., Koffel, B., LaPorte, D. & Tamminga, C. A. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacol 13, 9–19 (1995).
Ripke, S. et al. Biological insights from 108 schizophrenia-associated genetic loci. Nature 511, 421–427 (2014).
XiangWei, W., Jiang, Y. & Yuan, H. De novo mutations and rare variants occurring in NMDA receptors. Curr. Opin. Physiol. 2, 27–35 (2018).
Homayoun, H. & Moghaddam, B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J. Neurosci. 27, 11496–11500 (2007).
Cohen, S. M., Tsien, R. W., Goff, D. C. & Halassa, M. M. The impact of NMDA receptor hypofunction on GABAergic neurons in the pathophysiology of schizophrenia. Schizophr. Res. 167, 98–107 (2015).
Zhou, Q. & Sheng, M. NMDA receptors in nervous system diseases. Neuropharmacology 74, 69–75 (2013).
Hackos, D. H. et al. Positive allosteric modulators of GluN2A-containing NMDARs with distinct modes of action and impacts on circuit function. Neuron 89, 983–999 (2016).
Volgraf, M. et al. Discovery of GluN2A-selective NMDA receptor positive allosteric modulators (PAMs): tuning deactivation kinetics via structure-based design. J. Med. Chem. 59, 2760–2779 (2016).
Villemure, E. et al. GluN2A-selective pyridopyrimidinone series of NMDAR positive allosteric modulators with an improved in vivo profile. ACS Med. Chem. Lett. 8, 84–89 (2017).
Hanson, J. E. et al. GluN2A NMDA receptor enhancement improves brain oscillations, synchrony, and cognitive functions in Dravet syndrome and Alzheimer’s disease models. Cell Rep. 30, 381–396 (2020).
Hill, M. D. et al. SAGE-718: a first-in-class N-methyl-d-aspartate receptor positive allosteric modulator for the potential treatment of cognitive impairment. J. Med. Chem. 65, 9063–9075 (2022).
Huntley, M. A. et al. Genome-wide analysis of differential gene expression and splicing in excitatory neurons and interneuron subtypes. J. Neurosci. 40, 958–973 (2019).
Hanson, J. E. et al. Therapeutic potential of N-methyl-d-aspartate receptor modulators in psychiatry. Neuropsychopharmacology https://doi.org/10.1038/s41386-023-01614-3 (2023).
Yao, L., Grand, T., Hanson, J. E., Paoletti, P. & Zhou, Q. Higher ambient synaptic glutamate at inhibitory versus excitatory neurons differentially impacts NMDA receptor activity. Nat. Commun. 9, 4000 (2018).
Chao, M. V. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat. Rev. Neurosci. 4, 299–309 (2003).
Lu, B., Nagappan, G. & Lu, Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb. Exp. Pharmacol. 220, 223–250 (2014).
Greenberg, M. E., Xu, B., Lu, B. & Hempstead, B. L. New insights in the biology of BDNF synthesis and release: implications in CNS function. J. Neurosci. 29, 12764–12767 (2009).
Nagahara, A. H. & Tuszynski, M. H. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat. Rev. Drug Discov. 10, 209–219 (2011).
Lu, B., Nagappan, G., Guan, X., Nathan, P. J. & Wren, P. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat. Rev. Neurosci. 14, 401–416 (2013).
Wang, C. S., Kavalali, E. T. & Monteggia, L. M. BDNF signaling in context: from synaptic regulation to psychiatric disorders. Cell 185, 62–76 (2022).
Casarotto, P. C. et al. Antidepressant drugs act by directly binding to TRKB neurotrophin receptors. Cell 184, 1299–1313 (2021).
Moliner, R. et al. Psychedelics promote plasticity by directly binding to BDNF receptor TrkB. Nat. Neurosci. 26, 1032–1041 (2023).
Sakane, T. & Pardridge, W. M. Carboxyl-directed pegylation of brain-derived neurotrophic factor markedly reduces systemic clearance with minimal loss of biologic activity. Pharm. Res. 14, 1085–1091 (1997).
Poduslo, J. F. & Curran, G. L. Permeability at the blood-brain and blood-nerve barriers of the neurotrophic factors: NGF, CNTF, NT-3, BDNF. Mol. Brain Res. 36, 280–286 (1996).
Soderquist, R. G. et al. PEGylation of brain‐derived neurotrophic factor for preserved biological activity and enhanced spinal cord distribution. J. Biomed. Mater. Res. A 91A, 719–729 (2009).
Morse, J. et al. Brain-derived neurotrophic factor (BDNF) prevents the degeneration of medial septal cholinergic neurons following fimbria transection. J. Neurosci. 13, 4146–4156 (1993).
Dittrich, F. et al. Pharmacokinetics of intrathecally applied BDNF and effects on spinal motoneurons. Exp. Neurol. 141, 225–239 (1996).
Croll, S. D. et al. Co-infusion with a TrkB-Fc receptor body carrier enhances BDNF distribution in the adult rat brain. Exp. Neurol. 152, 20–33 (1998).
Hempstead, B. L. The many faces of p75NTR. Curr. Opin. Neurobiol. 12, 260–267 (2002).
Lu, B., Pang, P. T. & Woo, N. H. The yin and yang of neurotrophin action. Nat. Rev. Neurosci. 6, 603–614 (2005).
Henriques, A., Pitzer, C. & Schneider, A. Neurotrophic growth factors for the treatment of amyotrophic lateral sclerosis: where do we stand? Front. Neurosci. 4, 32 (2010).
Jang, S.-W. et al. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc. Natl Acad. Sci. USA 107, 2687–2692 (2010).
Longo, F. M. & Massa, S. M. Small-molecule modulation of neurotrophin receptors: a strategy for the treatment of neurological disease. Nat. Rev. Drug Discov. 12, 507–525 (2013).
Simmons, D. A. et al. A small molecule TrkB ligand reduces motor impairment and neuropathology in R6/2 and BACHD mouse models of Huntington’s disease. J. Neurosci. 33, 18712–18727 (2013).
Todd, D. et al. A monoclonal antibody TrkB receptor agonist as a potential therapeutic for Huntington’s disease. PLoS ONE 9, e87923 (2014).
Boltaev, U. et al. Multiplex quantitative assays indicate a need for reevaluating reported small-molecule TrkB agonists. Sci. Signal. 10, eaal1670 (2017).
Qian, M. D. et al. Novel agonist monoclonal antibodies activate Trkb receptors and demonstrate potent neurotrophic activities. J. Neurosci. 26, 9394–9403 (2006).
Bai, Y. et al. An agonistic TrkB mAb causes sustained TrkB activation, delays RGC death, and protects the retinal structure in optic nerve axotomy and in glaucoma. Invest. Ophthalmol. Vis. Sci. 51, 4722–4731 (2010).
Traub, S. et al. Pharmaceutical characterization of tropomyosin receptor kinase B-agonistic antibodies on human induced pluripotent stem (hiPS) cell-derived neurons. J. Pharmacol. Exp. Ther. 361, 355–365 (2017).
Merkouris, S. et al. Fully human agonist antibodies to TrkB using autocrine cell-based selection from a combinatorial antibody library. Proc. Natl Acad. Sci. USA 115, E7023–E7032 (2018).
Guo, W. et al. TrkB agonistic antibodies superior to BDNF: utility in treating motoneuron degeneration. Neurobiol. Dis. 132, 104590 (2019).
Kim, G. S., Cho, S., Nelson, J. W., Zipfel, G. J. & Han, B. H. TrkB agonist antibody pretreatment enhances neuronal survival and long-term sensory motor function following hypoxic ischemic injury in neonatal rats. PLoS ONE 9, e88962 (2014).
Han, F., Guan, X., Guo, W. & Lu, B. Therapeutic potential of a TrkB agonistic antibody for ischemic brain injury. Neurobiol. Dis. 127, 570–581 (2019).
Hu, Y., Cho, S. & Goldberg, J. L. Neurotrophic effect of a novel TrkB agonist on retinal ganglion cells. Investig. Opthalmol. Vis. Sci. 51, 1747 (2010).
Fouad, K., Vavrek, R. & Cho, S. A TrkB antibody agonist promotes plasticity after cervical spinal cord injury in adult rats. J. Neurotrauma 38, 1338–1348 (2021).
Yu, S. P., Jiang, M. Q., Shim, S. S., Pourkhodadad, S. & Wei, L. Extrasynaptic NMDA receptors in acute and chronic excitotoxicity: implications for preventive treatments of ischemic stroke and late-onset Alzheimer’s disease. Mol. Neurodegener. 18, 43 (2023).
Kumar, A. et al. S-Sulfocysteine/NMDA receptor-dependent signaling underlies neurodegeneration in molybdenum cofactor deficiency. J. Clin. Invest. 127, 4365–4378 (2017).
Verma, M., Lizama, B. N. & Chu, C. T. Excitotoxicity, calcium and mitochondria: a triad in synaptic neurodegeneration. Transl Neurodegener. 11, 3 (2022).
Parsons, M. P. & Raymond, L. A. Extrasynaptic NMDA receptor involvement in central nervous system disorders. Neuron 82, 279–293 (2014).
Simon, R. P., Swan, J. H., Griffiths, T. & Meldrum, B. S. Blockade of N-methyl-d-aspartate receptors may protect against ischemic damage in the brain. Science 226, 850–852 (1984).
Wieloch, T. Hypoglycemia-induced neuronal damage prevented by an N-methyl-d-aspartate antagonist. Science 230, 681–683 (1985).
Yurkewicz, L., Weaver, J., Bullock, M. R. & Marshall, L. F. The effect of the selective NMDA receptor antagonist traxoprodil in the treatment of traumatic brain injury. J. Neurotrauma 22, 1428–1443 (2005).
Merchant, R. E. et al. A double‐blind, placebo‐controlled study of the safety, tolerability and pharmacokinetics of CP‐101,606 in patients with a mild or moderate traumatic brain injury. Ann. NY Acad. Sci. 890, 42–50 (1999).
Ikonomidou, C. & Turski, L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. 1, 383–386 (2002).
Hanson, J. E. et al. Chronic GluN2B antagonism disrupts behavior in wild-type mice without protecting against synapse loss or memory impairment in Alzheimer’s disease mouse models. J. Neurosci. 34, 8277–8288 (2014).
Preskorn, S. H. et al. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-d-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J. Clin. Psychopharm. 28, 631–637 (2008).
Ghaemi, N., Sverdlov, A., Shelton, R. & Litman, R. Efficacy and safety of mij821 in patients with treatment-resistant depression: results from a randomized, placebo-controlled, proof-of-concept study. Eur. Psychiatry 64, S334–S335 (2021).
Xia, P., Chen, H. V., Zhang, D. & Lipton, S. A. Memantine preferentially blocks extrasynaptic over synaptic NMDA receptor currents in hippocampal autapses. J. Neurosci. 30, 11246–11250 (2010).
Johnson, J. W. & Kotermanski, S. E. Mechanism of action of memantine. Curr. Opin. Pharmacol. 6, 61–67 (2006).
Oliver, D. et al. Memantine inhibits efferent cholinergic transmission in the cochlea by blocking nicotinic acetylcholine receptors of outer hair cells. Mol. Pharmacol. 60, 183–189 (2001).
Rammes, G., Rupprecht, R., Ferrari, U., Zieglgänsberger, W. & Parsons, C. G. The N-methyl-d-aspartate receptor channel blockers memantine, MRZ 2/579 and other amino-alkyl-cyclohexanes antagonise 5-HT(3) receptor currents in cultured HEK-293 and N1E-115 cell systems in a non-competitive manner. Neurosci. Lett. 306, 81–84 (2001).
Aracava, Y., Pereira, E. F. R., Maelicke, A. & Albuquerque, E. X. Memantine blocks α7* nicotinic acetylcholine receptors more potently than N-methyl-d-aspartate receptors in rat hippocampal neurons. J. Pharmacol. Exp. Ther. 312, 1195–1205 (2004).
Hamilton, A., Esseltine, J. L., DeVries, R. A., Cregan, S. P. & Ferguson, S. S. G. Metabotropic glutamate receptor 5 knockout reduces cognitive impairment and pathogenesis in a mouse model of Alzheimer’s disease. Mol. Brain 7, 40 (2014).
Hamilton, A. et al. Chronic pharmacological mGluR5 inhibition prevents cognitive impairment and reduces pathogenesis in an Alzheimer disease mouse model. Cell Rep. 15, 1859–1865 (2016).
Um, J. W. et al. Metabotropic glutamate receptor 5 is a coreceptor for Alzheimer Aβ oligomer bound to cellular prion protein. Neuron 79, 887–902 (2013).
Haas, L. T. et al. Silent allosteric modulation of mGluR5 maintains glutamate signaling while rescuing Alzheimer’s mouse phenotypes. Cell Rep. 20, 76–88 (2017).
Spurrier, J. et al. Reversal of synapse loss in Alzheimer mouse models by targeting mGluR5 to prevent synaptic tagging by C1Q. Sci. Transl Med. 14, eabi8593 (2022).
Sturchler, E., Galichet, A., Weibel, M., Leclerc, E. & Heizmann, C. W. Site-specific blockade of RAGE-Vd prevents amyloid-β oligomer neurotoxicity. J. Neurosci. 28, 5149–5158 (2008).
Li, S. & Stern, A. M. Bioactive human Alzheimer brain soluble Aβ: pathophysiology and therapeutic opportunities. Mol. Psychiatry 27, 3182–3191 (2022).
Watkins, T. A. et al. DLK initiates a transcriptional program that couples apoptotic and regenerative responses to axonal injury. Proc. Natl Acad. Sci. USA 110, 4039–4044 (2013).
Osterloh, J. M. et al. dSarm/Sarm1 is required for activation of an injury-induced axon death pathway. Science 337, 481–484 (2012).
Summers, D. W., Frey, E., Walker, L. J., Milbrandt, J. & DiAntonio, A. DLK activation synergizes with mitochondrial dysfunction to downregulate axon survival factors and promote SARM1-dependent axon degeneration. Mol. Neurobiol. 57, 1146–1158 (2020).
Ghosh, A. S. et al. DLK induces developmental neuronal degeneration via selective regulation of proapoptotic JNK activity. J. Cell Biol. 194, 751–764 (2011).
Larhammar, M. et al. Dual leucine zipper kinase-dependent PERK activation contributes to neuronal degeneration following insult. eLife 6, e20725 (2017).
Siu, M., Ghosh, A. S. & Lewcock, J. W. Dual leucine zipper kinase inhibitors for the treatment of neurodegeneration. J. Med. Chem. 61, 8078–8087 (2018).
Welsbie, D. S. et al. Functional genomic screening identifies dual leucine zipper kinase as a key mediator of retinal ganglion cell death. Proc. Natl Acad. Sci. USA 110, 4045–4050 (2013).
Pozniak, C. D. et al. Dual leucine zipper kinase is required for excitotoxicity-induced neuronal degeneration. J. Exp. Med. 210, 2553–2567 (2013).
Pichon, C. E. L. et al. Loss of dual leucine zipper kinase signaling is protective in animal models of neurodegenerative disease. Sci. Transl Med. 9, eaag0394 (2017).
Patel, S. et al. Discovery of dual leucine zipper kinase (DLK, MAP3K12) inhibitors with activity in neurodegeneration models. J. Med. Chem. 58, 401–418 (2015).
Katz, J. S. et al. A phase 1 study of GDC‐0134, a dual leucine zipper kinase inhibitor, in ALS. Ann. Clin. Transl. Neurol. 9, 50–66 (2022).
Larhammar, M., Huntwork-Rodriguez, S., Rudhard, Y., Sengupta-Ghosh, A. & Lewcock, J. W. The Ste20 family kinases MAP4K4, MINK1, and TNIK converge to regulate stress-induced JNK signaling in neurons. J. Neurosci. 37, 11074–11084 (2017).
Bos, P. H. et al. Development of MAP4 kinase inhibitors as motor neuron-protecting agents. Cell Chem. Biol. 26, 1703–1715 (2019).
Coleman, M. P. & Freeman, M. R. Wallerian degeneration, WldS, and Nmnat. Annu. Rev. Neurosci. 33, 245–267 (2010).
Gilley, J., Orsomando, G., Nascimento-Ferreira, I. & Coleman, M. P. Absence of SARM1 rescues development and survival of NMNAT2-deficient axons. Cell Rep. 10, 1974–1981 (2015).
Gerdts, J., Brace, E. J., Sasaki, Y., DiAntonio, A. & Milbrandt, J. SARM1 activation triggers axon degeneration locally via NAD+ destruction. Science 348, 453–457 (2015).
Figley, M. D. et al. SARM1 is a metabolic sensor activated by an increased NMN/NAD+ ratio to trigger axon degeneration. Neuron 109, 1118–1136 (2021).
Essuman, K. et al. The SARM1 toll/interleukin-1 receptor domain possesses intrinsic NAD+ cleavage activity that promotes pathological axonal degeneration. Neuron 93, 1334–1343 (2017).
Summers, D. W., DiAntonio, A. & Milbrandt, J. Mitochondrial dysfunction induces Sarm1-dependent cell death in sensory neurons. J. Neurosci. 34, 9338–9350 (2014).
Geisler, S. et al. Prevention of vincristine-induced peripheral neuropathy by genetic deletion of SARM1 in mice. Brain 139, 3092–3108 (2016).
Henninger, N. et al. Attenuated traumatic axonal injury and improved functional outcome after traumatic brain injury in mice lacking Sarm1. Brain 139, 1094–1105 (2016).
Turkiew, E., Falconer, D., Reed, N. & Höke, A. Deletion of Sarm1 gene is neuroprotective in two models of peripheral neuropathy. J. Peripher. Nerv. Syst. 22, 162–171 (2017).
Marion, C. M., McDaniel, D. P. & Armstrong, R. C. Sarm1 deletion reduces axon damage, demyelination, and white matter atrophy after experimental traumatic brain injury. Exp. Neurol. 321, 113040 (2019).
White, M. A. et al. Sarm1 deletion suppresses TDP-43-linked motor neuron degeneration and cortical spine loss. Acta Neuropathol. Commun. 7, 166 (2019).
Bosanac, T. et al. Pharmacological SARM1 inhibition protects axon structure and function in paclitaxel-induced peripheral neuropathy. Brain 144, 3226–3238 (2021).
Hughes, R. O. et al. Small molecule SARM1 inhibitors recapitulate the SARM1−/− phenotype and allow recovery of a metastable pool of axons fated to degenerate. Cell Rep. 34, 108588 (2021).
Bratkowski, M. et al. Uncompetitive, adduct-forming SARM1 inhibitors are neuroprotective in preclinical models of nerve injury and disease. Neuron 110, 3711–3726.e16 (2022).
Verret, L. et al. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell 149, 708–721 (2012).
Lam, A. D. et al. Silent hippocampal seizures and spikes identified by foramen ovale electrodes in Alzheimer’s disease. Nat. Med. 23, 678–680 (2017).
Lynch, B. A. et al. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc. Natl Acad. Sci. USA 101, 9861–9866 (2004).
Crowder, K. M. et al. Abnormal neurotransmission in mice lacking synaptic vesicle protein 2A (SV2A). Proc. Natl Acad. Sci. USA 96, 15268–15273 (1999).
Custer, K. L., Austin, N. S., Sullivan, J. M. & Bajjalieh, S. M. Synaptic vesicle protein 2 enhances release probability at quiescent synapses. J. Neurosci. 26, 1303–1313 (2006).
Sanchez, P. E. et al. Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer’s disease model. Proc. Natl Acad. Sci. USA 109, E2895–E2903 (2012).
Nygaard, H. B. et al. Brivaracetam, but not ethosuximide, reverses memory impairments in an Alzheimer’s disease mouse model. Alzheimer’s Res. Ther. 7, 25 (2015).
Yassa, M. A. et al. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic mild cognitive impairment. Neuroimage 51, 1242–1252 (2010).
Leal, S. L., Landau, S. M., Bell, R. K. & Jagust, W. J. Hippocampal activation is associated with longitudinal amyloid accumulation and cognitive decline. eLife 6, e22978 (2017).
Bakker, A., Albert, M. S., Krauss, G., Speck, C. L. & Gallagher, M. Response of the medial temporal lobe network in amnestic mild cognitive impairment to therapeutic intervention assessed by fMRI and memory task performance. Neuroimage Clin. 7, 688–698 (2015).
Bakker, A. et al. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron 74, 467–474 (2012).
Vossel, K. et al. Effect of levetiracetam on cognition in patients with Alzheimer disease with and without epileptiform activity. JAMA Neurol. 78, 1345–1354 (2021).
Consortium, E. et al. De novo mutations in epileptic encephalopathies. Nature 501, 217–221 (2013).
Braat, S. & Kooy, R. F. The GABAA receptor as a therapeutic target for neurodevelopmental disorders. Neuron 86, 1119–1130 (2015).
Dejanovic, B. et al. Exonic microdeletions of the gephyrin gene impair GABAergic synaptic inhibition in patients with idiopathic generalized epilepsy. Neurobiol. Dis. 67, 88–96 (2014).
Reinthaler, E. M. et al. Rare variants in γ‐aminobutyric acid type A receptor genes in rolandic epilepsy and related syndromes. Ann. Neurol. 77, 972–986 (2015).
Guina, J. & Merrill, B. Benzodiazepines I: upping the care on downers: the evidence of risks, benefits and alternatives. J. Clin. Med. 7, 17 (2018).
Griessner, J. et al. Central amygdala circuit dynamics underlying the benzodiazepine anxiolytic effect. Mol. Psychiatry 26, 534–544 (2021).
McMackin, R. et al. Measuring network disruption in neurodegenerative diseases: new approaches using signal analysis. J. Neurol. Neurosurg. Psychiatry 90, 1011–1020 (2019).
Meltzer-Brody, S. et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet 392, 1058–1070 (2018).
Haas, S. L. et al. Pharmacodynamic and pharmacokinetic effects of TPA023, a GABAA α2,3 subtype-selective agonist, compared to lorazepam and placebo in healthy volunteers. J. Psychopharmacol. 21, 374–383 (2007).
Buchanan, R. W. et al. A randomized clinical trial of MK-0777 for the treatment of cognitive impairments in people with schizophrenia. Biol. Psychiatry 69, 442–449 (2011).
Jacob, T. C. Neurobiology and therapeutic potential of α5-GABA type A receptors. Front. Mol. Neurosci. 12, 179 (2019).
Koh, M. T., Rosenzweig-Lipson, S. & Gallagher, M. Selective GABAA α5 positive allosteric modulators improve cognitive function in aged rats with memory impairment. Neuropharmacology 64, 145–152 (2013).
Bernardo, A. M. et al. Positive allosteric modulation of α5-GABA A receptor in the 5XFAD mouse model has cognitive and neurotrophic benefits. Preprint at bioRxiv https://doi.org/10.1101/2022.09.30.510361 (2022).
Corbett, B. F. et al. Sodium channel cleavage is associated with aberrant neuronal activity and cognitive deficits in a mouse model of Alzheimer’s disease. J. Neurosci. 33, 7020–7026 (2013).
Osteen, J. D., Sampson, K., Iyer, V., Julius, D. & Bosmans, F. Pharmacology of the Nav1.1 domain IV voltage sensor reveals coupling between inactivation gating processes. Proc. Natl Acad. Sci. USA 114, 6836–6841 (2017).
Jensen, H. S., Grunnet, M. & Bastlund, J. F. Therapeutic potential of Na(V)1.1 activators. Trends Pharmacol. Sci. 35, 113–118 (2013).
Southwell, D. G. et al. Interneurons from embryonic development to cell-based therapy. Science 344, 1240622 (2014).
Baraban, S. C. et al. Reduction of seizures by transplantation of cortical GABAergic interneuron precursors into Kv1.1 mutant mice. Proc. Natl Acad. Sci. USA 106, 15472–15477 (2009).
Hunt, R. F., Girskis, K. M., Rubenstein, J. L., Alvarez-Buylla, A. & Baraban, S. C. GABA progenitors grafted into the adult epileptic brain control seizures and abnormal behavior. Nat. Neurosci. 16, 692–697 (2013).
Martinez-Losa, M. et al. Nav1.1-overexpressing interneuron transplants restore brain rhythms and cognition in a mouse model of Alzheimer’s disease. Neuron 98, 75–89 (2018).
Tong, L. M. et al. Inhibitory interneuron progenitor transplantation restores normal learning and memory in ApoE4 knock-in mice without or with Aβ accumulation. J. Neurosci. 34, 9506–9515 (2014).
Zhu, B., Eom, J. & Hunt, R. F. Transplanted interneurons improve memory precision after traumatic brain injury. Nat. Commun. 10, 5156 (2019).
Dyck, C. H. et al. Lecanemab in early Alzheimer’s disease. N. Engl. J. Med. 388, 9–21 (2022).
Brandebura, A. N., Paumier, A., Onur, T. S. & Allen, N. J. Astrocyte contribution to dysfunction, risk and progression in neurodegenerative disorders. Nat. Rev. Neurosci. 24, 23–39 (2023).
Stephan, A. H., Barres, B. A. & Stevens, B. The complement system: an unexpected role in synaptic pruning during development and disease. Neuroscience 35, 369–389 (2012).
Mastellos, D. C., Ricklin, D. & Lambris, J. D. Clinical promise of next-generation complement therapeutics. Nat. Rev. Drug Discov. 18, 707–729 (2019).
Ende, E. L. et al. Elevated CSF and plasma complement proteins in genetic frontotemporal dementia: results from the GENFI study. J. Neuroinflamm. 19, 217 (2022).
Kamitaki, N. et al. Complement genes contribute sex-biased vulnerability in diverse disorders. Nature 582, 577–581 (2020).
Consortium, S.W.G. of the P.G., Sekar et al. Schizophrenia risk from complex variation of complement component 4. Nature 530, 177–183 (2016).
Lambert, J.-C. et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat. Genet. 41, 1094–1099 (2009).
Shi, Q. et al. Complement C3-deficient mice fail to display age-related hippocampal decline. J. Neurosci. 35, 13029–13042 (2015).
Stephan, A. H. et al. A dramatic increase of C1q protein in the CNS during normal aging. J. Neurosci. 33, 13460–13474 (2013).
Litvinchuk, A. et al. Complement C3aR inactivation attenuates tau pathology and reverses an immune network deregulated in tauopathy models and Alzheimer’s disease. Neuron 100, 1337–1353.e5 (2018).
Hammond, J. W. et al. Complement-dependent synapse loss and microgliosis in a mouse model of multiple sclerosis. Brain Behav. Immun. 87, 739–750 (2020).
Yin, C. et al. ApoE attenuates unresolvable inflammation by complex formation with activated C1q. Nat. Med. 25, 496–506 (2019).
Zhong, L. et al. TREM2 receptor protects against complement-mediated synaptic loss by binding to complement C1q during neurodegeneration. Immunity 56, 1794–1808 (2023).
Vukojicic, A. et al. The classical complement pathway mediates microglia-dependent remodeling of spinal motor circuits during development and in SMA. Cell Rep. 29, 3087–3100 (2019).
Shi, Q. et al. Complement C3 deficiency protects against neurodegeneration in aged plaque-rich APP/PS1 mice. Sci. Transl Med. 9, eaaf6295 (2017).
Fonseca, M. I., Zhou, J., Botto, M. & Tenner, A. J. Absence of C1q leads to less neuropathology in transgenic mouse models of Alzheimer’s disease. J. Neurosci. 24, 6457–6465 (2004).
Fonseca, M. I. et al. Cell-specific deletion of C1qa identifies microglia as the dominant source of C1q in mouse brain. J. Neuroinflamm. 14, 48 (2017).
Pittock, S. J. et al. Eculizumab in AQP4-IgG-positive relapsing neuromyelitis optica spectrum disorders: an open-label pilot study. Lancet Neurol. 12, 554–562 (2013).
Howard, J. F. et al. Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol. 16, 976–986 (2017).
Carpanini, S. M. et al. Terminal complement pathway activation drives synaptic loss in Alzheimer’s disease models. Acta Neuropathol. Commun. 10, 99 (2022).
Gunner, G. et al. Sensory lesioning induces microglial synapse elimination via ADAM10 and fractalkine signaling. Nat. Neurosci. 22, 1075–1088 (2019).
Park, J. et al. Microglial MERTK eliminates phosphatidylserine‐displaying inhibitory post‐synapses. EMBO J. 40, e107121 (2021).
Li, T. et al. A splicing isoform of GPR56 mediates microglial synaptic refinement via phosphatidylserine binding. EMBO J. 39, e104136 (2020).
Ding, X. et al. Loss of microglial SIRPα promotes synaptic pruning in preclinical models of neurodegeneration. Nat. Commun. 12, 2030 (2021).
Lehrman, E. K. et al. CD47 protects synapses from excess microglia-mediated pruning during development. Neuron 100, 120–134.e6 (2018).
Guerreiro, R. et al. TREM2 variants in Alzheimer’s disease. N. Engl. J. Med. 368, 117–127 (2013).
Jonsson, T. et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. N. Engl. J. Med. 368, 107–116 (2013).
Condello, C., Yuan, P., Schain, A. & Grutzendler, J. Microglia constitute a barrier that prevents neurotoxic protofibrillar Aβ42 hotspots around plaques. Nat. Commun. 6, 6176 (2015).
Meilandt, W. J. et al. Trem2 deletion reduces late-stage amyloid plaque accumulation, elevates the Aβ42:Aβ40 ratio, and exacerbates axonal dystrophy and dendritic spine loss in the PS2APP Alzheimer’s mouse model. J. Neurosci. 40, 1956–1974 (2020).
Wang, Y. et al. TREM2-mediated early microglial response limits diffusion and toxicity of amyloid plaques. J. Exp. Med. 213, 667–675 (2016).
Wang, Y. et al. TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell 160, 1061–1071 (2015).
Scott‐Hewitt, N. et al. Local externalization of phosphatidylserine mediates developmental synaptic pruning by microglia. EMBO J. 39, e105380 (2020).
Filipello, F. et al. The microglial innate immune receptor TREM2 is required for synapse elimination and normal brain connectivity. Immunity 48, 979–991 (2018).
Rueda‐Carrasco, J. et al. Microglia‐synapse engulfment via PtdSer‐TREM2 ameliorates neuronal hyperactivity in Alzheimer’s disease models. EMBO J. 42, e113246 (2023).
Das, M. et al. Alzheimer risk-increasing TREM2 variant causes aberrant cortical synapse density and promotes network hyperexcitability in mouse models. Neurobiol. Dis. 186, 106263 (2023).
Brelstaff, J., Tolkovsky, A. M., Ghetti, B., Goedert, M. & Spillantini, M. G. Living neurons with tau filaments aberrantly expose phosphatidylserine and are phagocytosed by microglia. Cell Rep. 24, 1939–1948.e4 (2018).
Fracassi, A. et al. TREM2‐induced activation of microglia contributes to synaptic integrity in cognitively intact aged individuals with Alzheimer’s neuropathology. Brain Pathol. 33, e13108 (2023).
Wang, S. et al. Anti-human TREM2 induces microglia proliferation and reduces pathology in an Alzheimer’s disease model. J. Exp. Med. 217, e20200785 (2020).
Price, B. R. et al. Therapeutic Trem2 activation ameliorates amyloid-beta deposition and improves cognition in the 5XFAD model of amyloid deposition. J. Neuroinflamm. 17, 238 (2020).
Lengerich, B. et al. A TREM2-activating antibody with a blood–brain barrier transport vehicle enhances microglial metabolism in Alzheimer’s disease models. Nat. Neurosci. 26, 416–429 (2023).
Schlepckow, K. et al. Enhancing protective microglial activities with a dual function TREM2 antibody to the stalk region. EMBO Mol. Med. 12, e11227 (2020).
Ellwanger, D. C. et al. Prior activation state shapes the microglia response to antihuman TREM2 in a mouse model of Alzheimer’s disease. Proc. Natl Acad. Sci. USA 118, e2017742118 (2021).
Weber, M. et al. Cognitive deficits, changes in synaptic function, and brain pathology in a mouse model of normal aging. eNeuro https://doi.org/10.1523/eneuro.0047-15.2015 (2015).
Burke, S. N. & Barnes, C. A. Neural plasticity in the ageing brain. Nat. Rev. Neurosci. 7, 30–40 (2006).
Li, Y. et al. Proteomic profile of mouse brain aging contributions to mitochondrial dysfunction, DNA oxidative damage, loss of neurotrophic factor, and synaptic and ribosomal proteins. Oxidative Med. Cell. Longev. 2020, 5408452 (2020).
Bulovaite, E. et al. A brain atlas of synapse protein lifetime across the mouse lifespan. Neuron 110, 4057–4073 (2022).
Peters, A., Sethares, C. & Luebke, J. I. Synapses are lost during aging in the primate prefrontal cortex. Neuroscience 152, 970–981 (2008).
Jacobs, B., Driscoll, L. & Schall, M. Life‐span dendritic and spine changes in areas 10 and 18 of human cortex: a quantitative Golgi study. J. Comp. Neurol. 386, 661–680 (1997).
Pan, J., Ma, N., Yu, B., Zhang, W. & Wan, J. Transcriptomic profiling of microglia and astrocytes throughout aging. J. Neuroinflamm. 17, 97 (2020).
Clarke, L. E. et al. Normal aging induces A1-like astrocyte reactivity. Proc. Natl Acad. Sci. USA 115, E1896–E1905 (2018).
Lopes, K. et al. Genetic analysis of the human microglial transcriptome across brain regions, aging and disease pathologies. Nat. Genet. 54, 4–17 (2022).
Holtman, I. R. et al. Induction of a common microglia gene expression signature by aging and neurodegenerative conditions: a co-expression meta-analysis. Acta Neuropathol. Commun. 3, 31 (2015).
Villeda, S. A. et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477, 90–94 (2011).
Miguel, Z. D. et al. Exercise plasma boosts memory and dampens brain inflammation via clusterin. Nature 600, 494–499 (2021).
Villeda, S. A. et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat. Med. 20, 659–663 (2014).
Horowitz, A. M. et al. Blood factors transfer beneficial effects of exercise on neurogenesis and cognition to the aged brain. Science 369, 167–173 (2020).
Middeldorp, J. et al. Preclinical assessment of young blood plasma for Alzheimer disease. JAMA Neurol. 73, 1325 (2016).
Hannestad, J. et al. Safety and tolerability of GRF6019 in mild‐to‐moderate Alzheimer’s disease dementia. Alzheimer’s Dement. 6, e12115 (2020).
Hannestad, J. et al. Safety and tolerability of GRF6019 infusions in severe Alzheimer’s disease: a phase II double-blind placebo-controlled trial. J. Alzheimer’s Dis. 81, 1649–1662 (2021).
Gan, K. J. & Südhof, T. C. Specific factors in blood from young but not old mice directly promote synapse formation and NMDA-receptor recruitment. Proc. Natl Acad. Sci. USA 116, 12524–12533 (2019).
Nanasi, T., Feng, M., Braithwaite, S. P. & Lehallier, B. Deep plasma proteomics reveal age‐related molecular pathways modulated by GRF6019 treatment in Alzheimer’s disease patients. Alzheimer’s Dement. https://doi.org/10.1002/alz.061948 (2022).
Fu, H., Hardy, J. & Duff, K. E. Selective vulnerability in neurodegenerative diseases. Nat. Neurosci. 21, 1350–1358 (2018).
Finnema, S. J. et al. Imaging synaptic density in the living human brain. Sci. Transl Med. 8, 348ra96 (2016).
Onwordi, E. C. et al. Synaptic density marker SV2A is reduced in schizophrenia patients and unaffected by antipsychotics in rats. Nat. Commun. 11, 246 (2020).
Radhakrishnan, R. et al. In-vivo evidence of decreased synaptic density in schizophrenia: a [11C]UCB-J PET imaging study. Biol. Psychiatry 81, S389 (2017).
Holmes, S. E. et al. Lower synaptic density is associated with depression severity and network alterations. Nat. Commun. 10, 1529 (2019).
Vanhaute, H. et al. In vivo synaptic density loss is related to tau deposition in amnestic mild cognitive impairment. Neurology 95, e545–e553 (2020).
Holland, N. et al. Synaptic loss in primary tauopathies revealed by [11C]UCB‐J positron emission tomography. Mov. Disord. 35, 1834–1842 (2020).
Mecca, A. P. et al. In vivo measurement of widespread synaptic loss in Alzheimer’s disease with SV2A PET. Alzheimer’s Dement. 16, 974–982 (2020).
Chen, M.-K. et al. Assessing synaptic density in Alzheimer disease with synaptic vesicle glycoprotein 2A positron emission tomographic imaging. JAMA Neurol. 75, 1215–1224 (2018).
Radhakrishnan, R. et al. In vivo evidence of lower synaptic vesicle density in schizophrenia. Mol. Psychiatry 26, 7690–7698 (2021).
Mecca, A. P. et al. Synaptic density and cognitive performance in Alzheimer’s disease: a PET imaging study with [11C]UCB‐J. Alzheimer’s Dement. 18, 2527–2536 (2022).
Chen, Z. et al. Synaptic loss in spinocerebellar ataxia type 3 revealed by SV2A positron emission tomography. Mov. Disord. 38, 978–989 (2023).
Zhang, J. et al. In vivo synaptic density loss correlates with impaired functional and related structural connectivity in Alzheimer’s disease. J. Cereb. Blood Flow Metab. 43, 977–988 (2023).
Tang, Y. et al. Detection of changes in synaptic density in amyotrophic lateral sclerosis patients using 18F‐SynVesT‐1 positron emission tomography. Eur. J. Neurol. 29, 2934–2943 (2022).
Lleó, A. et al. Changes in synaptic proteins precede neurodegeneration markers in preclinical Alzheimer’s disease cerebrospinal fluid. Mol. Cell. Proteom. 18, 546–560 (2019).
Duits, F. H. et al. Synaptic proteins in CSF as potential novel biomarkers for prognosis in prodromal Alzheimer’s disease. Alzheimer’s Res. Ther. 10, 5 (2018).
Chatterjee, M. et al. Contactin-2, a synaptic and axonal protein, is reduced in cerebrospinal fluid and brain tissue in Alzheimer’s disease. Alzheimer’s Res. Ther. 10, 52 (2018).
Milà-Alomà, M. et al. CSF synaptic biomarkers in the preclinical stage of Alzheimer disease and their association with MRI and PET. Neurology 97, e2065–e2078 (2021).
Higginbotham, L. et al. Integrated proteomics reveals brain-based cerebrospinal fluid biomarkers in asymptomatic and symptomatic Alzheimer’s disease. Sci. Adv. 6, eaaz9360 (2020).
Kester, M. I. et al. Neurogranin as a cerebrospinal fluid biomarker for synaptic loss in symptomatic Alzheimer disease. JAMA Neurol. 72, 1275–1280 (2015).
Nilsson, J. et al. Cerebrospinal fluid biomarker panel for synaptic dysfunction in Alzheimer’s disease. Alzheimer’s Dement. 13, e12179 (2021).
Bader, J. M. et al. Proteome profiling in cerebrospinal fluid reveals novel biomarkers of Alzheimer’s disease. Mol. Syst. Biol. 16, e9356 (2020).
Pelkey, K. A. et al. Pentraxins coordinate excitatory synapse maturation and circuit integration of parvalbumin interneurons. Neuron 90, 661 (2016).
Chang, M. C. et al. Narp regulates homeostatic scaling of excitatory synapses on parvalbumin-expressing interneurons. Nat. Neurosci. 13, 1090–1097 (2010).
Xiao, M.-F. et al. A biomarker-authenticated model of schizophrenia implicating NPTX2 loss of function. Sci. Adv. 7, eabf6935 (2021).
Libiger, O. et al. Longitudinal CSF proteomics identifies NPTX2 as a prognostic biomarker of Alzheimer’s disease. Alzheimer’s Dement. 17, 1976–1987 (2021).
Xiao, M.-F. et al. NPTX2 and cognitive dysfunction in Alzheimer’s disease. eLife 6, e23798 (2017).
Ende, E. L. et al. Neuronal pentraxin 2: a synapse-derived CSF biomarker in genetic frontotemporal dementia. J. Neurol. Neurosurg. Psychiatry 91, 612–621 (2020).
Steenoven, I. et al. Identification of novel cerebrospinal fluid biomarker candidates for dementia with Lewy bodies: a proteomic approach. Mol. Neurodegener. 15, 36 (2020).
Galasko, D. R., Smirnov, D. S., Salmon, D. P. & Alzheimer’s Disease Neuroimaging Initiative. Longitudinal change in CSF biomarkers, especially NPTX2, in non‐demented elderly predicts cognitive decline and conversion to dementia. Alzheimer’s Dement. https://doi.org/10.1002/alz.046475 (2020).
Oeckl, P. et al. Targeted mass spectrometry suggests beta-synuclein as synaptic blood marker in Alzheimer’s disease. J. Proteome Res. 19, 1310–1318 (2020).
Oeckl, P. et al. Relationship of serum beta‐synuclein with blood biomarkers and brain atrophy. Alzheimer’s Dement. 19, 1358–1371 (2022).
Vrillon, A. et al. Plasma neuregulin 1 as a synaptic biomarker in Alzheimer’s disease: a discovery cohort study. Alzheimer’s Res. Ther. 14, 71 (2022).
Tian, C. et al. Blood extracellular vesicles carrying synaptic function‐ and brain‐related proteins as potential biomarkers for Alzheimer’s disease. Alzheimer’s Dement. 19, 909–923 (2023).
Winston, C. N. et al. Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile. Alzheimer’s Dement. 3, 63–72 (2016).
Acknowledgements
The authors thank C. Bohlen, F. Hinz, M.-C. Tsai, F. Yeh, D. Gray and M. Figley for the critical reading of the manuscript.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
B.D. is a full-time employee of Vigil Neuroscience. M.S. is scientific cofounder and member of the scientific advisory board of Neumora Therapeutics and serves on the scientific advisory board of Biogen, Vanqua Bio, ArcLight Therapeutics, Proximity Therapeutics and Cerevel Therapeutics. J.E.H. is a full-time employee of Genentech, a member of the Roche Group.
Peer review
Peer review information
Nature Reviews Drug Discovery thanks Karl Giese and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Astrocytes
-
A type of glial cell located in the CNS, responsible for a multitude of tasks such as supplying neurons with nutrients, managing the function of synapses, maintaining the balance of extracellular ions and chemicals and preserving the structural integrity of the blood–brain barrier.
- Interneurons
-
Inhibitory neurons that form nodes within neural circuitry and regulate neuronal activity by releasing the neurotransmitter GABA, which inhibits firing of other neurons.
- Medial ganglionic eminence
-
(MGE). Structure in the developing nervous system that produces inhibitory interneurons, which disperse widely throughout the brain.
- Membrane attack complex
-
A complex of complement proteins that forms cytolytic pores in the plasma membrane of targeted cells such as pathogens.
- Microglia
-
Resident immune cells of the CNS that respond to pathogens and damage and can engage in phagocytosis of protein aggregates, cellular structures and other substrates.
- Neurofilament light
-
Neuronal structural protein that is located primarily within myelinated axons.
- Synaptic plasticity
-
The ability of synapses to modify their strength over time in response to differing levels of activity.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dejanovic, B., Sheng, M. & Hanson, J.E. Targeting synapse function and loss for treatment of neurodegenerative diseases. Nat Rev Drug Discov 23, 23–42 (2024). https://doi.org/10.1038/s41573-023-00823-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41573-023-00823-1
This article is cited by
-
Role of MARK2 in the nervous system and cancer
Cancer Gene Therapy (2024)