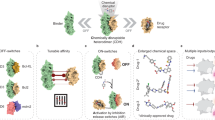
Abstract
Molecular chameleons possess a flexibility that allows them to dynamically shield or expose polar functionalities in response to the properties of the environment. Although the concept of molecular chameleons was introduced already in 1970, interest in them has grown considerably since the 2010s, when drug discovery has focused to an increased extent on new chemical modalities. Such modalities include cyclic peptides, macrocycles and proteolysis-targeting chimeras, all of which reside in a chemical space far from that of traditional small-molecule drugs. Both cell permeability and aqueous solubility are required for the oral absorption of drugs. Engineering these properties, and potent target binding, into the larger new modalities is a more daunting task than for traditional small-molecule drugs. The ability of chameleons to adapt to different environments may be essential for success. In this Review, we provide both general and theoretical insights into the realm of molecular chameleons. We discuss why chameleons have come into fashion and provide a do-it-yourself toolbox for their design; we then provide a glimpse of how advanced in silico methods can support molecular chameleon design.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hoy, K. L. New values of the solubility parameters from vapor pressure data. J. Paint. Technol. 42, 76–118 (1970).
Carrupt, P.-A. et al. Morphine 6-glucuronide and morphine 3-glucuronide as molecular chameleons with unexpected lipophilicity. J. Med. Chem. 34, 1272–1275 (1991).
Whitty, A. et al. Quantifying the chameleonic properties of macrocycles and other high-molecular-weight drugs. Drug Discov. Today 21, 712–717 (2016). An educational description of what a molecular chameleon is, the benefits chameleonicity provides in drug discovery and why chameleonicity becomes increasingly important as the MW of drugs increases.
Rossi Sebastiano, M. et al. Impact of dynamically exposed polarity on permeability and solubility of chameleonic drugs beyond the rule of 5. J. Med. Chem. 61, 4189–4202 (2018). Analysis of crystal structures for a large set of drugs reveals the drugs’ calculated minimum SA 3D PSA values to be useful descriptors of cell permeability, and highlights dynamic formation of intramolecular hydrogen bonds and shielding of polar groups by aromatic groups as particularly effective in conferring chameleonicity.
Over, B. et al. Structural and conformational determinants of macrocycle cell permeability. Nat. Chem. Biol. 12, 1065–1074 (2016).
Furukawa, A. et al. Drug-like properties in macrocycles above MW 1000: backbone rigidity versus side-chain lipophilicity. Angew. Chem. Int. Ed. 59, 21571–21577 (2020).
Danelius, E. et al. Solution conformations explain the chameleonic behavior of macrocyclic drugs. Chem. Eur. J. 26, 5231–5244 (2020). Solution-phase NMR spectroscopy reveals the chameleonic behaviour of three macrocyclic drugs residing in the chemical space far beyond Ro5 and confirms that such behaviour is not limited to the cyclic peptide cyclosporin A.
Over, B. et al. Impact of stereospecific intramolecular hydrogen bonding on cell permeability and physicochemical properties. J. Med. Chem. 57, 2746–2754 (2014).
Caron, G. & Ermondi, G. Updating molecular properties during early drug discovery. Drug Discov. Today 22, 835–840 (2017).
Shultz, M. D. Two decades under the influence of the rule of five and the changing properties of approved oral drugs. J. Med. Chem. 62, 1701–1714 (2019).
Villar, E. A. et al. How proteins bind macrocycles. Nat. Chem. Biol. 10, 723–732 (2014).
Arkin, M. R., Tang, Y. & Wells, J. A. Small-molecule inhibitors of protein-protein interactions: progressing toward the reality. Chem. Biol. 21, 1102–1114 (2014).
Valeur, E. et al. New modalities for challenging targets in drug discovery. Angew. Chem. Int. Ed. 56, 10294–10323 (2017).
Schreiber, S. L. The rise of molecular glues. Cell 184, 3–9 (2021).
Békés, M., Langley, D. R. & Crews, C. M. PROTAC targeted protein degraders: the past is prologue. Nat. Rev. Drug Discov. 21, 181–200 (2022).
Lipinski, C. A., Lombardo, F., Dominy, B. W. & Feeney, P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 23, 3–25 (1997).
Veber, D. F. et al. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 45, 2615–2623 (2002).
Hopkins, A. L. & Groom, C. R. The druggable genome. Nat. Rev. Drug Discov. 1, 727–730 (2002).
Driggers, E. M., Hale, S. P., Lee, J. & Terrett, N. F. The exploration of macrocycles for drug discovery — an underexploited structural class. Nat. Rev. Drug Discov. 7, 608–624 (2008).
Doak, B. C., Zheng, J., Dobritzsch, D. & Kihlberg, J. How beyond rule of 5 drugs and clinical candidates bind to their targets. J. Med. Chem. 59, 2312–2327 (2016).
Doak, B. C., Over, B., Giordanetto, F. & Kihlberg, J. Oral druggable space beyond the rule of 5: insights from drugs and clinical candidates. Chem. Biol. 21, 1115–1142 (2014).
DeGoey, D. A., Chen, H.-J., Cox, P. B. & Wendt, M. D. Beyond the rule of 5: lessons learned from AbbVie’s drugs and compound collection. J. Med. Chem. 61, 2636–2651 (2018).
García Jiménez, D., Poongavanam, V. & Kihlberg, J. Macrocycles in drug discovery–learning from the past for the future. J. Med. Chem. 66, 5377–5396 (2023).
Guimarães, C. R. W., Mathiowetz, A. M., Shalaeva, M., Goetz, G. & Liras, S. Use of 3D properties to characterize beyond rule-of-5 property space for passive permeation. J. Chem. Inf. Model. 52, 882–890 (2012).
Caron, G., Kihlberg, J. & Ermondi, G. Intramolecular hydrogen bonding: an opportunity for improved design in medicinal chemistry. Med. Res. Rev. 39, 1707–1729 (2019).
Poongavanam, V. et al. Predicting the permeability of macrocycles from conformational sampling – limitations of molecular flexibility. J. Pharm. Sci. 110, 301–313 (2021).
Hoang, H. N., Hill, T. A. & Fairlie, D. P. Connecting hydrophobic surfaces in cyclic peptides increases membrane permeability. Angew. Chem. Int. Ed. 60, 8385–8390 (2021).
Linker, S. M. et al. Lessons for oral bioavailability: how conformationally flexible cyclic peptides enter and cross lipid membranes. J. Med. Chem. 66, 2773–2788 (2023). MD simulations provide mechanistic understanding of how cyclic peptides can cross cell membranes by side chain-mediated anchoring followed by formation of a closed, nonpolar conformation that subsequently crosses the membrane.
Sugita, M. et al. Large-scale membrane permeability prediction of cyclic peptides crossing a lipid bilayer based on enhanced sampling molecular dynamics simulations. J. Chem. Inf. Model. 61, 3681–3695 (2021).
Rezai, T. et al. Conformational flexibility, internal hydrogen bonding, and passive membrane permeability: successful in silico prediction of the relative permeabilities of cyclic peptides. J. Am. Chem. Soc. 128, 14073–14080 (2006).
Witek, J. et al. Rationalization of the membrane permeability differences in a series of analogue cyclic decapeptides. J. Chem. Inf. Model. 59, 294–308 (2019).
Caron, G., Vallaro, M. & Ermondi, G. High throughput methods to measure the propensity of compounds to form intramolecular hydrogen bonding. MedChemComm 8, 1143–1151 (2017).
Ermondi, G., Vallaro, M., Goetz, G., Shalaeva, M. & Caron, G. Updating the portfolio of physicochemical descriptors related to permeability in the beyond the rule of 5 chemical space. Eur. J. Pharm. Sci. 146, 105274 (2020).
Kuhn, B., Mohr, P. & Stahl, M. Intramolecular hydrogen bonding in medicinal chemistry. J. Med. Chem. 53, 2601–2611 (2010).
Alex, A., Millan, D. S., Perez, M., Wakenhut, F. & Whitlock, G. A. Intramolecular hydrogen bonding to improve membrane permeability and absorption in beyond rule of five chemical space. MedChemComm 2, 669–674 (2011).
Wieske, L. H. E., Peintner, S. & Erdélyi, M. Ensemble determination by NMR data deconvolution. Nat. Rev. Chem. 7, 511–524 (2023).
Yang, Z. et al. Matched molecular pair analysis in drug discovery: methods and recent applications. J. Med. Chem. 66, 4361–4377 (2023).
Shalaeva, M. et al. Integrating intramolecular hydrogen bonding (IMHB) considerations in drug discovery using ΔlogP as a tool. J. Med. Chem. 56, 4870–4879 (2013).
Goetz, G. H. et al. High throughput method for the indirect detection of intramolecular hydrogen bonding. J. Med. Chem. 57, 2920–2929 (2014).
Garcia Jimenez, D. et al. Chamelogk: a chromatographic chameleonicity quantifier to design orally bioavailable beyond-rule-of‐5 drugs. J. Med. Chem. 66, 10681–10693 (2023).
Davis, A. M., Teague, S. J. & Kleywegt, G. J. Application and limitations of X-ray crystallographic data in structure-based ligand and drug design. Angew. Chem. Int. Ed. 42, 2718–2736 (2003).
Atilaw, Y. et al. Solution conformations shed light on PROTAC cell permeability. ACS Med. Chem. Lett. 12, 107–114 (2021).
Gramse, G., Dols-Perez, A., Edwards, M. A., Fumagalli, L. & Gomila, G. Nanoscale measurement of the dielectric constant of supported lipid bilayers in aqueous solutions with electrostatic force microscopy. Biophys. J. 104, 1257–1262 (2013).
Sheikh, A. Y. et al. Implications of the conformationally flexible, macrocyclic structure of the first-generation, direct-acting anti-viral paritaprevir on Its solid form complexity and chameleonic behavior. J. Am. Chem. Soc. 143, 17479–17491 (2021).
Yang, M. G. et al. Use of a conformational-switching mechanism to modulate exposed polarity: discovery of CCR2 antagonist BMS-741672. ACS Med. Chem. Lett. 10, 300–305 (2019).
Mackman, R. L. et al. Discovery of a potent and orally bioavailable cyclophilin inhibitor derived from the sanglifehrin macrocycle. J. Med. Chem. 61, 9473–9499 (2018). An example of how the insertion of an environment-dependent IMHB in lead optimization can improve both cell permeability and solubility, and thus contribute to the identification of a drug candidate suitable for progression into clinical studies.
Corbett, K. M., Ford, L., Warren, D. B., Pouton, C. W. & Chalmers, D. K. Cyclosporin structure and permeability: from A to Z and beyond. J. Med. Chem. 64, 13131–13151 (2021).
Wieske, L. H. E., Atilaw, Y., Poongavanam, V., Erdélyi, M. & Kihlberg, J. Going viral: an investigation into the chameleonic behaviour of antiviral compounds. Chem. Eur. J. 29, e202202798 (2023).
Poongavanam, V. et al. Linker-dependent folding rationalizes PROTAC cell permeability. J. Med. Chem. 65, 13029–13040 (2022).
Bockus, A. T. et al. Going out on a limb: delineating the effects of β-branching, N-methylation, and side chain size on the passive permeability, solubility, and flexibility of sanguinamide A analogues. J. Med. Chem. 58, 7409–7418 (2015).
Palian, M. M., Boguslavsky, V. I., O’Brien, D. F. & Polt, R. Glycopeptide-membrane interactions: glycosyl enkephalin analogues adopt turn conformations by NMR and CD in amphipathic media. J. Am. Chem. Soc. 125, 5823–5831 (2003).
Moxam, J. et al. Passive membrane permeability of sizable acyclic β‐hairpin peptides. ACS Med. Chem. Lett. 14, 278–284 (2023).
Ermondi, G. et al. Managing experimental 3D structures in the beyond-rule-of-5 chemical space: the case of rifampicin. Chem. Eur. J. 27, 10394–10404 (2021).
Choi, J., Chen, J., Schreiber, S. L. & Clardy, J. Structure of the FKBP12-rapamycin complex interacting with the binding domain of human FRAP. Science 273, 239–242 (1996).
Liang, J., Choi, J. & Clardy, J. Refined structure of the FKBP12–rapamycin–FRB ternary complex at 2.2 Å resolution. Acta Cryst. D55, 736–744 (1999).
März, A. M., Fabian, A.-K., Kozany, C., Bracher, A. & Hausch, F. Large FK506-binding proteins shape the pharmacology of rapamycin. Mol. Cell. Biol. 33, 1357–1367 (2013).
Ceymann, A. et al. Solution structure of the Legionella pneumophila Mip-rapamycin complex. BMC Struct. Biol. 8, 17 (2008).
Surleraux, D. L. N. G. et al. Discovery and selection of TMC114, a next generation HIV-1 protease inhibitor. J. Med. Chem. 48, 1813–1822 (2005).
Kovalevsky, A. Y. et al. Effectiveness of nonpeptide clinical inhibitor TMC-114 on HIV-1 protease with highly drug resistant mutations D30N, I50V, and L90M. J. Med. Chem. 49, 1379–1387 (2006).
Kovalevsky, A. Y. et al. Ultra-high resolution crystal structure of HIV-1 protease mutant reveals two binding sites for clinical inhibitor TMC114. J. Mol. Biol. 363, 161–173 (2006).
Zhang, Y. et al. Structures of darunavir-resistant HIV‐1 protease mutant reveal atypical binding of darunavir to wide open flaps. ACS Chem. Biol. 9, 1351–1358 (2014).
Chierrito, T. P. C. et al. Chameleon-like behavior of indolylpiperidines in complex with cholinesterases targets: potent butyrylcholinesterase inhibitors. Eur. J. Med. Chem. 145, 431–444 (2018).
Kuhnert, M. et al. Tracing binding modes in hit-to-lead optimization: chameleon-like poses of aspartic protease inhibitors. Angew. Chem. Int. Ed. 54, 2849–2853 (2015).
Limbach, M. N. et al. Atomic view of aqueous cyclosporine A: unpacking a decades-old mystery. J. Am. Chem. Soc. 144, 12602–12607 (2022).
Rezai, T., Yu, B., Millhauser, G. L., Jacobson, M. P. & Lokey, R. S. Testing the conformational hypothesis of passive membrane permeability using synthetic cyclic peptide diastereomers. J. Am. Chem. Soc. 128, 2510–2511 (2006). A pioneering study of how N-methylation of amide bonds and variation of stereochemistry in cyclic peptides can influence passive cell permeability by up to two orders of magnitude.
Taechalertpaisarn, J., Ono, S., Okada, O., Johnstone, T. C. & Lokey, R. S. A new amino acid for improving permeability and solubility in macrocyclic peptides through side chain-to-backbone hydrogen bonding. J. Med. Chem. 65, 5072–5084 (2022).
Rafi, S. B., Hearn, B. R., Vedantham, P., Jacobson, M. P. & Renslo, A. R. Predicting and improving the membrane permeability of peptidic small molecules. J. Med. Chem. 55, 3163–3169 (2011).
Birkinshaw, R. W. et al. Structures of BCL-2 in complex with venetoclax reveal the molecular basis of resistance mutations. Nat. Commun. 10, 2385 (2019).
Perdih, F., Žigart, N. & Časar, Z. Crystal structure and solid-state conformational analysis of active pharmaceutical ingredient venetoclax. Crystals 11, 261 (2021).
Tyagi, M. et al. Toward the design of molecular chameleons: flexible shielding of an amide bond enhances macrocycle cell permeability. Org. Lett. 20, 5737–5742 (2018).
Sethio, D. et al. Simulation reveals the chameleonic behavior of macrocycles. J. Chem. Inf. Model. 63, 138–146 (2023).
Caron, G., Digiesi, V., Solaro, S. & Ermondi, G. Flexibility in early drug discovery: focus on the beyond-Rule-of-5 chemical space. Drug Discov. Today 25, 621–627 (2020).
Poongavanam, V. et al. Conformational sampling of macrocyclic drugs in different environments: can we find the relevant conformations? ACS Omega 3, 11742–11757 (2018).
Hawkins, P. C. D. Conformation generation: the state of the art. J. Chem. Inf. Model. 57, 1747–1756 (2017).
Appavoo, S. D., Huh, S., Diaz, D. B. & Yudin, A. K. Conformational control of macrocycles by remote structural modification. Chem. Rev. 119, 9724–9752 (2019).
Bhardwaj, G. et al. Accurate de novo design of membrane-traversing macrocycles. Cell 185, 3520–3532 (2022). The first report of the comprehensive computational design and experimental characterization of structurally diverse cell-permeable cyclic peptides, some of which behave as molecular chameleons.
Wang, C. K., Swedberg, J. E., Harvey, P. J., Kaas, Q. & Craik, D. J. Conformational flexibility is a determinant of permeability for cyclosporin. J. Phys. Chem. B 122, 2261–2276 (2018).
Linker, S. M. et al. Polar/apolar interfaces modulate the conformational behavior of cyclic peptides with impact on their passive membrane permeability. RSC Adv. 12, 5782–5796 (2022).
Ono, S. et al. Conformation and permeability: cyclic hexapeptide diastereomers. J. Chem. Inf. Model. 59, 2952–2963 (2019).
Hosseinzadeh, P. et al. Comprehensive computational design of ordered peptide macrocycles. Science 358, 1461–1466 (2017).
Ramelot, T. A., Palmer, J., Montelione, G. T. & Bhardwaj, G. Cell-permeable chameleonic peptides: exploiting conformational dynamics in de novo cyclic peptide design. Curr. Opin. Struct. Biol. 80, 102603 (2023).
Miao, J., Descoteaux, M. L. & Lin, Y.-S. Structure prediction of cyclic peptides by molecular dynamics + machine learning. Chem. Sci. 12, 14927–14936 (2021).
Kola, I. & Landis, J. Can the pharmaceutical industry reduce attrition rates? Nat. Rev. Drug Discov. 3, 711–715 (2004).
Bergström, C. A. S. & Avdeef, A. Perspectives in solubility measurement and interpretation. ADMET DMPK 7, 88–105 (2019).
Di, L. et al. The critical role of passive permeability in designing successful drugs. ChemMedChem 15, 1862–1874 (2020).
Plant, N. Strategies for using in vitro screens in drug metabolism. Drug Discov. Today 9, 328–336 (2004).
Lipinski, C. & Hopkins, A. Navigating chemical space for biology and medicine. Nature 432, 855–861 (2004).
Poongavanam, V., Doak, B. C. & Kihlberg, J. Opportunities and guidelines for discovery of orally absorbed drugs in beyond rule of 5 space. Curr. Opin. Chem. Biol. 44, 23–29 (2018).
Acknowledgements
We thank A. Sheikh and R. Hong for helpful discussions on paritaprevir, and G. Bhardwaj for providing the two predicted low-energy conformations of cyclic peptide 27. The writing of this manuscript was supported by a grant from the Swedish Research Council (Grant No. 2021-04747).
Author information
Authors and Affiliations
Contributions
J.K. conceptualized and wrote the manuscript, with contributions from V.P., L.H.E.W., S.P. and M.E. V.P. performed the computational analysis, searched the literature and made the submitted figures. L.H.E.W., S.P. and V.P. downloaded and curated a dataset of crystal structures from which examples were chosen to illustrate different aspects of molecular chameleonicity.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Chemistry thanks Scott Lokey, Youla Tsantrizos, Sandor Vajda, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Poongavanam, V., Wieske, L.H.E., Peintner, S. et al. Molecular chameleons in drug discovery. Nat Rev Chem 8, 45–60 (2024). https://doi.org/10.1038/s41570-023-00563-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-023-00563-1