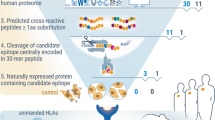
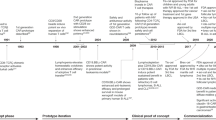
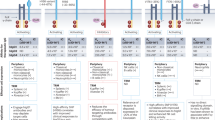
Abstract
The T cell receptor (TCR) complex is a naturally occurring antigen sensor that detects, amplifies and coordinates cellular immune responses to epitopes derived from cell surface and intracellular proteins. Thus, TCRs enable the targeting of proteins selectively expressed by cancer cells, including neoantigens, cancer germline antigens and viral oncoproteins. As such, TCRs have provided the basis for an emerging class of oncology therapeutics. Herein, we review the current cancer treatment landscape using TCRs and TCR-like molecules. This includes adoptive cell transfer of T cells expressing endogenous or engineered TCRs, TCR bispecific engagers and antibodies specific for human leukocyte antigen (HLA)-bound peptides (TCR mimics). We discuss the unique complexities associated with the clinical development of these therapeutics, such as HLA restriction, TCR retrieval, potency assessment and the potential for cross-reactivity. In addition, we highlight emerging clinical data that establish the antitumour potential of TCR-based therapies, including tumour-infiltrating lymphocytes, for the treatment of diverse human malignancies. Finally, we explore the future of TCR therapeutics, including emerging genome editing methods to safely enhance potency and strategies to streamline patient identification.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hedrick, S. M., Cohen, D. I., Nielsen, E. A. & Davis, M. M. Isolation of cDNA clones encoding T cell-specific membrane-associated proteins. Nature 308, 149–153 (1984).
Yanagi, Y. et al. A human T cell-specific cDNA clone encodes a protein having extensive homology to immunoglobulin chains. Nature 308, 145–149 (1984). Together with Hedrick et al. (1984), this work reports the genetic sequence for the TCRβ chain in mice and humans for the first time.
Rosenberg, S. A. IL-2: the first effective immunotherapy for human cancer. J. Immunol. 192, 5451–5458 (2014).
Ribas, A. & Wolchok, J. D. Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 (2018).
Guedan, S., Ruella, M. & June, C. H. Emerging cellular therapies for cancer. Annu. Rev. Immunol. 37, 145–171 (2019).
Chandran, S. S. & Klebanoff, C. A. T cell receptor-based cancer immunotherapy: emerging efficacy and pathways of resistance. Immunol. Rev. 290, 127–147 (2019).
Thul, P. J. et al. A subcellular map of the human proteome. Science 356, eaal3321 (2017).
Rossjohn, J. et al. T cell antigen receptor recognition of antigen-presenting molecules. Annu. Rev. Immunol. 33, 169–200 (2015).
Liddy, N. et al. Monoclonal TCR-redirected tumor cell killing. Nat. Med. 18, 980–987 (2012).
Hsiue, E. H. et al. Targeting a neoantigen derived from a common TP53 mutation. Science 371, eabc8697 (2021).
Walker, A. J. et al. Tumor antigen and receptor densities regulate efficacy of a chimeric antigen receptor targeting anaplastic lymphoma kinase. Mol. Ther. 25, 2189–2201 (2017).
Mansilla-Soto, J. et al. HLA-independent T cell receptors for targeting tumors with low antigen density. Nat. Med. 28, 345–352 (2022).
Huang, J. et al. A single peptide–major histocompatibility complex ligand triggers digital cytokine secretion in CD4+ T cells. Immunity 39, 846–857 (2013).
Purbhoo, M. A., Irvine, D. J., Huppa, J. B. & Davis, M. M. T cell killing does not require the formation of a stable mature immunological synapse. Nat. Immunol. 5, 524–530 (2004).
Sibille, C. et al. Structure of the gene of tum- transplantation antigen P198: a point mutation generates a new antigenic peptide. J. Exp. Med. 172, 35–45 (1990).
den Haan, J. M. et al. The minor histocompatibility antigen HA-1: a diallelic gene with a single amino acid polymorphism. Science 279, 1054–1057 (1998).
Lamb, J. R., Feldmann, M., Green, N. & Lerner, R. A. Influence of antigen structure on the activation and induction of unresponsiveness in cloned human T lymphocytes. Immunol 57, 331–335 (1986).
Nathan, P. et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N. Engl. J. Med. 385, 1196–1206 (2021). This paper reports on a randomized clinical trial in patients with metastatic uveal melanoma which led to the first FDA-approved TCR therapeutic for the treatment of cancer.
Carter, P. J. & Lazar, G. A. Next generation antibody drugs: pursuit of the ‘high-hanging fruit’. Nat. Rev. Drug. Discov. 17, 197–223 (2018).
Kalbasi, A. & Ribas, A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat. Rev. Immunol. 20, 25–39 (2020).
Peri, A. et al. The landscape of T cell antigens for cancer immunotherapy. Nat. Cancer 4, 937–954 (2023).
Susac, L. et al. Structure of a fully assembled tumor-specific T cell receptor ligated by pMHC. Cell 185, 3201–3213.e19 (2022).
Sebestyen, Z., Prinz, I., Dechanet-Merville, J., Silva-Santos, B. & Kuball, J. Translating gammadelta (γδ) T cells and their receptors into cancer cell therapies. Nat. Rev. Drug. Discov. 19, 169–184 (2020).
Davis, M. M. & Bjorkman, P. J. T-cell antigen receptor genes and T-cell recognition. Nature 334, 395–402 (1988).
Thomas, S. et al. Framework engineering to produce dominant T cell receptors with enhanced antigen-specific function. Nat. Commun. 10, 4451 (2019).
Cabaniols, J. P., Fazilleau, N., Casrouge, A., Kourilsky, P. & Kanellopoulos, J. M. Most α/β T cell receptor diversity is due to terminal deoxynucleotidyl transferase. J. Exp. Med. 194, 1385–1390 (2001).
Borrman, T. et al. ATLAS: a database linking binding affinities with structures for wild-type and mutant TCR–pMHC complexes. Proteins 85, 908–916 (2017).
Leem, J., de Oliveira, S. H. P., Krawczyk, K. & Deane, C. M. STCRDab: the structural T-cell receptor database. Nucleic Acids Res. 46, D406–D412 (2018).
Aleksic, M. et al. Different affinity windows for virus and cancer-specific T-cell receptors: implications for therapeutic strategies. Eur. J. Immunol. 42, 3174–3179 (2012).
Foote, J. & Eisen, H. N. Kinetic and affinity limits on antibodies produced during immune responses. Proc. Natl Acad. Sci. USA 92, 1254–1256 (1995).
Clay, T. M. et al. Efficient transfer of a tumor antigen-reactive TCR to human peripheral blood lymphocytes confers anti-tumor reactivity. J. Immunol. 163, 507–513 (1999). This paper provides the first demonstration that genetically modifying primary human T cells to express an exogenous TCR can confer tumour recognition.
Stanislawski, T. et al. Circumventing tolerance to a human MDM2-derived tumor antigen by TCR gene transfer. Nat. Immunol. 2, 962–970 (2001).
Morgan, R. A. et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 314, 126–129 (2006). This paper reports the first demonstration that adoptive transfer of TCR engineered T cells can lead to cancer regression in humans.
Johnson, L. A. et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood 114, 535–546 (2009).
Parkhurst, M. R. et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol. Ther. 19, 620–626 (2011).
Morgan, R. A. et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J. Immunother. 36, 133–151 (2013).
Kageyama, S. et al. Adoptive transfer of MAGE-A4 T-cell receptor gene-transduced lymphocytes in patients with recurrent esophageal cancer. Clin. Cancer Res. 21, 2268–2277 (2015).
Lu, Y. C. et al. Treatment of patients with metastatic cancer using a major histocompatibility complex class II-restricted T-cell receptor targeting the cancer germline antigen MAGE-A3. J. Clin. Oncol. 35, 3322–3329 (2017). This paper provides the first demonstration that adoptive transfer of CD4+ T cells genetically modified with an HLA class II-restricted TCR can cause cancer regression in diverse solid tumours.
Tawara, I. et al. Safety and persistence of WT1-specific T-cell receptor gene-transduced lymphocytes in patients with AML and MDS. Blood 130, 1985–1994 (2017).
Doran, S. L. et al. T-cell receptor gene therapy for human papillomavirus-associated epithelial cancers: a first-in-human, phase I/II study. J. Clin. Oncol. 37, 2759–2768 (2019).
Nagarsheth, N. B. et al. TCR-engineered T cells targeting E7 for patients with metastatic HPV-associated epithelial cancers. Nat. Med. 27, 419–425 (2021).
Linette, G. P. et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood 122, 863–871 (2013).
D’Angelo, S. P. et al. Antitumor activity associated with prolonged persistence of adoptively transferred NY-ESO-1 c259T cells in synovial sarcoma. Cancer Discov. 8, 944–957 (2018).
Chapuis, A. G. et al. T cell receptor gene therapy targeting WT1 prevents acute myeloid leukemia relapse post-transplant. Nat. Med. 25, 1064–1072 (2019).
Stadtmauer, E. A. et al. CRISPR-engineered T cells in patients with refractory cancer. Science 367, eaba7365 (2020).
Zhang, J. et al. Non-viral, specifically targeted CAR-T cells achieve high safety and efficacy in B-NHL. Nature 609, 369–374 (2022).
Foy, S. P. et al. Non-viral precision T cell receptor replacement for personalized cell therapy. Nature 615, 687–696 (2022). This paper provides the first clinical report demonstrating the feasibility of simultaneously targeting multiple neoantigens using a non-viral TCR integration approach.
Marcucci, K. T. et al. Retroviral and lentiviral safety analysis of gene-modified T cell products and infused HIV and oncology patients. Mol. Ther. 26, 269–279 (2018).
Eyquem, J. et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 543, 113–117 (2017).
Muller, T. R. et al. Targeted T cell receptor gene editing provides predictable T cell product function for immunotherapy. Cell Rep. Med. 2, 100374 (2021).
Kaiser, A. D. et al. Towards a commercial process for the manufacture of genetically modified T cells for therapy. Cancer Gene Ther. 22, 72–78 (2015).
Fraietta, J. A. et al. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature 558, 307–312 (2018).
Shah, N. N. et al. Clonal expansion of CAR T cells harboring lentivector integration in the CBL gene following anti-CD22 CAR T-cell therapy. Blood Adv. 3, 2317–2322 (2019).
Cohen, C. J., Zhao, Y., Zheng, Z., Rosenberg, S. A. & Morgan, R. A. Enhanced antitumor activity of murine-human hybrid T-cell receptor (TCR) in human lymphocytes is associated with improved pairing and TCR/CD3 stability. Cancer Res. 66, 8878–8886 (2006).
Heemskerk, M. H. et al. Efficiency of T-cell receptor expression in dual-specific T cells is controlled by the intrinsic qualities of the TCR chains within the TCR–CD3 complex. Blood 109, 235–243 (2007).
Ahmadi, M. et al. CD3 limits the efficacy of TCR gene therapy in vivo. Blood 118, 3528–3537 (2011).
van Loenen, M. M. et al. Mixed T cell receptor dimers harbor potentially harmful neoreactivity. Proc. Natl Acad. Sci. USA 107, 10972–10977 (2010).
Bendle, G. M. et al. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat. Med. 16, 565–570, 1p following 570 (2010).
Rosenberg, S. A. Of mice, not men: no evidence for graft-versus-host disease in humans receiving T-cell receptor-transduced autologous T cells. Mol. Ther. 18, 1744–1745 (2010).
Provasi, E. et al. Editing T cell specificity towards leukemia by zinc finger nucleases and lentiviral gene transfer. Nat. Med. 18, 807–815 (2012).
Berdien, B., Mock, U., Atanackovic, D. & Fehse, B. TALEN-mediated editing of endogenous T-cell receptors facilitates efficient reprogramming of T lymphocytes by lentiviral gene transfer. Gene Ther. 21, 539–548 (2014).
Roth, T. L. et al. Reprogramming human T cell function and specificity with non-viral genome targeting. Nature 559, 405–409 (2018).
Ruggiero, E. et al. CRISPR-based gene disruption and integration of high-avidity, WT1-specific T cell receptors improve antitumor T cell function. Sci. Transl. Med. 14, eabg8027 (2022).
Nahmad, A. D. et al. Frequent aneuploidy in primary human T cells after CRISPR–Cas9 cleavage. Nat. Biotechnol. 40, 1807–1813 (2022).
Ishihara, M. et al. NY-ESO-1-specific redirected T cells with endogenous TCR knockdown mediate tumor response and cytokine release syndrome. J. Immunother. Cancer 10, e003811 (2022).
Rees, H. A. & Liu, D. R. Base editing: precision chemistry on the genome and transcriptome of living cells. Nat. Rev. Genet. 19, 770–788 (2018).
Webber, B. R. et al. Highly efficient multiplex human T cell engineering without double-strand breaks using Cas9 base editors. Nat. Commun. 10, 5222 (2019).
Preece, R. et al. CRISPR-mediated base conversion allows discriminatory depletion of endogenous T cell receptors for enhanced synthetic immunity. Mol. Ther. Methods Clin. Dev. 19, 149–161 (2020).
Chiesa, R. et al. Base-edited CAR7 T cells for relapsed T-cell acute lymphoblastic leukemia. N. Engl. J. Med. 389, 899–910 (2023).
Schober, K. et al. Orthotopic replacement of T-cell receptor α- and β-chains with preservation of near-physiological T-cell function. Nat. Biomed. Eng. 3, 974–984 (2019).
Shy, B. R. et al. High-yield genome engineering in primary cells using a hybrid ssDNA repair template and small-molecule cocktails. Nat. Biotechnol. 41, 521–531 (2022).
Oh, S. A. et al. High-efficiency nonviral CRISPR/Cas9-mediated gene editing of human T cells using plasmid donor DNA. J. Exp. Med. 219, e20211530 (2022).
Nguyen, D. N. et al. Polymer-stabilized Cas9 nanoparticles and modified repair templates increase genome editing efficiency. Nat. Biotechnol. 38, 44–49 (2020).
Cohen, C. J. et al. Enhanced antitumor activity of T cells engineered to express T-cell receptors with a second disulfide bond. Cancer Res. 67, 3898–3903 (2007).
Kuball, J. et al. Facilitating matched pairing and expression of TCR chains introduced into human T cells. Blood 109, 2331–2338 (2007). Together with Cohen et al. (2006) and Cohen et al. (2007), this paper describes commonly used modifications to the TCR constant chains that enhance both the potency and the safety of exogenously expressed TCRs.
Bialer, G., Horovitz-Fried, M., Ya’acobi, S., Morgan, R. A. & Cohen, C. J. Selected murine residues endow human TCR with enhanced tumor recognition. J. Immunol. 184, 6232–6241 (2010).
Sommermeyer, D. & Uckert, W. Minimal amino acid exchange in human TCR constant regions fosters improved function of TCR gene-modified T cells. J. Immunol. 184, 6223–6231 (2010).
Davis, J. L. et al. Development of human anti-murine T-cell receptor antibodies in both responding and nonresponding patients enrolled in TCR gene therapy trials. Clin. Cancer Res. 16, 5852–5861 (2010).
Bethune, M. T. et al. Domain-swapped T cell receptors improve the safety of TCR gene therapy. eLife 5, e19095 (2016).
Kuball, J. et al. Increasing functional avidity of TCR-redirected T cells by removing defined N-glycosylation sites in the TCR constant domain. J. Exp. Med. 206, 463–475 (2009).
Haga-Friedman, A., Horovitz-Fried, M. & Cohen, C. J. Incorporation of transmembrane hydrophobic mutations in the TCR enhance its surface expression and T cell functional avidity. J. Immunol. 188, 5538–5546 (2012).
Robbins, P. F. et al. Single and dual amino acid substitutions in TCR CDRs can enhance antigen-specific T cell functions. J. Immunol. 180, 6116–6131 (2008).
Li, Y. et al. Directed evolution of human T-cell receptors with picomolar affinities by phage display. Nat. Biotechnol. 23, 349–354 (2005). This paper reports on the use of phage display libraries for the high-throughput evolution and selection of affinity-enhanced TCRs.
Holler, P. D. et al. In vitro evolution of a T cell receptor with high affinity for peptide/MHC. Proc. Natl Acad. Sci. USA 97, 5387–5392 (2000).
Dilchert, J., Hofmann, M., Unverdorben, F., Kontermann, R. & Bunk, S. Mammalian display platform for the maturation of bispecific TCR-based molecules. Antibodies 11, 34 (2022).
Cameron, B. J. et al. Identification of a Titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci. Transl. Med. 5, 197ra103 (2013).
Sanderson, J. P. et al. Preclinical evaluation of an affinity-enhanced MAGE-A4-specific T-cell receptor for adoptive T-cell therapy. OncoImmunol 9, 1682381 (2020).
Hellman, L. M. et al. Improving T cell receptor on-target specificity via structure-guided design. Mol. Ther. 27, 300–313 (2019).
Rosenberg, A. M. & Baker, B. M. Engineering the T cell receptor for fun and profit: uncovering complex biology, interrogating the immune system, and targeting disease. Curr. Opin. Struct. Biol. 74, 102358 (2022).
Zhao, X. et al. Tuning T cell receptor sensitivity through catch bond engineering. Science 376, eabl5282 (2022).
Sibener, L. V. et al. Isolation of a structural mechanism for uncoupling T cell receptor signaling from peptide-MHC binding. Cell 174, 672–687.e27 (2018).
Poole, A. et al. Therapeutic high affinity T cell receptor targeting a KRASG12D cancer neoantigen. Nat. Commun. 13, 5333 (2022).
Zhao, Y. et al. High-affinity TCRs generated by phage display provide CD4+ T cells with the ability to recognize and kill tumor cell lines. J. Immunol. 179, 5845–5854 (2007).
Border, E. C., Sanderson, J. P., Weissensteiner, T., Gerry, A. B. & Pumphrey, N. J. Affinity-enhanced T-cell receptors for adoptive T-cell therapy targeting MAGE-A10: strategy for selection of an optimal candidate. OncoImmunol 8, e1532759 (2019).
Docta, R. Y. et al. Tuning T-cell receptor affinity to optimize clinical risk–benefit when targeting α-fetoprotein-positive liver cancer. Hepatology 69, 2061–2075 (2019).
Huang, C. Receptor-Fc fusion therapeutics, traps, and MIMETIBODY technology. Curr. Opin. Biotechnol. 20, 692–699 (2009).
Dao, T. et al. Therapeutic bispecific T-cell engager antibody targeting the intracellular oncoprotein WT1. Nat. Biotechnol. 33, 1079–1086 (2015).
Hoogenboom, H. R. et al. Antibody phage display technology and its applications. Immunotechnology 4, 1–20 (1998).
Yang, X. et al. Facile repurposing of peptide–MHC-restricted antibodies for cancer immunotherapy. Nat. Biotechnol. 41, 932–943 (2023).
Holland, C. J. et al. Specificity of bispecific T cell receptors and antibodies targeting peptide-HLA. J. Clin. Invest. 130, 2673–2688 (2020).
Yarmarkovich, M. et al. Cross-HLA targeting of intracellular oncoproteins with peptide-centric CARs. Nature 599, 477–484 (2021). This paper describes the use of peptide-centric antibody binders that recognize the same epitope presented in the context of different HLA alleles as a strategy to enhance the scalability of TCR therapeutics.
Dunbar, J., Knapp, B., Fuchs, A., Shi, J. & Deane, C. M. Examining variable domain orientations in antigen receptors gives insight into TCR-like antibody design. PLoS Comput. Biol. 10, e1003852 (2014).
Cole, D. K. et al. Hotspot autoimmune T cell receptor binding underlies pathogen and insulin peptide cross-reactivity. J. Clin. Invest. 126, 3626 (2016).
Adams, J. J. et al. Structural interplay between germline interactions and adaptive recognition determines the bandwidth of TCR–peptide–MHC cross-reactivity. Nat. Immunol. 17, 87–94 (2016).
Liu, C. et al. Validation and promise of a TCR mimic antibody for cancer immunotherapy of hepatocellular carcinoma. Sci. Rep. 12, 12068 (2022).
Helsen, C. W. et al. The chimeric TAC receptor co-opts the T cell receptor yielding robust anti-tumor activity without toxicity. Nat. Commun. 9, 3049 (2018).
Long, A. H. et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat. Med. 21, 581–590 (2015).
Baeuerle, P. A. et al. Synthetic TRuC receptors engaging the complete T cell receptor for potent anti-tumor response. Nat. Commun. 10, 2087 (2019).
Hassan, R. et al. Mesothelin-targeting T cell receptor fusion construct cell therapy in refractory solid tumors: phase 1/2 trial interim results. Nat. Med. 29, 2099–2109 (2023).
Liu, Y. et al. Chimeric STAR receptors using TCR machinery mediate robust responses against solid tumors. Sci. Transl. Med. 13, eabb5191 (2021).
Oliveira, G. et al. Phenotype, specificity and avidity of antitumour CD8+ T cells in melanoma. Nature 596, 119–125 (2021).
Stevanovic, S. et al. Landscape of immunogenic tumor antigens in successful immunotherapy of virally induced epithelial cancer. Science 356, 200–205 (2017).
Creelan, B. C. et al. Tumor-infiltrating lymphocyte treatment for anti-PD-1-resistant metastatic lung cancer: a phase 1 trial. Nat. Med. 27, 1410–1418 (2021).
Linnemann, C. et al. High-throughput epitope discovery reveals frequent recognition of neo-antigens by CD4+ T cells in human melanoma. Nat. Med. 21, 81–85 (2015).
Parkhurst, M. R. et al. Unique neoantigens arise from somatic mutations in patients with gastrointestinal cancers. Cancer Discov. 9, 1022–1035 (2019).
Caushi, J. X. et al. Transcriptional programs of neoantigen-specific TIL in anti-PD-1-treated lung cancers. Nature 596, 126–132 (2021).
Scheper, W. et al. Low and variable tumor reactivity of the intratumoral TCR repertoire in human cancers. Nat. Med. 25, 89–94 (2019).
Pasetto, A. et al. Tumor- and neoantigen-reactive T-cell receptors can be identified based on their frequency in fresh tumor. Cancer Immunol. Res. 4, 734–743 (2016).
Simoni, Y. et al. Bystander CD8+ T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 557, 575–579 (2018).
Hanada, K. I. et al. A phenotypic signature that identifies neoantigen-reactive T cells in fresh human lung cancers. Cancer Cell 40, 479–493.e6 (2022).
Oliveira, G. et al. Landscape of helper and regulatory antitumour CD4+ T cells in melanoma. Nature 605, 532–538 (2022).
Duhen, T. et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat. Commun. 9, 2724 (2018).
Krishna, S. et al. Stem-like CD8 T cells mediate response of adoptive cell immunotherapy against human cancer. Science 370, 1328–1334 (2020).
Chow, A. et al. The ectonucleotidase CD39 identifies tumor-reactive CD8+ T cells predictive of immune checkpoint blockade efficacy in human lung cancer. Immunity 56, 93–106.e6 (2023).
Gros, A. et al. PD-1 identifies the patient-specific CD8+ tumor-reactive repertoire infiltrating human tumors. J. Clin. Invest. 124, 2246–2259 (2014).
Eberhardt, C. S. et al. Functional HPV-specific PD-1+ stem-like CD8 T cells in head and neck cancer. Nature 597, 279–284 (2021).
Veatch, J. R. et al. Neoantigen-specific CD4+ T cells in human melanoma have diverse differentiation states and correlate with CD8+ T cell, macrophage, and B cell function. Cancer Cell 40, 393–409.e9 (2022).
Ahmadzadeh, M. et al. Tumor-infiltrating human CD4+ regulatory T cells display a distinct TCR repertoire and exhibit tumor and neoantigen reactivity. Sci. Immunol. 4, eaao4310 (2019).
Cohen, C. J. et al. Isolation of neoantigen-specific T cells from tumor and peripheral lymphocytes. J. Clin. Invest. 125, 3981–3991 (2015).
Zhang, S. Q. et al. High-throughput determination of the antigen specificities of T cell receptors in single cells. Nat. Biotechnol. https://doi.org/10.1038/nbt.4282 (2018).
Gee, M. H. et al. Antigen identification for orphan T cell receptors expressed tumor-infiltrating lymphocytes. Cell 172, 549–563.e16 (2018).
Li, G. et al. T cell antigen discovery via trogocytosis. Nat. Methods 16, 183–190 (2019).
Kula, T. et al. T-scan: a genome-wide method for the systematic discovery of T cell epitopes. Cell 178, 1016–1028.e13 (2019).
Gejman, R. S. et al. Identification of the targets of T-cell receptor therapeutic agents and cells by use of a high-throughput genetic platform. Cancer Immunol. Res. 8, 672–684 (2020).
Dobson, C. S. et al. Antigen identification and high-throughput interaction mapping by reprogramming viral entry. Nat. Methods 19, 449–460 (2022).
Ye, Q. et al. CD137 accurately identifies and enriches for naturally occurring tumor-reactive T cells in tumor. Clin. Cancer Res. 20, 44–55 (2014).
Parkhurst, M. et al. Isolation of T-cell receptors specifically reactive with mutated tumor-associated antigens from tumor-infiltrating lymphocytes based on CD137 expression. Clin. Cancer Res. 23, 2491–2505 (2017).
Litchfield, K. et al. Meta-analysis of tumor- and T cell-intrinsic mechanisms of sensitization to checkpoint inhibition. Cell 184, 596–614.e14 (2021).
Lowery, F. J. et al. Molecular signatures of antitumor neoantigen-reactive T cells from metastatic human cancers. Science 375, 877–884 (2022).
Gros, A. et al. Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients. Nat. Med. 22, 433–438 (2016).
Cafri, G. et al. Memory T cells targeting oncogenic mutations detected in peripheral blood of epithelial cancer patients. Nat. Commun. 10, 449 (2019).
Neudorfer, J. et al. Reversible HLA multimers (streptamers) for the isolation of human cytotoxic T lymphocytes functionally active against tumor- and virus-derived antigens. J. Immunol. Methods 320, 119–131 (2007).
Perica, K. et al. Enrichment and expansion with nanoscale artificial antigen presenting cells for adoptive immunotherapy. ACS Nano 9, 6861–6871 (2015).
Stronen, E. et al. Targeting of cancer neoantigens with donor-derived T cell receptor repertoires. Science 352, 1337–1341 (2016).
Bassani-Sternberg, M. et al. Direct identification of clinically relevant neoepitopes presented on native human melanoma tissue by mass spectrometry. Nat. Commun. 7, 13404 (2016).
Pai, C. S. et al. Clonal deletion of tumor-specific T cells by interferon-γ confers therapeutic resistance to combination immune checkpoint blockade. Immunity 50, 477–492.e8 (2019).
Wolfl, M. & Greenberg, P. D. Antigen-specific activation and cytokine-facilitated expansion of naive, human CD8+ T cells. Nat. Protoc. 9, 950–966 (2014).
Chandran, S. S. et al. Immunogenicity and therapeutic targeting of a public neoantigen derived from mutated PIK3CA. Nat. Med. 28, 946–957 (2022).
Ali, M. et al. T cells targeted to TdT kill leukemic lymphoblasts while sparing normal lymphocytes. Nat. Biotechnol. 40, 488–498 (2022).
van der Lee, D. I. et al. Mutated nucleophosmin 1 as immunotherapy target in acute myeloid leukemia. J. Clin. Invest. 129, 774–785 (2019).
Jahn, L. et al. TCR-based therapy for multiple myeloma and other B-cell malignancies targeting intracellular transcription factor BOB1. Blood 129, 1284–1295 (2017).
Falkenburg, W. J. et al. Allogeneic HLA-A*02-restricted WT1-specific T cells from mismatched donors are highly reactive but show off-target promiscuity. J. Immunol. 187, 2824–2833 (2011).
Bijen, H. M. et al. Preclinical strategies to identify off-target toxicity of high-affinity TCRs. Mol. Ther. 26, 1206–1214 (2018).
Theobald, M. et al. Tolerance to p53 by A2.1-restricted cytotoxic T lymphocytes. J. Exp. Med. 185, 833–841 (1997).
Li, L. P. et al. Transgenic mice with a diverse human T cell antigen receptor repertoire. Nat. Med. 16, 1029–1034 (2010).
Moore, M. J. et al. Humanization of T cell-mediated immunity in mice. Sci. Immunol. 6, eabj4026 (2021).
Lonberg, N. Human antibodies from transgenic animals. Nat. Biotechnol. 23, 1117–1125 (2005).
Obenaus, M. et al. Identification of human T-cell receptors with optimal affinity to cancer antigens using antigen-negative humanized mice. Nat. Biotechnol. 33, 402–407 (2015).
Poncette, L., Chen, X., Lorenz, F. K. & Blankenstein, T. Effective NY-ESO-1-specific MHC II-restricted T cell receptors from antigen-negative hosts enhance tumor regression. J. Clin. Invest. 129, 324–335 (2019).
Klebanoff, C. A., Rosenberg, S. A. & Restifo, N. P. Prospects for gene-engineered T cell immunotherapy for solid cancers. Nat. Med. 22, 26–36 (2016).
Mortazavi, A., Williams, B. A., McCue, K., Schaeffer, L. & Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-seq. Nat. Methods 5, 621–628 (2008).
GTEx Consortium. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 45, 580–585 (2013).
Parker, K. R. et al. Single-cell analyses identify brain mural cells expressing CD19 as potential off-tumor targets for CAR-T immunotherapies. Cell 183, 126–142.e17 (2020).
Wu, L. et al. Variation and genetic control of protein abundance in humans. Nature 499, 79–82 (2013).
Haas, G. G. Jr., D’Cruz, O. J. & De Bault, L. E. Distribution of human leukocyte antigen-ABC and -D/DR antigens in the unfixed human testis. Am. J. Reprod. Immunol. Microbiol. 18, 47–51 (1988).
Felix, N. J. & Allen, P. M. Specificity of T-cell alloreactivity. Nat. Rev. Immunol. 7, 942–953 (2007).
van Amerongen, R. A. et al. Human iPSC-derived preclinical models to identify toxicity of tumor-specific T cells with clinical potential. Mol. Ther. Methods Clin. Dev. 28, 249–261 (2023).
Kunert, A., Obenaus, M., Lamers, C. H. J., Blankenstein, T. & Debets, R. T-cell receptors for clinical therapy: in vitro assessment of toxicity risk. Clin. Cancer Res. 23, 6012–6020 (2017).
Riley, T. P. et al. T cell receptor cross-reactivity expanded by dramatic peptide–MHC adaptability. Nat. Chem. Biol. 14, 934–942 (2018).
Birnbaum, M. E. et al. Deconstructing the peptide–MHC specificity of T cell recognition. Cell 157, 1073–1087 (2014).
Wilson, D. B. et al. Specificity and degeneracy of T cells. Mol. Immunol. 40, 1047–1055 (2004).
Whalley, T. et al. GPU-accelerated discovery of pathogen-derived molecular mimics of a T-cell insulin epitope. Front. Immunol. 11, 296 (2020).
Scholtalbers, J. et al. TCLP: an online cancer cell line catalogue integrating HLA type, predicted neo-epitopes, virus and gene expression. Genome Med. 7, 118 (2015).
Tran, E. et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 344, 641–645 (2014). This clinical case report provides the first evidence that HLA class II-restricted CD4+ T cells targeting a private neoantigen can mediate durable tumour regression in a patient with cancer.
Dijkstra, K. K. et al. Generation of tumor-reactive T cells by co-culture of peripheral blood lymphocytes and tumor organoids. Cell 174, 1586–1598.e12 (2018).
Demmers, L. C. et al. Single-cell derived tumor organoids display diversity in HLA class I peptide presentation. Nat. Commun. 11, 5338 (2020).
Tuveson, D. & Clevers, H. Cancer modeling meets human organoid technology. Science 364, 952–955 (2019).
Axelrod, M. L., Cook, R. S., Johnson, D. B. & Balko, J. M. Biological consequences of MHC-II expression by tumor cells in cancer. Clin. Cancer Res. 25, 2392–2402 (2019).
Hos, B. J. et al. Cancer-specific T helper shared and neo-epitopes uncovered by expression of the MHC class II master regulator CIITA. Cell Rep. 41, 111485 (2022).
Zeh, H. J. 3rd, Perry-Lalley, D., Dudley, M. E., Rosenberg, S. A. & Yang, J. C. High avidity CTLs for two self-antigens demonstrate superior in vitro and in vivo antitumor efficacy. J. Immunol. 162, 989–994 (1999).
Dutoit, V. et al. Heterogeneous T-cell response to MAGE-A10(254–262): high avidity-specific cytolytic T lymphocytes show superior antitumor activity. Cancer Res. 61, 5850–5856 (2001).
Johnson, L. A. et al. Gene transfer of tumor-reactive TCR confers both high avidity and tumor reactivity to nonreactive peripheral blood mononuclear cells and tumor-infiltrating lymphocytes. J. Immunol. 177, 6548–6559 (2006).
Slifka, M. K. & Whitton, J. L. Functional avidity maturation of CD8+ T cells without selection of higher affinity TCR. Nat. Immunol. 2, 711–717 (2001).
Gallegos, A. M. et al. Control of T cell antigen reactivity via programmed TCR downregulation. Nat. Immunol. 17, 379–386 (2016).
Guy, C. S. et al. Distinct TCR signaling pathways drive proliferation and cytokine production in T cells. Nat. Immunol. 14, 262–270 (2013).
Zhong, S. et al. T-cell receptor affinity and avidity defines antitumor response and autoimmunity in T-cell immunotherapy. Proc. Natl Acad. Sci. USA 110, 6973–6978 (2013).
Sim, M. J. W. et al. High-affinity oligoclonal TCRs define effective adoptive T cell therapy targeting mutant KRAS-G12D. Proc. Natl Acad. Sci. USA 117, 12826–12835 (2020).
Wu, D., Gallagher, D. T., Gowthaman, R., Pierce, B. G. & Mariuzza, R. A. Structural basis for oligoclonal T cell recognition of a shared p53 cancer neoantigen. Nat. Commun. 11, 2908 (2020).
Devlin, J. R. et al. Structural dissimilarity from self drives neoepitope escape from immune tolerance. Nat. Chem. Biol. 16, 1269–1276 (2020).
Margulies, D. H., Plaksin, D., Khilko, S. N. & Jelonek, M. T. Studying interactions involving the T-cell antigen receptor by surface plasmon resonance. Curr. Opin. Immunol. 8, 262–270 (1996).
Tian, S., Maile, R., Collins, E. J. & Frelinger, J. A. CD8+ T cell activation is governed by TCR–peptide/MHC affinity, not dissociation rate. J. Immunol. 179, 2952–2960 (2007).
Huang, J. et al. The kinetics of two-dimensional TCR and pMHC interactions determine T-cell responsiveness. Nature 464, 932–936 (2010).
Liu, B. et al. 2D TCR–pMHC–CD8 kinetics determines T-cell responses in a self-antigen-specific TCR system. Eur. J. Immunol. 44, 239–250 (2014).
Gannon, P. O. et al. Quantitative TCR:pMHC dissociation rate assessment by NTAmers reveals antimelanoma T cell repertoires enriched for high functional competence. J. Immunol. 195, 356–366 (2015).
Hebeisen, M. et al. Identification of rare high-avidity, tumor-reactive CD8+ T cells by monomeric TCR-ligand off-rates measurements on living cells. Cancer Res. 75, 1983–1991 (2015).
Nauerth, M. et al. Flow cytometry-based TCR-ligand Koff rate assay for fast avidity screening of even very small antigen-specific T cell populations ex vivo. Cytom. A 89, 816–825 (2016).
Laugel, B. et al. Different T cell receptor affinity thresholds and CD8 coreceptor dependence govern cytotoxic T lymphocyte activation and tetramer binding properties. J. Biol. Chem. 282, 23799–23810 (2007).
Schmidt, J. et al. Neoantigen-specific CD8 T cells with high structural avidity preferentially reside in and eliminate tumors. Nat. Commun. 14, 3188 (2023).
Jin, B. Y. et al. Engineered T cells targeting E7 mediate regression of human papillomavirus cancers in a murine model. JCI Insight 3, e99488 (2018).
Allard, M. et al. TCR-ligand dissociation rate is a robust and stable biomarker of CD8+ T cell potency. JCI Insight 2, e92570 (2017).
Gattinoni, L. et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J. Exp. Med. 20S2, 907–912 (2005).
Klebanoff, C. A., Khong, H. T., Antony, P. A., Palmer, D. C. & Restifo, N. P. Sinks, suppressors and antigen presenters: how lymphodepletion enhances T cell-mediated tumor immunotherapy. Trends Immunol. 26, 111–117 (2005).
Paulos, C. M. et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J. Clin. Invest. 117, 2197–2204 (2007).
Klebanoff, C. A. et al. Determinants of successful CD8+ T-cell adoptive immunotherapy for large established tumors in mice. Clin. Cancer Res. 17, 5343–5352 (2011).
Chandran, S. S. et al. Treatment of metastatic uveal melanoma with adoptive transfer of tumour-infiltrating lymphocytes: a single-centre, two-stage, single-arm, phase 2 study. Lancet Oncol. 18, 792–802 (2017).
Arnaud, M. et al. Sensitive identification of neoantigens and cognate TCRs in human solid tumors. Nat. Biotechnol. 40, 656–660 (2021).
Leko, V. et al. Identification of neoantigen-reactive tumor-infiltrating lymphocytes in primary bladder cancer. J. Immunol. 202, 3458–3467 (2019).
Zacharakis, N. et al. Breast cancers are immunogenic: immunologic analyses and a phase II pilot clinical trial using mutation-reactive autologous lymphocytes. J. Clin. Oncol. 40, 1741–1754 (2022).
Stevanovic, S. et al. A phase II study of tumor-infiltrating lymphocyte therapy for human papillomavirus-associated epithelial cancers. Clin. Cancer Res. 25, 1486–1493 (2019).
Hanada, K., Perry-Lalley, D. M., Ohnmacht, G. A., Bettinotti, M. P. & Yang, J. C. Identification of fibroblast growth factor-5 as an overexpressed antigen in multiple human adenocarcinomas. Cancer Res. 61, 5511–5516 (2001).
McGranahan, N. et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 351, 1463–1469 (2016).
Goff, S. L. et al. Randomized, prospective evaluation comparing intensity of lymphodepletion before adoptive transfer of tumor-infiltrating lymphocytes for patients with metastatic melanoma. J. Clin. Oncol. 34, 2389–2397 (2016).
Sarnaik, A. A. et al. Lifileucel, a tumor-infiltrating lymphocyte therapy, in metastatic melanoma. J. Clin. Oncol. 39, 2656–2666 (2021).
Rohaan MW et al. Tumor-Infiltrating Lymphocyte Therapy or Ipilimumab in Advanced Melanoma. N Engl J Med. 387, 2113-2125 (2022). This randomized phase III clinical trial demonstrates that adoptive transfer of TILs results in significantly longer progression-free survival compared with anti-CTLA4 as a salvage therapy following anti-PD1 treatment failure.
Dafni, U. et al. Efficacy of adoptive therapy with tumor-infiltrating lymphocytes and recombinant interleukin-2 in advanced cutaneous melanoma: a systematic review and meta-analysis. Ann. Oncol. 30, 1902–1913 (2019).
Radvanyi, L. G. et al. Specific lymphocyte subsets predict response to adoptive cell therapy using expanded autologous tumor-infiltrating lymphocytes in metastatic melanoma patients. Clin. Cancer Res. 18, 6758–6770 (2012).
Seitter, S. J. et al. Impact of prior treatment on the efficacy of adoptive transfer of tumor-infiltrating lymphocytes in patients with metastatic melanoma. Clin. Cancer Res. 27, 5289–5298 (2021).
Besser, M. J. et al. Adoptive transfer of tumor-infiltrating lymphocytes in patients with metastatic melanoma: intent-to-treat analysis and efficacy after failure to prior immunotherapies. Clin. Cancer Res. 19, 4792–4800 (2013).
Levi, S. T. et al. Neoantigen identification and response to adoptive cell transfer in anti PD-1 naive and experienced patients with metastatic melanoma. Clin. Cancer Res. 28, 3042–3052 (2022).
Samstein, R. M. et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 51, 202–206 (2019).
Lauss, M. et al. Mutational and putative neoantigen load predict clinical benefit of adoptive T cell therapy in melanoma. Nat. Commun. 8, 1738 (2017).
Kristensen, N. P. et al. Neoantigen-reactive CD8+ T cells affect clinical outcome of adoptive transfer with tumor-infiltrating lymphocytes in melanoma. J. Clin. Invest. 132, e150535 (2021).
Algazi, A. P. et al. Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer 122, 3344–3353 (2016).
Tran, E. et al. T-cell transfer therapy targeting mutant KRAS in cancer. N. Engl. J. Med. 375, 2255–2262 (2016). Together with Doran et al. (2019) and Nagarsheth et al. (2021), this clinical report highlights diverse mechanisms of acquired immune resistance to adoptively transferred CD8+ T cells expressing TCRs specific for HLA class I-restricted epitopes.
Klebanoff, C. A. & Wolchok, J. D. Shared cancer neoantigens: making private matters public. J. Exp. Med. 215, 5–7 (2018).
Butler, M. O. et al. Establishment of antitumor memory in humans using in vitro-educated CD8+ T cells. Sci. Transl. Med. 3, 80ra34 (2011).
Chapuis, A. G. et al. T-cell therapy using interleukin-21-primed cytotoxic T-cell lymphocytes combined with cytotoxic T-cell lymphocyte antigen-4 blockade results in long-term cell persistence and durable tumor regression. J. Clin. Oncol. 34, 3787–3795 (2016).
Yee, C. et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc. Natl Acad. Sci. USA 99, 16168–16173 (2002).
Khammari, A. et al. Treatment of metastatic melanoma with autologous Melan-A/MART-1-specific cytotoxic T lymphocyte clones. J. Invest. Dermatol. 129, 2835–2842 (2009).
Hunder, N. N. et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N. Engl. J. Med. 358, 2698–2703 (2008).
Chandran, S. S. et al. Persistence of CTL clones targeting melanocyte differentiation antigens was insufficient to mediate significant melanoma regression in humans. Clin. Cancer Res. 21, 534–543 (2015).
Dudley, M. E. et al. Adoptive transfer of cloned melanoma-reactive T lymphocytes for the treatment of patients with metastatic melanoma. J. Immunother. 24, 363–373 (2001).
Chapuis, A. G. et al. Transferred melanoma-specific CD8+ T cells persist, mediate tumor regression, and acquire central memory phenotype. Proc. Natl Acad. Sci. USA 109, 4592–4597 (2012).
Caballero, O. L. & Chen, Y. T. Cancer/testis (CT) antigens: potential targets for immunotherapy. Cancer Sci. 100, 2014–2021 (2009).
De Smet, C., Lurquin, C., Lethe, B., Martelange, V. & Boon, T. DNA methylation is the primary silencing mechanism for a set of germ line- and tumor-specific genes with a CpG-rich promoter. Mol. Cell Biol. 19, 7327–7335 (1999).
Chapuis, A. G. et al. Tracking the fate and origin of clinically relevant adoptively transferred CD8+ T cells in vivo. Sci. Immunol. 2, eaal2568 (2017).
Klebanoff, C. A., Gattinoni, L. & Restifo, N. P. Sorting through subsets: which T-cell populations mediate highly effective adoptive immunotherapy? J. Immunother. 35, 651–660 (2012).
Chandran, S. S. et al. Tumor-specific effector CD8+ T cells that can establish immunological memory in humans after adoptive transfer are marked by expression of IL7 receptor and c-myc. Cancer Res. 75, 3216–3226 (2015).
Wang, A. et al. The stoichiometric production of IL-2 and IFN-γ mRNA defines memory T cells that can self-renew after adoptive transfer in humans. Sci. Transl. Med. 4, 149ra120 (2012).
Klebanoff, C. A. et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc. Natl Acad. Sci. USA 102, 9571–9576 (2005).
Gattinoni, L. et al. A human memory T cell subset with stem cell-like properties. Nat. Med. 17, 1290–1297 (2011).
Graef, P. et al. Serial transfer of single-cell-derived immunocompetence reveals stemness of CD8+ central memory T cells. Immunity 41, 116–126 (2014).
Yamamoto, T. N. et al. T cells genetically engineered to overcome death signaling enhance adoptive cancer immunotherapy. J. Clin. Invest. 129, 1551–1565 (2019).
Oda, S. K. et al. A Fas–4-1BB fusion protein converts a death to a pro-survival signal and enhances T cell therapy. J. Exp. Med. 217, e20191166 (2020).
Roth, T. L. et al. Pooled knockin targeting for genome engineering of cellular immunotherapies. Cell 181, 728–744.e21 (2020).
Silk, J. D. et al. Engineering cancer antigen-specific T cells to overcome the immunosuppressive effects of TGF-β. J. Immunol. 208, 169–180 (2022).
Stromnes, I. M. et al. Abrogating Cbl-b in effector CD8+ T cells improves the efficacy of adoptive therapy of leukemia in mice. J. Clin. Invest. 120, 3722–3734 (2010).
Palmer, D. C. et al. Cish actively silences TCR signaling in CD8+ T cells to maintain tumor tolerance. J. Exp. Med. 212, 2095–2113 (2015).
Shifrut, E. et al. Genome-wide CRISPR screens in primary human T cells reveal key regulators of immune function. Cell 175, 1958–1971.e15 (2018).
Carnevale, J. et al. RASA2 ablation in T cells boosts antigen sensitivity and long-term function. Nature 609, 174–182 (2022).
Duval, L. et al. Adoptive transfer of allogeneic cytotoxic T lymphocytes equipped with a HLA-A2 restricted MART-1 T-cell receptor: a phase I trial in metastatic melanoma. Clin. Cancer Res. 12, 1229–1236 (2006).
Borbulevych, O. Y., Santhanagopolan, S. M., Hossain, M. & Baker, B. M. TCRs used in cancer gene therapy cross-react with MART-1/Melan-A tumor antigens via distinct mechanisms. J. Immunol. 187, 2453–2463 (2011).
Sugiyama, H. WT1 (Wilms’ tumor gene 1): biology and cancer Immunotherapy. Jpn. J. Clin. Oncol. 40, 377–387 (2010).
Levine, A. J., Momand, J. & Finlay, C. A. The p53 tumour suppressor gene. Nature 351, 453–456 (1991).
Theobald, M., Biggs, J., Dittmer, D., Levine, A. J. & Sherman, L. A. Targeting p53 as a general tumor antigen. Proc. Natl Acad. Sci. USA 92, 11993–11997 (1995).
Cohen, C. J. et al. Recognition of fresh human tumor by human peripheral blood lymphocytes transduced with a bicistronic retroviral vector encoding a murine anti-p53 TCR. J. Immunol. 175, 5799–5808 (2005).
Theoret, M. R. et al. Relationship of p53 overexpression on cancers and recognition by anti-p53 T cell receptor-transduced T cells. Hum. Gene Ther. 19, 1219–1232 (2008).
Robbins, P. F. et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: long-term follow-up and correlates with response. Clin. Cancer Res. 21, 1019–1027 (2015).
D’Angelo, S. P. et al. Primary efficacy and safety of letetresgene autoleucel (lete-cel; GSK3377794) pilot study in patients with advanced and metastatic myxoid/round cell liposarcoma (MRCLS). J. Clin. Oncol. 40, 11500–11500 (2022).
Kerkar, S. P. et al. MAGE-A is more highly expressed than NY-ESO-1 in a systematic immunohistochemical analysis of 3668 cases. J. Immunother. 39, 181–187 (2016).
Chinnasamy, N. et al. A TCR targeting the HLA-A*0201-restricted epitope of MAGE-A3 recognizes multiple epitopes of the MAGE-A antigen superfamily in several types of cancer. J. Immunol. 186, 685–696 (2011).
D’Angelo, S. P. et al. Identification of response stratification factors from pooled efficacy analyses of afamitresgene autoleucel (“Afami-cel” [formerly ADP-A2M4]) in metastatic synovial sarcoma and myxoid/round cell liposarcoma phase 1 and phase 2 trials. J. Clin. Oncol. 40 (Suppl. 16), 11562 (2022).
Hong, D. S. et al. Autologous T cell therapy for MAGE-A4+ solid cancers in HLA-A*02+ patients: a phase 1 trial. Nat. Med. 29, 104–114 (2023).
Hong, D. S. et al. 540P safety and efficacy from the SURPASS trial with ADP-A2M4CD8, a SPEAR T-cell therapy incorporating a CD8α co-receptor and an affinity optimized TCR targeting MAGE-A4. Ann. Oncol. 32, S604–S605 (2021).
Hong, D. S. et al. Phase 1 clinical trial evaluating the safety and anti-tumor activity of ADP-A2M10 SPEAR T-cells in patients with MAGE-A10+ head and neck, melanoma, or urothelial tumors. Front. Oncol. 12, 818679 (2022).
Blumenschein, G. R. et al. Phase I clinical trial evaluating the safety and efficacy of ADP-A2M10 SPEAR T cells in patients with MAGE-A10+ advanced non-small cell lung cancer. J. Immunother. Cancer 10, e003581 (2022).
Xu, Y., Zou, R., Wang, J., Wang, Z. W. & Zhu, X. The role of the cancer testis antigen PRAME in tumorigenesis and immunotherapy in human cancer. Cell Prolif. 53, e12770 (2020).
Wermke, M. et al. Safety and anti-tumor activity of TCR-engineered autologous, PRAME-directed T cells across multiple advanced solid cancers at low doses—clinical update on the ACTengine® IMA203 trial. J. Immunother. Cancer 9, A1009–A1009 (2021).
Schiller, J. T. & Lowy, D. R. Vaccines to prevent infections by oncoviruses. Annu. Rev. Microbiol. 64, 23–41 (2010).
Feng, H. C., Shuda, M., Chang, Y. & Moore, P. S. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 319, 1096–1100 (2008).
Jing, L. C. et al. Prevalent and diverse intratumoral oncoprotein-specific CD8+ T cells within polyomavirus-driven Merkel cell carcinomas. Cancer Immunol. Res. 8, 648–659 (2020).
Miller, N. J. et al. Tumor-infiltrating merkel cell polyomavirus-specific T cells are diverse and associated with improved patient survival. Cancer Immunol. Res. 5, 137–147 (2017).
Veatch, J. et al. Merkel polyoma virus specific T-cell receptor transgenic T-cell therapy in PD-1 inhibitor refractory Merkel cell carcinoma. J. Clin. Oncol. 40, 9549 (2022).
Leidner, R. et al. Neoantigen T-cell receptor gene therapy in pancreatic cancer. N. Engl. J. Med. 386, 2112–2119 (2022). Together with Tran et al. (2016), this paper demonstrates that adoptive transfer of T cells targeting an epitope derived from the recurrent KRAS G12D hotspot mutation can mediate tumour regression in common solid tumours.
Kim, S. P. et al. Adoptive cellular therapy with autologous tumor-infiltrating lymphocytes and T-cell receptor-engineered T cells targeting common p53 neoantigens in human solid tumors. Cancer Immunol. Res. 10, 932–946 (2022).
Lo, W. et al. Immunologic recognition of a shared p53 mutated neoantigen in a patient with metastatic colorectal cancer. Cancer Immunol. Res. 7, 534–543 (2019).
Middleton, M. R. et al. Tebentafusp, a TCR/anti-CD3 bispecific fusion protein targeting gp100, potently activated antitumor immune responses in patients with metastatic melanoma. Clin. Cancer Res. 26, 5869–5878 (2020).
Carvajal, R. D. et al. Clinical and molecular response to tebentafusp in previously treated patients with metastatic uveal melanoma: a phase 2 trial. Nat. Med. 28, 2364–2373 (2022).
Kantoff, P. W. et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 363, 411–422 (2010).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Neelapu, S. S. et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 377, 2531–2544 (2017).
Maude, S. L. et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 378, 439–448 (2018).
Schuster, S. J. et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N. Engl. J. Med. 380, 45–56 (2019).
Wang, M. et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N. Engl. J. Med. 382, 1331–1342 (2020).
Abramson, J. S. et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet 396, 839–852 (2020).
Shah, B. D. et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet 398, 491–502 (2021).
Munshi, N. C. et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N. Engl. J. Med. 384, 705–716 (2021).
Mungall, A. J. et al. The DNA sequence and analysis of human chromosome 6. Nature 425, 805–811 (2003).
Donoghue, M. T. A., Schram, A. M., Hyman, D. M. & Taylor, B. S. Discovery through clinical sequencing in oncology. Nat. Cancer 1, 774–783 (2020).
Rosenbaum, E. et al. HLA genotyping in synovial sarcoma: identifying HLA-A*02 and its association with clinical outcome. Clin. Cancer Res. 26, 5448–5455 (2020).
Montesion, M. et al. Somatic HLA class I loss is a widespread mechanism of immune evasion which refines the use of tumor mutational burden as a biomarker of checkpoint inhibitor response. Cancer Discov. 11, 282–292 (2021).
Doebele, R. C. et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1–2 trials. Lancet Oncol. 21, 271–282 (2020).
Hong, D. S. et al. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 21, 531–540 (2020).
Le, D. T. et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357, 409–413 (2017). This paper provides the first comprehensive demonstration that a genomic biomarker (MSI-high) can be used to enrich for patients with diverse common solid cancers who are likely to respond to a single-agent cancer immunotherapy, such as anti-PD1 immune checkpoint blockade.
Marabelle, A. et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 21, 1353–1365 (2020).
Lipsitz, Y. Y. et al. A roadmap for cost-of-goods planning to guide economic production of cell therapy products. Cytotherapy 19, 1383–1391 (2017).
Iriguchi, S. et al. A clinically applicable and scalable method to regenerate T-cells from iPSCs for off-the-shelf T-cell immunotherapy. Nat. Commun. 12, 430 (2021).
Morton, L. T. et al. T cell receptor engineering of primary NK cells to therapeutically target tumors and tumor immune evasion. J. Immunother. Cancer 10, e003715 (2022).
Moore, T. et al. Clinical and immunologic evaluation of three metastatic melanoma patients treated with autologous melanoma-reactive TCR-transduced T cells. Cancer Immunol. Immunother. 67, 311–325 (2018).
Martino, M.D. et al. Translating Science into Survival: Report on the Sixth International Cancer Immunotherapy Conference. Cancer Immunol Res. 11, 145–149 (2023).
Arstila, T. P. et al. A direct estimate of the human αβ T cell receptor diversity. Science 286, 958–961 (1999).
Qi, Q. et al. Diversity and clonal selection in the human T-cell repertoire. Proc. Natl Acad. Sci. USA 111, 13139–13144 (2014).
Merkenschlager, M. et al. How many thymocytes audition for selection? J. Exp. Med. 186, 1149–1158 (1997).
Mason, D. A very high level of crossreactivity is an essential feature of the T-cell receptor. Immunol. Today 19, 395–404 (1998).
Sewell, A. K. Why must T cells be cross-reactive? Nat. Rev. Immunol. 12, 669–677 (2012).
Wooldridge, L. et al. A single autoimmune T cell receptor recognizes more than a million different peptides. J. Biol. Chem. 287, 1168–1177 (2012).
Scott, D. R., Borbulevych, O. Y., Piepenbrink, K. H., Corcelli, S. A. & Baker, B. M. Disparate degrees of hypervariable loop flexibility control T-cell receptor cross-reactivity, specificity, and binding mechanism. J. Mol. Biol. 414, 385–400 (2011).
Colf, L. A. et al. How a single T cell receptor recognizes both self and foreign MHC. Cell 129, 135–146 (2007).
Borbulevych, O. Y., Piepenbrink, K. H. & Baker, B. M. Conformational melding permits a conserved binding geometry in TCR recognition of foreign and self molecular mimics. J. Immunol. 186, 2950–2958 (2011).
Sarkizova, S. et al. A large peptidome dataset improves HLA class I epitope prediction across most of the human population. Nat. Biotechnol. 38, 199–209 (2020).
Acknowledgements
This study was supported, in part, by National Institutes of Health (NIH) grants R37 CA259177 (C.A.K.), R01 CA269733 (C.A.K.), R01 CA286507 (C.A.K.), R35 GM118166 (B.M.B.), R35 CA197633 (A.R.), P01 CA168585 (A.R.), P30 CA008748 (C.A.K.), and the NIH SPORE in Soft Tissue Sarcoma P50 CA217694 (C.A.K.); the Parker Institute for Cancer Immunotherapy (C.A.K. and A.R.); the Cancer Research Institute CRI3176 (C.A.K.); The Sarcoma Center at Memorial Sloan Kettering Cancer Center (MSKCC) (C.A.K.); the Mr. William H. Goodwin and Mrs Alice Goodwin and the Commonwealth Foundation for Cancer Research (C.A.K and S.S.C.); and The Center for Experimental Therapeutics at Memorial Sloan Kettering Cancer Center (C.A.K and S.S.C.). S.A.Q. is funded by a CRUK Senior Cancer Research Fellowship (C36463/A22246) and a CRUK Biotherapeutic Programme Grant (C36463/A20764).
Author information
Authors and Affiliations
Contributions
C.A.K. wrote an initial draft of the manuscript. All authors edited and revised the final manuscript.
Corresponding author
Ethics declarations
Competing interests
C.A.K. and S.S.C. are inventors on patents related to T cell receptor (TCR) discovery and public neoantigen-specific TCRs and are recipients of licensing revenue from Intima Bioscience shared according to Memorial Sloan Kettering Cancer Center (MSKCC) institutional policies. C.A.K. has consulted for or is on the scientific advisory boards for Achilles Therapeutics, Affini-T Therapeutics, Aleta BioTherapeutics, Bellicum Pharmaceuticals, Bristol Myers Squibb, Catamaran Bio, Cell Design Labs, Decheng Capital, G1 Therapeutics, Klus Pharma, Obsidian Therapeutics, PACT Pharma, Roche/Genentech and T-knife, and is a scientific co-founder and equity holder in Affini-T Therapeutics. S.S.C. is a scientific advisor and equity holder in Affini-T Therapeutics. B.M.B. is an inventor on patents related to TCR engineering and neoantigen discovery, has consulted for Eureka Therapeutics and EnaraBio, and is on the scientific advisory board of T-cure Bioscience. S.A.Q is an inventor in patents related to the use of T cell therapies targeting tumour clonal neoantigens in cancer, and is also a founder, CSO and equity holder of Achilles Therapeutics. A.R. reports personal fees from Amgen, Chugai, Genentech, Merck, Novartis, Roche, Sanofi, Vedanta, 4C Biomed, Appia, Apricity, Arcus, Highlight, Compugen, ImaginAb, Kalthera/ImmPACT Bio, MapKure, Merus, Rgenix, Lutris, Nextech, PACT Pharma, Synthekine, Tango, Advaxis, CytomX, Five Prime, RAPT, Isoplexis and Kite/Gilead, and has received grants from Agilent and Bristol Myers Squibb outside the submitted work.
Peer review
Peer review information
Nature Reviews Drug Discovery thanks Mirjam Heemskerk, Paul Robbins and the other, anonymous, reviewer for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- γδ T cells
-
Approximately 1–5% of circulating T cells express a somatically recombined γδ T cell receptor (TCR) that associates with the CD3 subunits and mediates antigen-specific cellular immunity. γδ T cells recognize a limited number of ligands presented in the context of non-polymorphic antigen presentation molecules.
- Catch bond
-
A property of many low-affinity cell surface adhesion systems, including selectins, integrins, adhesins and T cell receptors, in which a bond’s likelihood of separating is reduced as tensile force is applied. This property contrasts with the more common slip bond in which a bond disassociates rapidly following application of sheer force.
- Cellular indexing of transcriptomes and epitopes by sequencing
-
(CITE-seq). A sequencing-based method that enables the simultaneous detection and quantification of cell surface proteins, immune receptor binding specificity and transcriptomic data at single-cell resolution.
- Common γ-chain cytokine
-
A member of a family of six cytokines that share the common γ-chain (γc; CD132) as part of a receptor complex. Members of this cytokine family include IL-2, IL-7, IL-9, IL-15 and IL-21.
- DNA-barcoded p/HLA multimers
-
A synthetic peptide/human leukocyte antigen molecule conjugated to a unique oligonucleotide sequence that is detected and quantified using next-generation sequencing methods. This reagent enables the parallel detection of >1,000 T cell specificities in a single sample.
- DNA mismatch repair proficiency
-
The vast majority (~95%) of colorectal cancers and other gastrointestinal malignancies are proficient in DNA mismatch repair enzymatic function. These cancers are associated with a modest tumour mutational burden and are largely unresponsive to immune checkpoint inhibitors (ICIs).
- HLA-restricted tumour antigens
-
Cancer-specific and cancer-associated polypeptides resulting from proteasomal, endosomal or lysosomal protein degradation. These polypeptides are bound non-covalently within the binding groove of a human leukocyte antigen (HLA) class I or class II molecule and facilitate the activation of antigen-specific T cells.
- Human leukocyte antigen
-
(HLA). A family of highly polymorphic, germline-encoded transmembrane proteins that bind proteolytically degraded polypeptides. In vertebrate species, related proteins are referred to as the major histocompatibility complex (MHC).
- Immune receptor tyrosine-based activation motif
-
(ITAM). A conserved four amino acid sequence (YxxL/I) contained in the cytoplasmic tails of non-catalytic tyrosine-phosphorylated receptors found in immune cells.
- Investigational new drug application
-
Request from a clinical study sponsor to obtain authorization from the US Food and Drug Administration to administer an investigational drug or biological product to humans. An investigational new drug application comprises preclinical data establishing whether the product is reasonably safe and can be manufactured consistently alongside a protocol for the study’s conduct that ensures patients are not exposed to unnecessary risks.
- Leukapheresis
-
An outpatient procedure to obtain large numbers of circulating T cells, B cells, NK cells and monocytes for downstream clinical applications, including genetic engineering and in vitro stimulation. In this procedure, mononuclear cells are separated from red blood cells, platelets and plasma through differential centrifugation.
- Melanoma antigen recognized by T cells 1
-
(MART-1; also known as Melan-A). A transmembrane protein associated with normal melanocytes and expressed by the majority of melanomas.
- Paramagnetic artificial APC
-
An iron-dextran nanoparticle conjugated to a synthetic peptide/human leukocyte antigen complex and an anti-CD28 co-stimulatory antibody. In the presence of a magnetic column, this reagent simultaneously enriches for rare antigen-specific T cells and induces T cell proliferation.
- Sleeping Beauty transposon/transposase system
-
A gene therapy method that uses co-transfer of two DNA plasmids to achieve stable transgene genomic integration and expression. One plasmid transiently expresses a transposase enzyme that digests the second plasmid, the Sleeping Beauty transposon, at inverted/direct repeats and ligates the transposon cassette containing a gene of interest into TA dinucleotide repeats within the genome.
- Somatic recombination
-
The genes for the variable (V), diversity (D), junctional (J) and constant (C) segments of the T cell receptor-α (TCRα) and TCRβ hemichains do not encode functional proteins in their germline configuration. Rather, each segment undergoes site-specific recombination with the aid of recombination activation genes to assemble a single functional frame.
- Strep-tag II sequence
-
A short polypeptide that binds with intermediate affinity to the biotin binding site of a mutated form of streptavidin. In the presence of excess d-biotin, Strep-tag II multimers dissociate into monomers. When peptide/human leukocyte antigen molecules are conjugated to Strep-tag II, this reagent facilitates the capture, enrichment and release of antigen-specific T cells.
- Tumour organoids
-
Multicellular in vitro structures that preserve the genetic diversity, phenotype and structural features of a tumour in vivo. In vitro responses of tumour organoids to different treatments, including immunotherapies, often correlate with patient responses.
- Uveal melanoma
-
A rare malignancy that arises from melanocytes within the uveal tract of the eye, which includes the iris, ciliary body and choroid. Unlike cutaneous melanoma, uveal melanomas are modestly mutated and generally respond poorly to immune checkpoint blockade.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Klebanoff, C.A., Chandran, S.S., Baker, B.M. et al. T cell receptor therapeutics: immunological targeting of the intracellular cancer proteome. Nat Rev Drug Discov 22, 996–1017 (2023). https://doi.org/10.1038/s41573-023-00809-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41573-023-00809-z
This article is cited by
-
Discovery of tumor-reactive T cell receptors by massively parallel library synthesis and screening
Nature Biotechnology (2024)
-
Immunotherapy for Brain Tumors: Where We Have Been, and Where Do We Go From Here?
Current Treatment Options in Oncology (2024)