Abstract
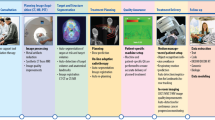
Artificial intelligence (AI) has the potential to fundamentally alter the way medicine is practised. AI platforms excel in recognizing complex patterns in medical data and provide a quantitative, rather than purely qualitative, assessment of clinical conditions. Accordingly, AI could have particularly transformative applications in radiation oncology given the multifaceted and highly technical nature of this field of medicine with a heavy reliance on digital data processing and computer software. Indeed, AI has the potential to improve the accuracy, precision, efficiency and overall quality of radiation therapy for patients with cancer. In this Perspective, we first provide a general description of AI methods, followed by a high-level overview of the radiation therapy workflow with discussion of the implications that AI is likely to have on each step of this process. Finally, we describe the challenges associated with the clinical development and implementation of AI platforms in radiation oncology and provide our perspective on how these platforms might change the roles of radiotherapy medical professionals.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Delaney, G., Jacob, S., Featherstone, C. & Barton, M. The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 104, 1129–1137 (2005).
Pan, H. Y. et al. Supply and demand for radiation oncology in the United States: updated projections for 2015 to 2025. Int. J. Radiat. Oncol. Biol. Phys. 96, 493–500 (2016).
Ferlay, J. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 136, E359–E386 (2015).
Miller, K. D. et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 66, 271–289 (2016).
Atun, R. et al. Expanding global access to radiotherapy. Lancet Oncol. 16, 1153–1186 (2015).
Grover, S. et al. A systematic review of radiotherapy capacity in low- and middle-income countries. Front. Oncol. 4, 380 (2015).
Elmore, S. N. C., Ben Prajogi, G., Rubio, J. A. P. & Zubizarreta, E. The global radiation oncology workforce in 2030: estimating physician training needs and proposing solutions to scale up capacity in low- and middle-income countries. Adv. Radiat. Oncol. 1–8 (2019).
Kresl, J. J. & Drummond, R. L. A historical perspective of the radiation oncology workforce and ongoing initiatives to impact recruitment and retention. J. Am. Coll. Radiol. 1, 641–648 (2004).
Peters, L. J. et al. Critical impact of radiotherapy protocol compliance and quality in the treatment of advanced head and neck cancer: results from TROG 02.02. J. Clin. Oncol. 28, 2996–3001 (2010).
Brade, A. M. et al. Radiation therapy quality assurance (RTQA) of concurrent chemoradiation therapy for locally advanced non-small cell lung cancer in the PROCLAIM phase 3 trial. Int. J. Radiat. Oncol. Biol. Phys. 101, 927–934 (2018).
Kalet, I. J. & Paluszynski, W. Knowledge-based computer systems for radiotherapy planning. Am. J. Clin. Oncol. 13, 344–351 (1990).
Laramore, G. E. et al. Applications of data bases and AI/expert systems in radiation therapy. Am. J. Clin. Oncol. 11, 387–393 (1988).
Sanders, G. D. & Lyons, E. A. The potential use of expert systems to enable physicians to order more cost-effective diagnostic imaging examinations. J. Digit. Imaging 4, 112–122 (1991).
Hosny, A., Parmar, C., Quackenbush, J., Schwartz, L. H. & Aerts, H. J. W. L. Artificial intelligence in radiology. Nat. Rev. Cancer. 18, 500–510 (2018).
Dreyfus, S. The numerical solution of variational problems. J. Math. Anal. Appl. 5, 30–45 (1962).
Fukushima, K. Neocognitron: a self organizing neural network model for a mechanism of pattern recognition unaffected by shift in position. Biol. Cybern. 36, 193–202 (1980).
LeCun, Y., Haffner, P., Bottou, L. & Bengio, Y. in Shape, Contour and Grouping in Computer Vision (eds Forsyth, D. A., Mundy, J. L., di Gesú, V. & Cipolla, R.) 319–345 (Springer, 1999).
Litjens, G. et al. A survey on deep learning in medical image analysis. Med. Image Anal. 42, 60–88 (2017).
Ngiam, J. et al. Multimodal deep learning. in Proceedings of the 28th international conference on machine learning (ICML-11) 689–696 (2011).
Feng, M., Valdes, G., Dixit, N. & Solberg, T. D. Machine learning in radiation oncology: opportunities, requirements, and needs. Front. Oncol. 8, 110 (2018).
Kann, B. H. et al. Pretreatment identification of head and neck cancer nodal metastasis and extranodal extension using deep learning neural networks. Sci. Rep. 8, 14036 (2018).
Savova, G. K. et al. DeepPhe: a natural language processing system for extracting cancer phenotypes from clinical records. Cancer Res. 77, e115–e118 (2017).
Hong, J. C., Niedzwiecki, D., Palta, M. & Tenenbaum, J. D. Predicting emergency visits and hospital admissions during radiation and chemoradiation: an internally validated pretreatment machine learning algorithm. JCO Clin. Cancer Inform. 2, 1–11 (2018).
Oberije, C. et al. A validated prediction model for overall survival from stage III non-small cell lung cancer: toward survival prediction for individual patients. Int. J. Radiat. Oncol. Biol. Phys. 92, 935–944 (2015).
Jochems, A. et al. Developing and validating a survival prediction model for NSCLC patients through distributed learning across 3 countries. Int. J. Radiat. Oncol. Biol. Phys. 99, 344–352 (2017).
Deist, T. M. et al. Expert knowledge and data-driven Bayesian networks to predict post-RT dyspnea and 2-year survival. Radiother. Oncol. 118, S29–S30 (2016).
Deist, T. M. et al. Machine learning algorithms for outcome prediction in (chemo)radiotherapy: an empirical comparison of classifiers. Med. Phys. 45, 3449–3459 (2018).
Gilmer, V., Timothy, D. S., Marina, H., Lyle, U. & Charles, B. S. II. Using machine learning to predict radiation pneumonitis in patients with stage I non-small cell lung cancer treated with stereotactic body radiation therapy. Phys. Med. Biol. 61, 6105 (2016).
Lou, B. et al. An image-based deep learning framework for individualising radiotherapy dose: a retrospective analysis of outcome prediction. Lancet Digital Health 1, e136–e147 (2019).
Nguyen, D. et al. A feasibility study for predicting optimal radiation therapy dose distributions of prostate cancer patients from patient anatomy using deep learning. Sci. Rep. 9, 1076 (2019).
Campbell, W. G. et al. Neural network dose models for knowledge-based planning in pancreatic SBRT. Med. Phys. 44, 6148–6158 (2017).
Häring, M., Großhans, J., Wolf, F. & Eule, S. Automated segmentation of epithelial tissue using cycle-consistent generative adversarial networks. Preprint at bioRxiv (2018).
Dinkla, A. M. et al. MR-only brain radiation therapy: dosimetric evaluation of synthetic CTs generated by a dilated convolutional neural network. Int. J. Radiat. Oncol. Biol. Phys. 102, 801–812 (2018).
Han, X. MR-based synthetic CT generation using a deep convolutional neural network method. Med. Phys. 44, 1408–1419 (2017).
Maspero, M. et al. Dose evaluation of fast synthetic-CT generation using a generative adversarial network for general pelvis MR-only radiotherapy. Phys. Med. Biol. 63, 185001 (2018).
Chandarana, H., Wang, H., Tijssen, R. H. N. & Das, I. J. Emerging role of MRI in radiation therapy. J. Magn. Reson. Imaging 48, 1468–1478 (2018).
Rai, R. et al. The integration of MRI in radiation therapy: collaboration of radiographers and radiation therapists. J. Med. Radiat. Sci. 64, 61–68 (2017).
Kerkmeijer, L. G. W. et al. The MRI-linear accelerator consortium: evidence-based clinical introduction of an innovation in radiation oncology connecting researchers, methodology, data collection, quality assurance, and technical development. Front. Oncol. 6, 215 (2016).
Hyun, C. M., Kim, H. P., Lee, S. M., Lee, S. & Seo, J. K. Deep learning for undersampled MRI reconstruction. Phys. Med. Biol. 63, 135007 (2018).
Wang, S. et al. Accelerating magnetic resonance imaging via deep learning. Proc. IEEE Int. Symp. Biomed. Imaging 514–517 (2016).
Zhu, B., Liu, J. Z., Cauley, S. F., Rosen, B. R. & Rosen, M. S. Image reconstruction by domain-transform manifold learning. Nature 555, 487–492 (2018).
Schlemper, J., Caballero, J., Hajnal, J. V., Price, A. N. & Rueckert, D. A deep cascade of convolutional neural networks for dynamic MR image reconstruction. IEEE Trans. Med. Imaging 37, 491–503 (2018).
Fallone, B. G. The rotating biplanar linac–magnetic resonance imaging system. Semin. Radiat. Oncol. 24, 200–202 (2014).
Mutic, S. & Dempsey, J. F. The ViewRay system: magnetic resonance-guided and controlled radiotherapy. Semin. Radiat. Oncol. 24, 196–199 (2014).
Raaymakers, B. W. et al. Integrating a 1.5 T MRI scanner with a 6 MV accelerator: proof of concept. Phys. Med. Biol. 54, N229–N237 (2009).
Bahrami, K., Shi, F., Rekik, I. & Shen, D. in Deep Learning and Data Labeling for Medical Applications 39–47 (Springer, 2016).
de Tournemire P. et al. An artificial agent for robust image registration. Proceedings of the Thirty-First AAAI Conference on Artificial Intelligence 4168–4175 (2017).
Wu, G., Kim, M., Wang, Q., Munsell, B. C. & Shen, D. Scalable high-performance image registration framework by unsupervised deep feature representations learning. IEEE Trans. Biomed. Eng. 63, 1505–1516 (2016).
Miao, S., et al. Dilated FCN for multi-agent 2D/3D medical image registration. Thirty-Second AAAI Conference on Artificial Intelligence (2018).
Hou, B. et al. Predicting slice-to-volume transformation in presence of arbitrary subject motion. International Conference on Medical Image Computing and Computer-Assisted Intervention 296–304 (2017).
Yang, X., Kwitt, R., Styner, M. & Niethammer, M. Fast predictive multimodal image registration. 2017 IEEE 14th International Symposium on Biomedical Imaging (ISBI) 858–862 (2017).
Miao, S., Jane Wang, Z., Zheng, Y. & Liao, R. Real-time 2D/3D registration via CNN regression. 2016 IEEE 13th International Symposium on Biomedical Imaging (ISBI)1430–1434 (2016).
Kearney, V., Haaf, S., Sudhyadhom, A., Valdes, G. & Solberg, T. D. An unsupervised convolutional neural network-based algorithm for deformable image registration. Phys. Med. Biol. 63, 185017 (2018).
Ma, K. et al. Multimodal image registration with deep context reinforcement learning. in Lecture Notes in Computer Science 240–248 (2017).
Suk, H.-I., Lee, S.-W. & Shen, D. Hierarchical feature representation and multimodal fusion with deep learning for AD/MCI diagnosis. Neuroimage 101, 569–582 (2014).
Van de Steene, J. et al. Definition of gross tumor volume in lung cancer: inter-observer variability. Radiother. Oncol. 62, 37–49 (2002).
Cui, Y. et al. Contouring variations and the role of atlas in non-small cell lung cancer radiation therapy: analysis of a multi-institutional preclinical trial planning study. Pract. Radiat. Oncol. 5, e67–e75 (2015).
Wuthrick, E. J. et al. Institutional clinical trial accrual volume and survival of patients with head and neck cancer. J. Clin. Oncol. 33, 156–164 (2015).
Ohri, N. et al. Radiotherapy protocol deviations and clinical outcomes: a meta-analysis of Cooperative Group clinical trials. J. Clin. Oncol. 30, 181–181 (2012).
Delpon, G. et al. Comparison of automated atlas-based segmentation software for postoperative prostate cancer radiotherapy. Front. Oncol. 6, 178 (2016).
Kim, Y. et al. Impact of contouring accuracy on expected tumor control probability for head and neck cancer: semiautomated segmentation versus manual contouring. Int. J. Radiat. Oncol. Biol. Phys. 96, E545 (2016).
Men, K. et al. Deep deconvolutional neural network for target segmentation of nasopharyngeal cancer in planning computed tomography images. Front. Oncol. 7, 315 (2017).
Mak, R. H. et al. Use of crowd innovation to develop an artificial intelligence-based solution for radiation therapy targeting. JAMA Oncol. 5, 654 (2019).
Cardenas, C. E. et al. Deep learning algorithm for auto-delineation of high-risk oropharyngeal clinical target volumes with built-in dice similarity coefficient parameter optimization function. Int. J. Radiat. Oncol. Biol. Phys. 101, 468–478 (2018).
Ibragimov, B. & Xing, L. Segmentation of organs-at-risks in head and neck CT images using convolutional neural networks. Med. Phys. 44, 547–557 (2017).
Lustberg, T. et al. Clinical evaluation of atlas and deep learning based automatic contouring for lung cancer. Radiother. Oncol. 126, 312–317 (2018).
Jackson, P. et al. Deep learning renal segmentation for fully automated radiation dose estimation in unsealed source therapy. Front. Oncol. 8, 215 (2018).
Peijun, H., Fa, W., Jialin, P., Ping, L. & Dexing, K. Automatic 3D liver segmentation based on deep learning and globally optimized surface evolution. Phys. Med. Biol. 61, 8676 (2016).
Ibragimov, B., Toesca, D., Chang, D., Koong, A. & Xing, L. Combining deep learning with anatomical analysis for segmentation of the portal vein for liver SBRT planning. Phys. Med. Biol. 62, 8943–8958 (2017).
Morris, E. D. et al. Cardiac substructure segmentation with deep learning for improved cardiac sparing. Med. Phys. 47, 576–586 (2020).
Nikolov, S. et al. Deep learning to achieve clinically applicable segmentation of head and neck anatomy for radiotherapy. Preprint at arXiv (2018).
Zhang, J., Ates, O. & Li, A. Implementation of a machine learning-based automatic contour quality assurance tool for online adaptive radiation therapy of prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 96, E668 (2016).
Berry, S. L., Boczkowski, A., Ma, R., Mechalakos, J. & Hunt, M. Interobserver variability in radiation therapy plan output: results of a single-institution study. Pract. Radiat. Oncol. 6, 442–449 (2016).
Appenzoller, L. M., Michalski, J. M., Thorstad, W. L., Mutic, S. & Moore, K. L. Predicting dose-volume histograms for organs-at-risk in IMRT planning. Med. Phys. 39, 7446–7461 (2012).
Babier, A., Boutilier, J. J., McNiven, A. L. & Chan, T. C. Y. Knowledge-based automated planning for oropharyngeal cancer. Med. Phys. 45, 2875–2883 (2018).
Boutilier, J. J., Lee, T., Craig, T., Sharpe, M. B. & Chan, T. C. Y. Models for predicting objective function weights in prostate cancer IMRT. Med. Phys. 42, 1586–1595 (2015).
Voet, P. W. J. et al. Toward fully automated multicriterial plan generation: a prospective clinical study. Int. J. Radiat. Oncol. Biol. Phys. 85, 866–872 (2013).
Hussein, M., Heijmen, B. J. M., Verellen, D. & Nisbet, A. Automation in intensity modulated radiotherapy treatment planning — a review of recent innovations. Br. J. Radiol. 91, 20180270 (2018).
Xing, Y., Nguyen, D., Lu, W., Yang, M. & Jiang, S. Technical note: a feasibility study on deep learning-based radiotherapy dose calculation. Med. Phys. 47, 753–758 (2019).
Mnih, V. et al. Human-level control through deep reinforcement learning. Nature 518, 529–533 (2015).
Chen, J. X. The evolution of computing: AlphaGo. Comput. Sci. Eng. 18, 4–7 (2016).
Shen, C. et al. Intelligent inverse treatment planning via deep reinforcement learning, a proof-of-principle study in high dose-rate brachytherapy for cervical cancer. Phys. Med. Biol. 64, 115013 (2019).
Tseng, H.-H. et al. Deep reinforcement learning for automated radiation adaptation in lung cancer. Med. Phys. 44, 6690–6705 (2017).
Valdes, G. et al. A mathematical framework for virtual IMRT QA using machine learning. Med. Phys. 43, 4323 (2016).
Valdes, G. et al. IMRT QA using machine learning: a multi-institutional validation. J. Appl. Clin. Med. Phys. 18, 279–284 (2017).
Carlson, J. N. K. et al. A machine learning approach to the accurate prediction of multi-leaf collimator positional errors. Phys. Med. Biol. 61, 2514–2531 (2016).
Li, Q. & Chan, M. F. Predictive time-series modeling using artificial neural networks for Linac beam symmetry: an empirical study. Ann. N. Y. Acad. Sci. 1387, 84–94 (2017).
Valdes, G. et al. Use of TrueBeam developer mode for imaging QA. J. Appl. Clin. Med. Phys. 16, 322–333 (2015).
Paul, C. et al. Cancer patients’ concerns regarding access to cancer care: perceived impact of waiting times along the diagnosis and treatment journey. Eur. J. Cancer Care 21, 321–329 (2012).
Joseph, A., Hijal, T., Kildea, J., Hendren, L. & Herrera, D. in Machine Learning and Applications (ICMLA), 2017 16th IEEE International Conference 1024–1029 (McGill Univ. Health Centre, 2018).
Kida, S. et al. Cone beam computed tomography image quality improvement using a deep convolutional neural network. Cureus 10, e2548 (2018).
Langen, K. M. & Jones, D. T. Organ motion and its management. Int. J. Radiat. Oncol. Biol. Phys. 50, 265–278 (2001).
Isaksson, M., Jalden, J. & Murphy, M. J. On using an adaptive neural network to predict lung tumor motion during respiration for radiotherapy applications. Med. Phys. 32, 3801–3809 (2005).
Kakar, M., Nyström, H., Aarup, L. R., Nøttrup, T. J. & Olsen, D. R. Respiratory motion prediction by using the adaptive neuro fuzzy inference system (ANFIS). Phys. Med. Biol. 50, 4721–4728 (2005).
Murphy, M. J. & Pokhrel, D. Optimization of an adaptive neural network to predict breathing. Med. Phys. 36, 40–47 (2009).
Guidi, G. et al. A support vector machine tool for adaptive tomotherapy treatments: prediction of head and neck patients criticalities. Phys. Med. 31, 442–451 (2015).
Guidi, G. et al. A machine learning tool for re-planning and adaptive RT: a multicenter cohort investigation. Phys. Med. 32, 1659–1666 (2016).
Varfalvy, N., Piron, O., Cyr, M. F., Dagnault, A. & Archambault, L. Classification of changes occurring in lung patient during radiotherapy using relative γ analysis and hidden Markov models. Med. Phys. 44, 5043–5050 (2017).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Xu, Y. et al. Deep learning predicts lung cancer treatment response from serial medical imaging. Clin. Cancer Res. 25, 3266–3275 (2019).
Hosny, A. et al. Deep learning for lung cancer prognostication: a retrospective multi-cohort radiomics study. PLoS Med. 15, e1002711 (2018).
Cha, K. H. et al. Bladder cancer treatment response assessment in CT using radiomics with deep-learning. Sci. Rep. 7, 8738 (2017).
Chen, X. et al. Assessment of treatment response during chemoradiation therapy for pancreatic cancer based on quantitative radiomic analysis of daily CTs: an exploratory study. PLoS ONE 12, e0178961 (2017).
Horvat, N. et al. MR imaging of rectal cancer: radiomics analysis to assess treatment response after neoadjuvant therapy. Radiology 287, 833–843 (2018).
Mattonen, S. A. et al. Detection of local cancer recurrence after stereotactic ablative radiation therapy (SABR) for lung cancer: physician performance versus radiomic assessment. Int. J. Radiat. Oncol. Biol. Phys. 96, S48 (2016).
Lambin, P. et al. Predicting outcomes in radiation oncology — multifactorial decision support systems. Nat. Rev. Clin. Oncol. 10, 27–40 (2013).
Lee, S. et al. Machine learning on a genome-wide association study to predict late genitourinary toxicity after prostate radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 101, 128–135 (2018).
Dean, J. et al. Incorporating spatial dose metrics in machine learning-based normal tissue complication probability (NTCP) models of severe acute dysphagia resulting from head and neck radiotherapy. Clin. Transl. Radiat. Oncol. 8, 27–39 (2018).
Gabryś, H. S., Buettner, F., Sterzing, F., Hauswald, H. & Bangert, M. Design and selection of machine learning methods using radiomics and dosiomics for normal tissue complication probability modeling of xerostomia. Front. Oncol. 8, 35 (2018).
Dean, J. A. et al. Normal tissue complication probability (NTCP) modelling using spatial dose metrics and machine learning methods for severe acute oral mucositis resulting from head and neck radiotherapy. Radiother. Oncol. 120, 21–27 (2016).
Cunliffe, A. et al. Lung texture in serial thoracic computed tomography scans: correlation of radiomics-based features with radiation therapy dose and radiation pneumonitis development. Int. J. Radiat. Oncol. Biol. Phys. 91, 1048–1056 (2015).
Chen, S., Zhou, S., Yin, F.-F., Marks, L. B. & Das, S. K. Investigation of the support vector machine algorithm to predict lung radiation-induced pneumonitis. Med. Phys. 34, 3808–3814 (2007).
Moran, A., Daly, M. E., Yip, S. S. F. & Yamamoto, T. Radiomics-based assessment of radiation-induced lung injury after stereotactic body radiotherapy. Clin. Lung Cancer 18, e425–e431 (2017).
Luna, J. M. et al. Novel use of machine learning for predicting radiation esophagitis in locally advanced stage II–III non-small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 99, E476–E477 (2017).
Zhen, X. et al. Deep convolutional neural networks with transfer learning for rectum toxicity prediction in combined brachytherapy and external beam radiation therapy for cervical cancer. Int. J. Radiat. Oncol. Biol. Phys. 99, S168 (2017).
Liu, Z. et al. Radiomics analysis allows for precise prediction of epilepsy in patients with low-grade gliomas. NeuroImage Clin. 19, 271–278 (2018).
Wright, J. L. et al. Standardizing normal tissue contouring for radiation therapy treatment planning: an ASTRO consensus paper. Pract. Radiat. Oncol. 9, 65–72 (2019).
Covington, E. L. et al. Improving treatment plan evaluation with automation. J. Appl. Clin. Med. Phys. 17, 16–31 (2016).
Evans, S. B. et al. Standardizing dose prescriptions: an ASTRO white paper. Pract. Radiat. Oncol. 6, e369–e381 (2016).
Clark, K. et al. The cancer imaging archive (TCIA): maintaining and operating a public information repository. J. Digit. Imaging 26, 1045–1057 (2013).
Mayo, C. S. et al. American Association of Physicists in Medicine task group 263: standardizing nomenclatures in radiation oncology. Int. J. Radiat. Oncol. Biol. Phys. 100, 1057–1066 (2018).
Hayman, J. A. et al. Minimum data elements for radiation oncology: an ASTRO consensus paper. Pract. Radiat. Oncol. 9, 395–401 (2019).
Kim, D. W., Jang, H. Y., Kim, K. W., Shin, Y. & Park, S. H. Design characteristics of studies reporting the performance of artificial intelligence algorithms for diagnostic analysis of medical images: results from recently published papers. Korean J. Radiol. 20, 405 (2019).
Allen, B. Jr et al. A road map for translational research on artificial intelligence in medical imaging: from the 2018 National Institutes of Health/RSNA/ACR/the Academy workshop. J. Am. Coll. Radiol. 16, 1179–1189 (2019).
Gilpin, L. H. et al. in 2018 IEEE 5th International Conference on Data Science and Advanced Analytics (DSAA) 80–89 (2018).
Goodman, B. & Flaxman, S. European Union regulations on algorithmic decision-making and a ‘right to explanation’. AI Mag. 38, 50–57 (2017).
Kaminski, M. E. The right to explanation, explained. Berkeley Technol. Law J. 34, 1 (2019).
Harned, Z., Lungren, M. P. & Rajpurkar, P. Machine Vision, Medical AI, and Malpractice (JOLT, 2019).
Buolamwini, J. & Gebru, T. in Proceedings of the 1st Conference on Fairness, Accountability and Transparency Vol. 81 (eds Friedler, S. A. & Wilson, C.) 77–91 (PMLR, 2018).
Angwin, J., Larson, J., Mattu, S. & Kirchner, L. Machine bias. ProPublica 23, 2016 (2016).
Obermeyer, Z., Powers, B., Vogeli, C. & Mullainathan, S. Dissecting racial bias in an algorithm used to manage the health of populations. Science 366, 447–453 (2019).
Char, D. S., Shah, N. H. & Magnus, D. Implementing machine learning in health care — addressing ethical challenges. N. Engl. J. Med. 378, 981–983 (2018).
IMDRF. “Software as a Medical Device”: Possible Framework for Risk Categorization and Corresponding Considerations. http://www.imdrf.org/docs/imdrf/final/technical/imdrf-tech-140918-samd-framework-risk-categorization-141013.pdf (2014).
FDA. Proposed Regulatory Framework for Modifications to Artificial Intelligence/Machine Learning (AI/ML)-Based Software as a Medical Device (SaMD) — Discussion Paper and Request for Feedback. https://www.fda.gov/files/medical%20devices/published/US-FDA-Artificial-Intelligence-and-Machine-Learning-Discussion-Paper.pdf (2019).
FDA. Draft Guidance for Industry and Food and Drug Administration Staff. https://www.fda.gov/media/109618/download (2019).
Hwang, T. J., Kesselheim, A. S. & Vokinger, K. N. Lifecycle regulation of artificial intelligence- and machine learning-based software devices in medicine. JAMA 322, 2285–2286 (2019).
Bitterman, D. S. et al. Master protocol trial design for efficient and rational evaluation of novel therapeutic oncology devices. J. Natl Cancer Inst. 112, 229–237 (2020).
Schuller, B. W., Hendrickson, K. R. G. & Rong, Y. Medical physicists should meet with patients as part of the initial consult. J. Appl. Clin. Med. Phys. 19, 6–9 (2018).
Brown, D. W. et al. A program to train medical physicists for direct patient care responsibilities. J. Appl. Clin. Med. Phys. 19, 332–335 (2018).
Atwood, T. F. et al. Establishing a new clinical role for medical physicists: a prospective phase II trial. Int. J. Radiat. Oncol. Biol. Phys. 102, 635–641 (2018).
Nelms, B. E. et al. Variation in external beam treatment plan quality: an inter-institutional study of planners and planning systems. Practical Radiat. Oncol. 2, 296–305 (2012).
Adams, R. D. The future of medical dosimetry. Med. Dosim. 40, 159–165 (2015).
American Association of Medical Dosimetrists. 2017 Salary Survey of Currently Active Medical Dosimetrists. (American Association of Medical Dosimetrists, 2018).
Center for Medicare & Medicaid Services. Radiation Oncology Model. https://innovation.cms.gov/initiatives/radiation-oncology-model (2019).
Hosny, A. & Hugo, J. W. Artificial intelligence for global health. Science 366, 955–956 (2019).
Barton, M. B., Frommer, M. & Shafiq, J. Role of radiotherapy in cancer control in low-income and middle-income countries. Lancet Oncol. 7, 584–595 (2006).
Zubizarreta, E. H., Fidarova, E., Healy, B. & Rosenblatt, E. Need for radiotherapy in low and middle income countries — the silent crisis continues. Clin. Oncol. 27, 107–114 (2015).
Aerts, H. J. W. L. et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat. Commun. 5, 4006 (2014).
Wang, J. et al. A predictive model of radiation-related fibrosis based on radiomic features of magnetic resonance imaging. Int. J. Radiat. Oncol. Biol. Phys. 105, E599 (2019).
Lin, H. et al. A super-learner model for tumor motion prediction and management in radiation therapy: development and feasibility evaluation. Sci. Rep. 9, 14868 (2019).
Mahdavi, S. R. et al. Use of artificial neural network for pretreatment verification of intensity modulation radiation therapy fields. Br. J. Radiol. 92, 20190355 (2019).
Zhen, X. et al. Deep convolutional neural network with transfer learning for rectum toxicity prediction in cervical cancer radiotherapy: a feasibility study. Phys. Med. Biol. 62, 8246–8263 (2017).
Tomori, S. et al. A deep learning-based prediction model for gamma evaluation in patient-specific quality assurance. Med. Phys. 45, 4055–4065 (2018).
Kearney, V., Chan, J. W., Haaf, S., Descovich, M. & Solberg, T. D. DoseNet: a volumetric dose prediction algorithm using 3D fully-convolutional neural networks. Phys. Med. Biol. 63, 235022 (2018).
Chen, X., Men, K., Li, Y., Yi, J. & Dai, J. A feasibility study on an automated method to generate patient-specific dose distributions for radiotherapy using deep learning. Med. Phys. 46, 56–64 (2019).
Cui, S., Luo, Y., Tseng, H., Ten Haken, R. K. & El Naqa, I. Combining handcrafted features with latent variables in machine learning for prediction of radiation-induced lung damage. Med. Phys. 46, 2497–2511 (2019).
Wei, L. et al. Variational autoencoder graph-based radiomics outcome modeling of intrahepatic progression risk and overall survival for HCC post-SBRT patients. Int. J. Radiat. Oncol. Biol. Phys. 105, S83–S84 (2019).
Mahmood, R., Babier, A., McNiven, A., Diamant, A. & Chan, T. C. Y. Automated treatment planning in radiation therapy using generative adversarial networks. Preprint at arXiv (2018).
Acknowledgements
The authors acknowledge financial support from the US NIH (grants U24CA194354, U01CA190234, U01CA209414 and R35CA220523).
Author information
Authors and Affiliations
Contributions
E.H., A.H. and R.H.M. researched data for the article; E.H., A.H., H.J.W.L.A. and R.H.M. contributed substantially to the discussion of content; and E.H., A.H., C.G., D.S.B., H.J.W.L.A. and R.H.M. wrote the article. All authors reviewed/edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing Interests
A.H. is a shareholder of and receives consulting fees from Altis Labs. The other authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Clinical Oncology thanks I. El Naqa, G. Valdes and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huynh, E., Hosny, A., Guthier, C. et al. Artificial intelligence in radiation oncology. Nat Rev Clin Oncol 17, 771–781 (2020). https://doi.org/10.1038/s41571-020-0417-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-020-0417-8
This article is cited by
-
Edge roughness quantifies impact of physician variation on training and performance of deep learning auto-segmentation models for the esophagus
Scientific Reports (2024)
-
Artificial intelligence in liver imaging: methods and applications
Hepatology International (2024)
-
Artificial intelligence and multimodal data fusion for smart healthcare: topic modeling and bibliometrics
Artificial Intelligence Review (2024)
-
Artificial intelligence in the treatment of cancer: Changing patterns, constraints, and prospects
Health and Technology (2024)
-
A swallowable X-ray dosimeter for the real-time monitoring of radiotherapy
Nature Biomedical Engineering (2023)