Abstract
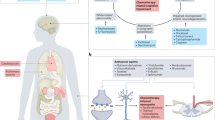
Despite the enthusiasm surrounding novel targeted agents and immunotherapies, chemotherapy remains the mainstay treatment for most human malignancies, either alone or in combination. Yet, the burden of chemotherapy-associated adverse events (CAAEs) remains high and, importantly, is associated with considerable morbidity, mortality and costs that affect patients across multiple dimensions, including physical, emotional and social functioning. CAAEs can directly affect patient outcomes and indirectly increase the risk of cancer recurrence by compromising treatment intensity and continuity. Systematic efforts to identify and critically summarize the evidence on management approaches for CAAEs remain limited. Herein, we review the most common acute CAAEs having a major effect on survival, quality of life, function and/or continuation of optimal therapy. We focus on selected acute toxicities that occur during treatment, summarizing their underlying pathophysiology, multifactorial aetiologies, evidenced-based treatments, prevention strategies and management recommendations. We also summarize the available evidence on risk factors, validated risk assessment tools and other efforts to optimize symptom control in patients most likely to benefit in order to personalize the prevention and treatment of acute CAAEs. Finally, we discuss innovative symptom monitoring and supportive care interventions that are under development to further improve the outcomes of patients with cancer.
Key points
-
The burden of chemotherapy-associated adverse events (CAAEs) remains high and is associated with considerable morbidity, mortality and costs.
-
CAAEs are frequently multifactorial in nature and often adversely affect multiple dimensions, including physical, emotional and social functioning domains.
-
CAAEs have a direct effect on patient symptoms, quality of life, and both physical and psychosocial functioning and can also compromise treatment intensity and continuity, potentially increasing the risk of cancer recurrence.
-
Continuing research into the underlying pathophysiology, emerging biomarkers, supportive care therapies and advances in patient risk stratification will further enable the personalized management of CAAEs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Flowers, C. R. et al. Antimicrobial prophylaxis and outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 31, 794–810 (2013).
NCCN. Prevention and Treatment of Cancer-Related Infections https://www.nccn.org/professionals/physician_gls/pdf/growthfactors.pdf (NCCN, 2021).
Klastersky, J. et al. Management of febrile neutropaenia: ESMO Clinical Practice Guidelines. Ann. Oncol. 27, v111–v118 (2016).
Taplitz, R. A. et al. Outpatient management of fever and neutropenia in adults treated for malignancy: American society of clinical oncology and infectious diseases society of America clinical practice guideline update. J. Clin. Oncol. 36, 1443–1453 (2018).
Lyman, G. H. & Sparreboom, A. Chemotherapy dosing in overweight and obese patients with cancer. Nat. Rev. Clin. Oncol. 10, 451–459 (2013).
Denduluri, N. et al. Dose delays, dose reductions, and relative dose intensity in patients with cancer who received adjuvant or neoadjuvant chemotherapy in community oncology practices. J. Natl Compr. Cancer Netw. 13, 1383–1393 (2015).
Kuderer, N. M. et al. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 106, 2258–2266 (2006).
Pfreundschuh, M. et al. Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of elderly patients with aggressive lymphomas: results of the NHL-B2 trial of the DSHNHL. Blood 104, 634–641 (2004).
Pettengell, R., Schwenkglenks, M. & Bosly, A. Association of reduced relative dose intensity and survival in lymphoma patients receiving CHOP-21 chemotherapy. Ann. Hematol. 87, 429–430 (2008).
Kwak, L. W. et al. Prognostic significance of actual dose intensity in diffuse large-cell lymphoma: results of a tree-structured survival analysis. J. Clin. Oncol. 8, 963–977 (1990).
Hanna, R. K. et al. Predictors of reduced relative dose intensity and its relationship to mortality in women receiving multi-agent chemotherapy for epithelial ovarian cancer. Gynecol. Oncol. 129, 74–80 (2013).
Citron, M. L. et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J. Clin. Oncol. 21, 1431–1439 (2003).
Budman, D. R. et al. Dose and dose intensity as determinants of outcome in the adjuvant treatment of breast cancer. The Cancer and Leukemia Group B. J. Natl Cancer Inst. 90, 1205–1211 (1998).
Bosly, A. et al. Achievement of optimal average relative dose intensity and correlation with survival in diffuse large B-cell lymphoma patients treated with CHOP. Ann. Hematol. 87, 277–283 (2008).
Bonneterre, J. et al. Epirubicin increases long-term survival in adjuvant chemotherapy of patients with poor-prognosis, node-positive, early breast cancer: 10-year follow-up results of the French Adjuvant Study Group 05 randomized trial. J. Clin. Oncol. 23, 2686–2693 (2005).
Bonadonna, G. et al. Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer: the results of 20 years of follow-up. N. Engl. J. Med. 332, 901–906 (1995).
Hryniuk, W. & Levine, M. N. Analysis of dose intensity for adjuvant chemotherapy trials in stage II breast cancer. J. Clin. Oncol. 4, 1162–1170 (1986).
Hryniuk, W., Frei, E. III & Wright, F. A. A single scale for comparing dose-intensity of all chemotherapy regimens in breast cancer: summation dose-intensity. J. Clin. Oncol. 16, 3137–3147 (1998).
Hryniuk, W. & Bush, H. The importance of dose intensity in chemotherapy of metastatic breast cancer. J. Clin. Oncol. 2, 1281–1288 (1984).
Frei, E. III et al. The relationship between high-dose treatment and combination chemotherapy: the concept of summation dose intensity. Clin. Cancer Res. 4, 2027–2037 (1998).
Lyman, G. H., Dale, D. C. & Crawford, J. Incidence and predictors of low dose-intensity in adjuvant breast cancer chemotherapy: a nationwide study of community practices. J. Clin. Oncol. 21, 4524–4531 (2003).
Lyman, G. H. et al. Incidence and predictors of low chemotherapy dose-intensity in aggressive non-Hodgkin’s lymphoma: a nationwide study. J. Clin. Oncol. 22, 4302–4311 (2004).
Lyman, G. H. Impact of chemotherapy dose intensity on cancer patient outcomes. J. Natl Compr. Cancer Netw. 7, 99–108 (2009).
Shayne, M. et al. Dose intensity and hematologic toxicity in older breast cancer patients receiving systemic chemotherapy. Cancer 115, 5319–5328 (2009).
Shayne, M. et al. Dose intensity and hematologic toxicity in older cancer patients receiving systemic chemotherapy. Cancer 110, 1611–1620 (2007).
Lyman, G. H. Issues on the use of white blood cell growth factors in oncology practice. Am. Soc. Clin. Oncol. Educ. Book 35, e528–e532 (2016).
Lyman, G. H. Risk assessment in oncology clinical practice. From risk factors to risk models. Oncology 17, 8–13 (2003).
Dale, D., Crawford, J. & Lyman, G. H. Myelotoxicity and dose intensity of chemotherapy: reporting practices from randomized clinical trials. J. Natl Compr. Cancer Netw. 1, 440–454 (2003).
Kuderer, N. M. & Wolff, A. C. Enhancing therapeutic decision making when options abound: toxicities matter. J. Clin. Oncol. 32, 1990–1993 (2014).
Truong, J. et al. Interpreting febrile neutropenia rates from randomized controlled trials for consideration of primary prophylaxis in the real world: a systematic review and meta-analysis. Ann. Oncol. 27, 608–618 (2015).
Gonzalez-Barca, E. et al. Prognostic factors influencing mortality in cancer patients with neutropenia and bacteremia. Eur. J. Clin. Microbiol. Infect. Dis. 18, 539–544 (1999).
Darmon, M. et al. Impact of neutropenia duration on short-term mortality in neutropenic critically ill cancer patients. Intensive Care Med. 28, 1775–1780 (2002).
Carratala, J. et al. Bacteremic pneumonia in neutropenic patients with cancer: causes, empirical antibiotic therapy, and outcome. Arch. Intern. Med. 158, 868–872 (1998).
Kuderer, N. M. et al. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J. Clin. Oncol. 25, 3158–3167 (2007).
Bodey, G. P. et al. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann. Intern. Med. 64, 328–340 (1966).
Crawford, J., Dale, D. C. & Lyman, G. H. Chemotherapy-induced neutropenia: risks, consequences, and new directions for its management. Cancer 100, 228–237 (2004).
Blackwell S., Crawford J. In Filgrastim (r-metHuG-CSF) in Clinical Practice (eds Morsten G., Dexter T.) pp 103–116 (Marcel Dekker, 1994).
Lyman, G. H. et al. Risk of febrile neutropenia among patients with intermediate-grade non-Hodgkin’s lymphoma receiving CHOP chemotherapy. Leuk. Lymphoma 44, 2069–2076 (2003).
Lyman, G. H. & Delgado, D. J. Risk and timing of hospitalization for febrile neutropenia in patients receiving CHOP, CHOP-R, or CNOP chemotherapy for intermediate-grade non-Hodgkin lymphoma. Cancer 98, 2402–2409 (2003).
Crawford, J. et al. Risk and timing of neutropenic events in adult cancer patients receiving chemotherapy: the results of a prospective nationwide study of oncology practice. J. Natl Compr. Cancer Netw. 6, 109–118 (2008).
Culakova, E. et al. Patterns of chemotherapy-associated toxicity and supportive care in US oncology practice: a nationwide prospective cohort study. Cancer Med. 3, 434–444 (2014).
Crawford, J. et al. Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N. Engl. J. Med. 325, 164–170 (1991).
Lyman, G. H. et al. Predicting individual risk of neutropenic complications in patients receiving cancer chemotherapy. Cancer 117, 1917–1927 (2011).
Jenkins, P., Scaife, J. & Freeman, S. Validation of a predictive model that identifies patients at high risk of developing febrile neutropaenia following chemotherapy for breast cancer. Ann. Oncol. 23, 1766–1771 (2012).
Bozcuk, H. et al. A prospectively validated nomogram for predicting the risk of chemotherapy-induced febrile neutropenia: a multicenter study. Support. Care Cancer 23, 1759–1767 (2015).
Becker, P. S. et al. NCCN Guidelines Insights: hematopoietic growth factors, version 1.2020. J. Natl Compr. Cancer Netw. 18, 12–22 (2020).
Lyman, G. H., Abella, E. & Pettengell, R. Risk factors for febrile neutropenia among patients with cancer receiving chemotherapy: a systematic review. Crit. Rev. Oncol. Hematol. 90, 190–199 (2014).
Lyman, G. H., Lyman, C. H. & Agboola, O. Risk models for predicting chemotherapy-induced neutropenia. Oncologist 10, 427–437 (2005).
Smith, T. J. et al. Recommendations for the use of WBC growth factors: American Society of Clinical Oncology clinical practice guideline update. J. Clin. Oncol. 33, 3199–3212 (2015).
Lyman, G. H. et al. Assessing patients’ risk of febrile neutropenia: is there a correlation between physician-assessed risk and model-predicted risk? Cancer Med. 4, 1153–1160 (2015).
Kahneman, D., Sibony, O. & Sunstein, C. Noise: A Flaw in Human Judgment (Little Brown & Company, 2021).
Pawloski, P. A. et al. Predicting neutropenia risk in patients with cancer using electronic data. J. Am. Med. Inf. Assoc. 24, e129–e135 (2017).
Li, Y. et al. Value of incorporating newly identified risk factors into risk prediction for chemotherapy-induced febrile neutropenia. Cancer Med. 7, 4121–4131 (2018).
Laskey, R. A. et al. Predictors of severe and febrile neutropenia during primary chemotherapy for ovarian cancer. Gynecol. Oncol. 125, 625–630 (2012).
Weycker, D. et al. Importance of risk factors for febrile neutropenia among patients receiving chemotherapy regimens not classified as high-risk in guidelines for myeloid growth factor use. J. Natl Compr. Cancer Netw. 13, 979–986 (2015).
Weycker, D. et al. Risk of febrile neutropenia in patients receiving emerging chemotherapy regimens. Support. Care Cancer 22, 3275–3285 (2014).
Crawford, J. et al. Myeloid growth factors. Clinical practice guidelines in oncology. J. Natl Compr. Cancer Netw. 5, 188–202 (2007).
Aapro, M. S. et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur. J. Cancer 47, 8–32 (2011).
Kuderer, N. M. & Lyman, G. H. Personalized medicine and cancer supportive care: appropriate use of colony-stimulating factor support of chemotherapy. J. Natl Cancer Inst. 103, 910–913 (2011).
Lyman, G. H. et al. Risk of mortality in patients with cancer who experience febrile neutropenia. Cancer 116, 5555–5563 (2010).
Klastersky, J. & Paesmans, M. The multinational association for supportive care in cancer (MASCC) risk index score: 10 years of use for identifying low-risk febrile neutropenic cancer patients. Support. Care Cancer 21, 1487–1495 (2013).
Klastersky, J. et al. The Multinational Association for supportive care in cancer risk index: a multinational scoring system for identifying low-risk febrile neutropenic cancer patients. J. Clin. Oncol. 18, 3038–3051 (2000).
de Souza Viana, L. et al. Performance of a modified MASCC index score for identifying low-risk febrile neutropenic cancer patients. Support. Care Cancer 16, 841–846 (2008).
Hui, E. P. et al. Prediction of outcome in cancer patients with febrile neutropenia: a prospective validation of the Multinational Association for supportive care in cancer risk index in a Chinese population and comparison with the Talcott model and artificial neural network. Support. Care Cancer 19, 1625–1635 (2011).
Carmona-Bayonas, A. et al. Prediction of serious complications in patients with seemingly stable febrile neutropenia: validation of the Clinical Index of Stable Febrile Neutropenia in a prospective cohort of patients from the FINITE study. J. Clin. Oncol. 33, 465–471 (2015).
Carmona-Bayonas, A. et al. Prognostic evaluation of febrile neutropenia in apparently stable adult cancer patients. Br. J. Cancer 105, 612–617 (2011).
Taplitz, R. A. et al. Antimicrobial prophylaxis for adult patients with cancer-related immunosuppression: ASCO and IDSA clinical practice guideline update. J. Clin. Oncol. 36, 3043–3054 (2018).
Cullen, M. et al. Antibacterial prophylaxis after chemotherapy for solid tumors and lymphomas. N. Engl. J. Med. 353, 988–998 (2005).
Lyman, G. H. et al. Acute myeloid leukemia or myelodysplastic syndrome in randomized controlled clinical trials of cancer chemotherapy with granulocyte colony-stimulating factor: a systematic review. J. Clin. Oncol. 28, 2914–2924 (2010).
Lyman, G. H. et al. The impact of the granulocyte colony-stimulating factor on chemotherapy dose intensity and cancer survival: a systematic review and meta-analysis of randomized controlled trials. Ann. Oncol. 24, 2475–2484 (2013).
Rajan, S. S. et al. Short-term costs associated with primary prophylactic G-CSF use during chemotherapy. Am. J. Manag. Care 19, 150–159 (2013).
Hershman, D. et al. Acute myeloid leukemia or myelodysplastic syndrome following use of granulocyte colony-stimulating factors during breast cancer adjuvant chemotherapy. J. Natl Cancer Inst. 99, 196–205 (2007).
Agiro, A. et al. Risk of neutropenia-related hospitalization in patients who received colony-stimulating factors with chemotherapy for breast cancer. J. Clin. Oncol. 34, 3872–3879 (2016).
Weycker, D. et al. Burden of chemotherapy-induced febrile neutropenia hospitalizations in US Clinical Practice, by use and patterns of prophylaxis with colony-stimulating factor. Support. Care Cancer 25, 439–447 (2017).
Dinan, M. A., Hirsch, B. R. & Lyman, G. H. Management of chemotherapy-induced neutropenia: measuring quality, cost, and value. J. Natl Compr. Cancer Netw. 13, e1–e7 (2015).
Lyman, G. H. et al. Changing patterns of chemotherapy relative dose intensity and supportive care for aggressive B-cell non-Hodgkin lymphoma. Leuk. Lymphoma 57, 283–290 (2015).
Fust, K. et al. Granulocyte colony-stimulating factors in the prevention of febrile neutropenia: review of cost-effectiveness models. Expert Rev. Pharmacoecon. Outcomes Res. 17, 39–52 (2017).
Schnipper, L. E. et al. American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: the top five list for oncology. J. Clin. Oncol. 30, 1715–1724 (2012).
Crawford, J. et al. Myeloid growth factors. J. Natl Compr. Cancer Netw. 11, 1266–1290 (2013).
Vogel, C. L. et al. First and subsequent cycle use of pegfilgrastim prevents febrile neutropenia in patients with breast cancer: a multicenter, double-blind, placebo-controlled phase III study. J. Clin. Oncol. 23, 1178–1184 (2005).
Mahtani, R. L. et al. A prospective cohort study to evaluate the incidence of febrile neutropenia in patients receiving pegfilgrastim on-body injector versus other options for prophylaxis of febrile neutropenia: breast cancer subgroup analysis. Support. Care Cancer 30, 6135–6144 (2022).
Lyman, G. H., Kuderer, N. M. & Aapro, M. Improving outcomes of chemotherapy: established and novel options for myeloprotection in the COVID-19 era. Front. Oncol. 11, 697908 (2021).
Shayne, M., Harvey, R. D. & Lyman, G. H. Prophylaxis and treatment strategies for optimizing chemotherapy relative dose intensity. Expert Rev. Anticancer Ther. 21, 1145–1159 (2021).
Natoli, M. et al. Plinabulin, a distinct microtubule-targeting chemotherapy, promotes M1-like macrophage polarization and anti-tumor immunity. Front. Oncol. 11, 644608 (2021).
Blayney, D. W. et al. Efficacy of plinabulin vs pegfilgrastim for prevention of chemotherapy-induced neutropenia in adults with non-small cell lung cancer: a phase 2 randomized clinical trial. JAMA Oncol. 6, e204429 (2020).
Blayney, D. W., Mohanlal, R. & Huang, L. Protective-2 (BPI-2358-106): a confirmatory trial to demonstrate superiority of the plinabulin+pegfilgrastim (Plin/Peg) combination versus standard of care pegfilgrastim for the prevention of chemotherapy-induced neutropenia (CIN) in breast cancer (BC) patients (pts). Blood 136 (Suppl. 1), 16 (2020).
Mohile, S. G. et al. Evaluation of geriatric assessment and management on the toxic effects of cancer treatment (GAP70+): a cluster-randomised study. Lancet 398, 1894–1904 (2021).
Ambrus, J. L. et al. Causes of death in cancer patients. J. Med. 6, 61–64 (1975).
Chew, H. K. et al. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch. Intern. Med. 166, 458–464 (2006).
Lyman, G. H. et al. Morbidity, mortality and costs associated with venous thromboembolism in hospitalized patients with cancer. Thromb. Res. 164 (Suppl. 1), S112–S118 (2018).
Guerin, L. et al. Prevalence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. Prevalence of CTEPH after pulmonary embolism. Thromb. Haemost. 112, 598–605 (2014).
Rabinovich, A. & Kahn, S. R. The postthrombotic syndrome: current evidence and future challenges. J. Thromb. Haemost. 15, 230–241 (2017).
Elting, L. S. et al. Outcomes and cost of deep venous thrombosis among patients with cancer. Arch. Intern. Med. 164, 1653–1661 (2004).
Kuderer, N. M., Ortel, T. L. & Francis, C. W. Impact of venous thromboembolism and anticoagulation on cancer and cancer survival. J. Clin. Oncol. 27, 4902–4911 (2009).
Falanga, A. Mechanisms of hypercoagulation in malignancy and during chemotherapy. Haemostasis 28 (Suppl. 3), 50–60 (1998).
Dhami, S. P. S., Patmore, S. & O’Sullivan, J. M. Advances in the management of cancer-associated thrombosis. Semin. Thromb. Hemost. 47, 139–149 (2021).
Lyman, G. H. & Khorana, A. A. Cancer, clots and consensus: new understanding of an old problem. J. Clin. Oncol. 27, 4821–4826 (2009).
Ay, C. et al. Prediction of venous thromboembolism in patients with cancer by measuring thrombin generation: results from the Vienna Cancer and Thrombosis Study. J. Clin. Oncol. 29, 2099–2103 (2011).
Dunbar, A. et al. Genomic profiling identifies somatic mutations predicting thromboembolic risk in patients with solid tumors. Blood 137, 2103–2113 (2021).
Ahlbrecht, J. et al. Tumor grade is associated with venous thromboembolism in patients with cancer: results from the Vienna Cancer and Thrombosis Study. J. Clin. Oncol. 30, 3870–3875 (2012).
White, R. H. et al. Incidence of venous thromboembolism in the year before the diagnosis of cancer in 528,693 adults. Arch. Intern. Med. 165, 1782–1787 (2005).
Lyman, G. H. et al. Venous thromboembolism risk in patients with cancer receiving chemotherapy: a real-world analysis. Oncologist 18, 1321–1329 (2013).
Lyman, G. H. The incidence of venous thromboembolism in cancer patients: a real-world analysis. Clin. Adv. Hematol. Oncol. 10, 40–42 (2012).
Khorana, A. A. et al. Risk factors for chemotherapy-associated venous thromboembolism in a prospective observational study. Cancer 104, 2822–2829 (2005).
Cavo, M. et al. Deep-vein thrombosis in patients with multiple myeloma receiving first-line thalidomide-dexamethasone therapy. Blood 100, 2272–2273 (2002).
Kuenen, B. C. et al. Potential role of platelets in endothelial damage observed during treatment with cisplatin, gemcitabine, and the angiogenesis inhibitor SU5416. J. Clin. Oncol. 21, 2192–2198 (2003).
Hurwitz, H. I. et al. Venous thromboembolic events with chemotherapy plus bevacizumab: a pooled analysis of patients in randomized phase II and III studies. J. Clin. Oncol. 29, 1757–1764 (2011).
Khorana, A. A. et al. Cancer associated thrombosis and mortality in patients with cancer stratified by Khorana score risk levels. Cancer Med. 9, 8062–8073 (2020).
Kuderer, N. M. et al. Venous thromboembolism represents a major risk factor for early all-cause mortality in patients receiving cancer chemotherapy. J. Clin. Oncol. 26 (Suppl. 15), 9521 (2008).
Lyman, G. H. Venous thromboembolism in the patient with cancer: focus on burden of disease and benefits of thromboprophylaxis. Cancer 117, 1334–1349 (2011).
Bohlius, J. et al. Recombinant human erythropoietins and cancer patients: updated meta-analysis of 57 studies including 9353 patients. J. Natl Cancer Inst. 98, 708–714 (2006).
Khorana, A. A. et al. Blood transfusions, thrombosis, and mortality in hospitalized patients with cancer. Arch. Intern. Med. 168, 2377–2381 (2008).
Khorana, A. A. et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 111, 4902–4907 (2008).
Ay, C. et al. High plasma levels of soluble P-selectin are predictive of venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS). Blood 112, 2703–2708 (2008).
van Es, N. et al. The Khorana score for prediction of venous thromboembolism in cancer patients: an individual patient data meta-analysis. J. Thromb. Haemost. 18, 1940–1951 (2020).
Ay, C. et al. Prediction of venous thromboembolism in cancer patients. Blood 116, 5377–5382 (2010).
Verso, M. et al. A modified Khorana risk assessment score for venous thromboembolism in cancer patients receiving chemotherapy: the Protecht score. Intern. Emerg. Med. 7, 291–292 (2012).
Kuderer, N. M. et al. Predictors of venous thromboembolism and early mortality in lung cancer: results from a global prospective study (CANTARISK). Oncologist 23, 247–255 (2018).
Mansfield, A. S. et al. Predictors of active cancer thromboembolic outcomes: validation of the Khorana score among patients with lung cancer. J. Thromb. Haemost. 14, 1773–1778 (2016).
Rupa-Matysek, J. et al. Evaluation of risk factors and assessment models for predicting venous thromboembolism in lung cancer patients. Med. Oncol. 35, 63 (2018).
Kuderer, N. M. et al. A validated risk score for venous thromboembolism is predictive of cancer progression and mortality. Oncologist 21, 861–867 (2016).
Pabinger, I. et al. A clinical prediction model for cancer-associated venous thromboembolism: a development and validation study in two independent prospective cohorts. Lancet Haematol. 5, e289–e298 (2018).
Li, A. et al. Derivation and validation of a risk assessment model for immunomodulatory drug-associated thrombosis among patients with multiple myeloma. J. Natl Compr. Cancer Netw. 17, 840–847 (2019).
Sanfilippo, K. M. et al. Predicting venous thromboembolism in multiple myeloma: development and validation of the IMPEDE VTE score. Am. J. Hematol. 94, 1176–1184 (2019).
Lyman, G. H. & Kuderer, N. M. Clinical practice guidelines for the treatment and prevention of cancer-associated thrombosis. Thromb. Res. 191 (Suppl. 1), S79–S84 (2020).
Schunemann, H. J. et al. Use of heparins in patients with cancer: individual participant data meta-analysis of randomised trials study protocol. BMJ Open 6, e010569 (2016).
Khorana, A. A. et al. Dalteparin thromboprophylaxis in cancer patients at high risk for venous thromboembolism: a randomized trial. Thromb. Res. 151, 89–95 (2017).
Carrier, M. et al. Apixaban to prevent venous thromboembolism in patients with cancer. N. Engl. J. Med. 380, 711–719 (2019).
Khorana, A. A. et al. Rivaroxaban for thromboprophylaxis in high-risk ambulatory patients with cancer. N. Engl. J. Med. 380, 720–728 (2019).
Li, A. et al. Direct oral anticoagulant for the prevention of thrombosis in ambulatory patients with cancer: a systematic review and meta-analysis. J. Thromb. Haemost. 17, 2141–2151 (2019).
Key, N. S. et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J. Clin. Oncol. 38, 496–520 (2019).
Lyman, G. H. et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer. Blood Adv. 5, 927–974 (2021).
Verso, M. & Di Nisio, M. Management of venous thromboembolism in cancer patients: Considerations about the clinical practice guideline update of the American Society of Clinical Oncology. Eur. J. Intern. Med. 71, 4–7 (2020).
Aapro, M. et al. Supportive care in patients with cancer during the COVID-19 pandemic. ESMO Open 6, 100038 (2021).
Navari, R. M. & Aapro, M. Antiemetic prophylaxis for chemotherapy-induced nausea and vomiting. N. Engl. J. Med. 374, 1356–1367 (2016).
Trigg, M. E. & Inverso, D. M. Nausea and vomiting with high-dose chemotherapy and stem cell rescue therapy: a review of antiemetic regimens. Bone Marrow Transpl. 42, 501–506 (2008).
Wickham, R. J. Revisiting the physiology of nausea and vomiting-challenging the paradigm. Support. Care Cancer 28, 13–21 (2020).
Smith, P., Lavery, A. & Turkington, R. C. An overview of acute gastrointestinal side effects of systemic anti-cancer therapy and their management. Best Pract. Res. Clin. Gastroenterol. 48-49, 101691 (2020).
Gupta, K., Walton, R. & Kataria, S. P. Chemotherapy-induced nausea and vomiting: pathogenesis, recommendations, and new trends. Cancer Treat. Res. Commun. 26, 100278 (2021).
Crowder, S. L. et al. Metagenomics and chemotherapy-induced nausea: a roadmap for future research. Cancer 128, 461–470 (2021).
Clemons, M. et al. Risk model-guided antiemetic prophylaxis vs physician’s choice in patients receiving chemotherapy for early-stage breast cancer: a randomized clinical trial. JAMA Oncol. 2, 225–231 (2016).
Schwartzberg, L. et al. Resource utilization for chemotherapy-induced nausea and vomiting events in patients with solid tumors treated with antiemetic regimens. Am. Health Drug Benefits 8, 273–282 (2015).
Hesketh, P. J. et al. Antiemetics: ASCO guideline update. J. Clin. Oncol. 38, 2782–2797 (2020).
Roila, F. et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann. Oncol. 27, v119–v133 (2016).
Benson, A. B. III et al. Recommended guidelines for the treatment of cancer treatment-induced diarrhea. J. Clin. Oncol. 22, 2918–2926 (2004).
Andreyev, J. et al. Guidance on the management of diarrhoea during cancer chemotherapy. Lancet Oncol. 15, e447–e460 (2014).
O’Brien, B. E., Kaklamani, V. G. & Benson, A. B. 3rd. The assessment and management of cancer treatment-related diarrhea. Clin. Colorectal Cancer 4, 375–381 (2005).
Rothenberg, M. L. et al. Phase II trial of irinotecan in patients with progressive or rapidly recurrent colorectal cancer. J. Clin. Oncol. 14, 1128–1135 (1996).
Wadler, S. et al. Recommended guidelines for the treatment of chemotherapy-induced diarrhea. J. Clin. Oncol. 16, 3169–3178 (1998).
Rutledge, D. N. & Engelking, C. Cancer-related diarrhea: selected findings of a national survey of oncology nurse experiences. Oncol. Nurs. Forum 25, 861–873 (1998).
Ikuno, N. et al. Irinotecan (CPT-11) and characteristic mucosal changes in the mouse ileum and cecum. J. Natl Cancer Inst. 87, 1876–1883 (1995).
Abigerges, D. et al. Phase I and pharmacologic studies of the camptothecin analog irinotecan administered every 3 weeks in cancer patients. J. Clin. Oncol. 13, 210–221 (1995).
Saliba, F. et al. Pathophysiology and therapy of irinotecan-induced delayed-onset diarrhea in patients with advanced colorectal cancer: a prospective assessment. J. Clin. Oncol. 16, 2745–2751 (1998).
Arbuckle, R. B., Huber, S. L. & Zacker, C. The consequences of diarrhea occurring during chemotherapy for colorectal cancer: a retrospective study. Oncologist 5, 250–259 (2000).
Anand, A. & Glatt, A. E. Clostridium difficile infection associated with antineoplastic chemotherapy: a review. Clin. Infect. Dis. 17, 109–113 (1993).
Aksoy, D. Y. et al. Diarrhea in neutropenic patients: a prospective cohort study with emphasis on neutropenic enterocolitis. Ann. Oncol. 18, 183–189 (2007).
Zidan, J. et al. Octreotide in the treatment of severe chemotherapy-induced diarrhea. Ann. Oncol. 12, 227–229 (2001).
Morton, A. J. & Durrant, S. T. Efficacy of octreotide in controlling refractory diarrhea following bone marrow transplantation. Clin. Transpl. 9, 205–208 (1995).
Kornblau, S. et al. Management of cancer treatment-related diarrhea. Issues and therapeutic strategies. J. Pain Symptom Manag. 19, 118–129 (2000).
Grace, E. et al. Review article: small intestinal bacterial overgrowth–prevalence, clinical features, current and developing diagnostic tests, and treatment. Aliment. Pharmacol. Ther. 38, 674–688 (2013).
Sonis, S. T. The pathobiology of mucositis. Nat. Rev. Cancer 4, 277–284 (2004).
Bowen, J. et al. The pathogenesis of mucositis: updated perspectives and emerging targets. Support. Care Cancer 27, 4023–4033 (2019).
Sonis, S. T. et al. Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 100, 1995–2025 (2004).
Menezes-Garcia, Z. et al. Mechanisms underlying chemotherapy-associated mucositis: the role of inflammatory mediators and potential therapeutic targets. EMJ Gastroenterol. 7, 82–91 (2018).
Epstein, J. B. & Schubert, M. M. Oropharyngeal mucositis in cancer therapy. Review of pathogenesis, diagnosis, and management. Oncology 17, 1767–1779 (2003).
National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v5.0 (NCI, 2017).
Maria, O. M., Eliopoulos, N. & Muanza, T. Radiation-induced oral mucositis. Front. Oncol. 7, 89 (2017).
Rapoport, A. P. et al. Analysis of factors that correlate with mucositis in recipients of autologous and allogeneic stem-cell transplants. J. Clin. Oncol. 17, 2446–2453 (1999).
Sonis, S. T. et al. Validation of a new scoring system for the assessment of clinical trial research of oral mucositis induced by radiation or chemotherapy. Cancer 85, 2103–2113 (1999).
Kushner, J. A. et al. Development and validation of a patient-reported oral mucositis symptom (PROMS) scale. J. Can. Dent. Assoc. 74, 59 (2008).
Wardill, H. R. et al. Prediction of mucositis risk secondary to cancer therapy: a systematic review of current evidence and call to action. Support. Care Cancer 28, 5059–5073 (2020).
Elting, L. S. et al. Risk of oral and gastrointestinal mucosal injury among patients receiving selected targeted agents: a meta-analysis. Support. Care Cancer 21, 3243–3254 (2013).
Watters, A. L., Epstein, J. B. & Agulnik, M. Oral complications of targeted cancer therapies: a narrative literature review. Oral. Oncol. 47, 441–448 (2011).
Riley, P. et al. Interventions for preventing oral mucositis in patients with cancer receiving treatment: oral cryotherapy. Cochrane Database Syst. Rev. 2015, CD011552 (2015).
Nawi, R. I. M. et al. Oral cryotherapy: prevention of oral mucositis and pain among patients with colorectal cancer undergoing chemotherapy. Clin. J. Oncol. Nurs. 22, 555–560 (2018).
Farrell, C. L. et al. Keratinocyte growth factor protects mice from chemotherapy and radiation-induced gastrointestinal injury and mortality. Cancer Res. 58, 933–939 (1998).
Spielberger, R. et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N. Engl. J. Med. 351, 2590–2598 (2004).
Logan, R. M. et al. Systematic review of growth factors and cytokines for the management of oral mucositis in cancer patients and clinical practice guidelines. Support. Care Cancer 28, 2485–2498 (2020).
Blijlevens, N. et al. In a high-dose melphalan setting, palifermin compared with placebo had no effect on oral mucositis or related patient’s burden. Bone Marrow Transpl. 48, 966–971 (2013).
Rosen, L. S. et al. Palifermin reduces the incidence of oral mucositis in patients with metastatic colorectal cancer treated with fluorouracil-based chemotherapy. J. Clin. Oncol. 24, 5194–5200 (2006).
Zadik, Y. et al. Systematic review of photobiomodulation for the management of oral mucositis in cancer patients and clinical practice guidelines. Support. Care Cancer 27, 3969–3983 (2019).
Elad, S. et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 126, 4423–4431 (2020).
Peterson, D. E. et al. Management of oral and gastrointestinal mucosal injury: ESMO Clinical Practice Guidelines for diagnosis, treatment, and follow-up. Ann. Oncol. 26 (Suppl. 5), v139–v151 (2015).
Uderzo, C. et al. Glutamine-enriched nutrition does not reduce mucosal morbidity or complications after stem-cell transplantation for childhood malignancies: a prospective randomized study. Transplantation 91, 1321–1325 (2011).
McGinnis, W. L. et al. Placebo-controlled trial of sucralfate for inhibiting radiation-induced esophagitis. J. Clin. Oncol. 15, 1239–1243 (1997).
Movsas, B. et al. Randomized trial of amifostine in locally advanced non–small-cell lung cancer patients receiving chemotherapy and hyperfractionated radiation: Radiation Therapy Oncology Group trial 98-01. J. Clin. Oncol. 23, 2145–2154 (2005).
Dodd, M. J. et al. Randomized clinical trial of the effectiveness of 3 commonly used mouthwashes to treat chemotherapy-induced mucositis. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 90, 39–47 (2000).
McGuire, D. B. et al. Systematic review of basic oral care for the management of oral mucositis in cancer patients. Support. Care Cancer 21, 3165–3177 (2013).
Lalla, R. V. et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 120, 1453–1461 (2014).
Nursing AAo. Magic Mouthwash (2021).
Uberoi, A. S., Brown, T. J. & Gupta, A. Magic mouthwash for oral mucositis: a teachable moment. JAMA Intern. Med. 179, 104–105 (2019).
Rugo, H. S. et al. Prevention of everolimus-related stomatitis in women with hormone receptor-positive, HER2-negative metastatic breast cancer using dexamethasone mouthwash (SWISH): a single-arm, phase 2 trial. Lancet Oncol. 18, 654–662 (2017).
Carnel, S. B. et al. Treatment of radiation-and chemotherapy-induced stomatitis. Otolaryngol. Head Neck Surg. 102, 326–330 (1990).
Epstein, J. B. et al. Oral topical doxepin rinse: analgesic effect in patients with oral mucosal pain due to cancer or cancer therapy. Oral. Oncol. 37, 632–637 (2001).
Saunders, D. P. et al. Systematic review of antimicrobials, mucosal coating agents, anesthetics, and analgesics for the management of oral mucositis in cancer patients and clinical practice guidelines. Support. Care Cancer 28, 2473–2484 (2020).
Cerchietti, L. C. et al. Effect of topical morphine for mucositis-associated pain following concomitant chemoradiotherapy for head and neck carcinoma. Cancer 95, 2230–2236 (2002).
Coda, B. A. et al. Comparative efficacy of patient-controlled administration of morphine, hydromorphone, or sufentanil for the treatment of oral mucositis pain following bone marrow transplantation. Pain 72, 333–346 (1997).
Yarom, N. et al. Systematic review of natural and miscellaneous agents for the management of oral mucositis in cancer patients and clinical practice guidelines-part 1: vitamins, minerals, and nutritional supplements. Support. Care Cancer 27, 3997–4010 (2019).
Payne, A. S., James, W. D. & Weiss, R. B. Dermatologic toxicity of chemotherapeutic agents. Semin. Oncol. 33, 86–97 (2006).
Susser, W. S., Whitaker-Worth, D. L. & Grant-Kels, J. M. Mucocutaneous reactions to chemotherapy. J. Am. Acad. Dermatol. 40, 367–398 (1999).
Thodtmann, R. et al. A phase II trial of pemetrexed in patients with metastatic renal cancer. Invest. New Drugs 21, 353–358 (2003).
Hanna, N. et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J. Clin. Oncol. 22, 1589–1597 (2004).
Singal, R. et al. Discrete pigmentation after chemotherapy. Pediatr. Dermatol. 8, 231–235 (1991).
Issaivanan, M. et al. Cutaneous manifestations of hydroxyurea therapy in childhood: case report and review. Pediatr. Dermatol. 21, 124–127 (2004).
Hendrix, J. D. Jr. & Greer, K. E. Cutaneous hyperpigmentation caused by systemic drugs. Int. J. Dermatol. 31, 458–466 (1992).
Bronner, A. K. & Hood, A. F. Cutaneous complications of chemotherapeutic agents. J. Am. Acad. Dermatol. 9, 645–663 (1983).
Rosen, A. C. et al. Life-threatening dermatologic adverse events in oncology. Anticancer. Drugs 25, 225–234 (2014).
Nassif, A. et al. Toxic epidermal necrolysis: effector cells are drug-specific cytotoxic T cells. J. Allergy Clin. Immunol. 114, 1209–1215 (2004).
Frey, N. et al. The epidemiology of Stevens-Johnson syndrome and toxic epidermal necrolysis in the UK. J. Invest. Dermatol. 137, 1240–1247 (2017).
Alley, E., Green, R. & Schuchter, L. Cutaneous toxicities of cancer therapy. Curr. Opin. Oncol. 14, 212–216 (2002).
Robert, C. et al. Nail toxicities induced by systemic anticancer treatments. Lancet Oncol. 16, e181–e189 (2015).
Alimonti, A. et al. Nail disorders in a woman treated with ixabepilone for metastatic breast cancer. Anticancer. Res. 25, 3531–3532 (2005).
Marks, D. H., Qureshi, A. & Friedman, A. Evaluation of prevention interventions for taxane-induced dermatologic adverse events: a systematic review. JAMA Dermatol. 154, 1465–1472 (2018).
Ishiguro, H. et al. Degree of freezing does not affect efficacy of frozen gloves for prevention of docetaxel-induced nail toxicity in breast cancer patients. Support. Care Cancer 20, 2017–2024 (2012).
Miller, K. K., Gorcey, L. & McLellan, B. N. Chemotherapy-induced hand-foot syndrome and nail changes: a review of clinical presentation, etiology, pathogenesis, and management. J. Am. Acad. Dermatol. 71, 787–794 (2014).
Farr, K. P. & Safwat, A. Palmar-plantar erythrodysesthesia associated with chemotherapy and its treatment. Case Rep. Oncol. 4, 229–235 (2011).
Drake, R. D. et al. Oral dexamethasone attenuates Doxil-induced palmar-plantar erythrodysesthesias in patients with recurrent gynecologic malignancies. Gynecol. Oncol. 94, 320–324 (2004).
Hesketh, P. J. et al. Chemotherapy-induced alopecia: psychosocial impact and therapeutic approaches. Support. Care Cancer 12, 543–549 (2004).
Choi, E. K. et al. Impact of chemotherapy-induced alopecia distress on body image, psychosocial well-being, and depression in breast cancer patients. Psychooncology 23, 1103–1110 (2014).
Prevezas, C. et al. Irreversible and severe alopecia following docetaxel or paclitaxel cytotoxic therapy for breast cancer. Br. J. Dermatol. 160, 883–885 (2009).
Paus, R. & Cotsarelis, G. The biology of hair follicles. N. Engl. J. Med. 341, 491–497 (1999).
Kluger, N. et al. Permanent scalp alopecia related to breast cancer chemotherapy by sequential fluorouracil/epirubicin/cyclophosphamide (FEC) and docetaxel: a prospective study of 20 patients. Ann. Oncol. 23, 2879–2884 (2012).
Hrin, M. L. & McMichael, A. J. Chemotherapy-induced alopecia in African American women: a literature review demonstrates a knowledge gap. J. Am. Acad. Dermatol. 86, 1434–1435 (2022).
Westgate, G. E., Ginger, R. S. & Green, M. R. The biology and genetics of curly hair. Exp. Dermatol. 26, 483–490 (2017).
Dilawari, A. et al. Does scalp cooling have the same efficacy in black patients receiving chemotherapy for breast cancer? Oncologist 26, 292–e548 (2021).
Rugo, H. S., Melin, S. A. & Voigt, J. Scalp cooling with adjuvant/neoadjuvant chemotherapy for breast cancer and the risk of scalp metastases: systematic review and meta-analysis. Breast Cancer Res. Treat. 163, 199–205 (2017).
Kruse, M. & Abraham, J. Management of chemotherapy-induced alopecia with scalp cooling. J. Oncol. Pract. 14, 149–154 (2018).
Nangia, J. et al. Effect of a scalp cooling device on alopecia in women undergoing chemotherapy for breast cancer: the SCALP randomized clinical trial. JAMA 317, 596–605 (2017).
Cairo, M. S. & Bishop, M. Tumour lysis syndrome: new therapeutic strategies and classification. Br. J. Haematol. 127, 3–11 (2004).
Howard, S. C., Pui, C.-H. & Ribeiro, R. C. Tumor Lysis Syndrome. Renal Disease in Cancer Patients. 39–64 (Elsevier, 2014).
Coiffier, B. et al. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J. Clin. Oncol. 26, 2767–2778 (2008).
Cairo, M. S. et al. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br. J. Haematol. 149, 578–586 (2010).
Halfdanarson, T. R., Hogan, W. J. & Moynihan, T. J. In Mayo Clinic Proceedings Vol. 81, pp 835–848 (Elsevier, 2006).
Krakoff, I. H. & Meyer, R. L. Prevention of hyperuricemia in leukemia and lymphoma: use of alopurinol, a xanthine oxidase inhibitor. JAMA 193, 1–6 (1965).
Ko, T. M. et al. Use of HLA-B*58:01 genotyping to prevent allopurinol induced severe cutaneous adverse reactions in Taiwan: national prospective cohort study. BMJ 351, h4848 (2015).
Cortes, J. et al. Control of plasma uric acid in adults at risk for tumor lysis syndrome: efficacy and safety of rasburicase alone and rasburicase followed by allopurinol compared with allopurinol alone–results of a multicenter phase III study. J. Clin. Oncol. 28, 4207–4213 (2010).
Bellos, I. et al. Febuxostat administration for the prevention of tumour lysis syndrome: a meta-analysis. J. Clin. Pharm. Ther. 44, 525–533 (2019).
Ganz, P. A. Cancer survivors: a look backward and forward. J. Oncol. Pract. 10, 289–293 (2014).
Gyawali, B. et al. Reporting harms more transparently in trials of cancer drugs. BMJ 363, k4383 (2018).
Gyawali, B. et al. In American Society of Clinical Oncology Educational Book 374–387 (ASCO, 2019).
Roy, U. B. et al. Learning from patients: reflection on use of patient-reported outcomes in lung cancer trials. J. Thorac. Oncol. 13, 1815–1817 (2018).
Wallis, C. J. D. et al. Association of treatment modality, functional outcomes, and baseline characteristics with treatment-related regret among men with localized prostate cancer. JAMA Oncol. 8, 50–59 (2021).
Basch, E. et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J. Clin. Oncol. 34, 557–565 (2016).
Basch, E., Charlot, M. & Dueck, A. C. Population-level evidence of survival benefits of patient-reported outcome symptom monitoring software systems in routine cancer care. Cancer Med. 9, 7797–7799 (2020).
Mohile, S. G. et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J. Clin. Oncol. 36, 2326–2347 (2018).
Kabbinavar, F. et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J. Clin. Oncol. 21, 60–65 (2003).
Scappaticci, F. A. et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J. Natl Cancer Inst. 99, 1232–1239 (2007).
Nalluri, S. R. et al. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA 300, 2277–2285 (2008).
Zangari, M. et al. Thrombotic events in patients with cancer receiving antiangiogenesis agents. J. Clin. Oncol. 27, 4865–4873 (2009).
Lyman, G. H. Impact of venous thromboembolism on survival in patients with advanced cancer: an unmet clinical need. Intern. Emerg. Med. 9, 497–499 (2014).
Acknowledgements
The authors want to thank the peer reviewers for their thoughtful and constructive comments.
Author information
Authors and Affiliations
Contributions
All the authors contributed equally to all aspects of the preparation of the article.
Corresponding authors
Ethics declarations
Competing interests
N.M.K. reports personal fees from BeyondSpring, BMS, G1 Therapeutics, Invitae, Sandoz, Seattle Genetics, Spectrum and Total Health, all outside the submitted work. M.B.L. is on the physician advisory board for Infinite Strength, Project Life, and The Right Dose and has been a consultant for AstraZeneca, Biotheranostics, Novartis, and Pfizer outside of the current subject. G.H.L. reports institutional research funding from Amgen, honoraria for lectures from Merck, Partners Healthcare, ER Squibb, Samsung, Sandoz, Seattle Genetics and TEVA, and honoraria for consulting from BeyondSpring, G1 Therapeutics and Jazz Pharm. A.D. declares no competing interests.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks P. Gascon, M. Shayne and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kuderer, N.M., Desai, A., Lustberg, M.B. et al. Mitigating acute chemotherapy-associated adverse events in patients with cancer. Nat Rev Clin Oncol 19, 681–697 (2022). https://doi.org/10.1038/s41571-022-00685-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-022-00685-3
This article is cited by
-
Synergistic anticancer effect of Pistacia lentiscus essential oils and 5-Fluorouracil co-loaded onto biodegradable nanofibers against melanoma and breast cancer
Discover Nano (2024)
-
Autophagy, a critical element in the aging male reproductive disorders and prostate cancer: a therapeutic point of view
Reproductive Biology and Endocrinology (2023)
-
Whey-based diet containing medium chain triglycerides modulates the gut microbiota and protects the intestinal mucosa from chemotherapy while maintaining therapy efficacy
Cell Death & Disease (2023)
-
Mitigating long-term and delayed adverse events associated with cancer treatment: implications for survivorship
Nature Reviews Clinical Oncology (2023)
-
A prospective, real-world, multinational study of febrile neutropenia (FN) occurrence in oncology patients receiving chemotherapy with intermediate risk of FN: a MASCC Neutropenia, Infection, and Myelosuppression Study Group initiative
Supportive Care in Cancer (2023)