Abstract
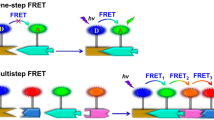
Phosphorescence energy transfer systems have been applied in encryption, biomedical imaging and chemical sensing. These systems exhibit ultra-large Stokes shifts, high quantum yields and are colour-tuneable with long-wavelength afterglow fluorescence (particularly in the near-infrared) under ambient conditions. This review discusses triplet-to-singlet PRET or triplet-to-singlet-to-singlet cascaded PRET systems based on macrocyclic or assembly-confined purely organic phosphorescence introducing the critical toles of supramolecular noncovalent interactions in the process. These interactions promote intersystem crossing, restricting the motion of phosphors, minimizing non-radiative decay and organizing donor–acceptor pairs in close proximity. We discuss the applications of these systems and focus on the challenges ahead in facilitating their further development.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Guo, J., Yang, C. & Zhao, Y. Long-lived organic room-temperature phosphorescence from amorphous polymer systems. Acc. Chem. Res. 55, 1160–1170 (2022).
Shi, H. et al. Ultralong organic phosphorescence: from material design to applications. Acc. Chem. Res. 55, 3445–3459 (2022).
Zhang, T. et al. Molecular engineering for metal-free amorphous materials with room-temperature phosphorescence. Angew. Chem. Int. Ed. Engl. 59, 11206–11216 (2020).
Yang, J., Fang, M. & Li, Z. Stimulus-responsive room temperature phosphorescence materials: internal mechanism, design strategy, and potential application. Acc. Mater. Res. 2, 644–654 (2021).
Garain, S. et al. Arylene diimide phosphors: aggregation modulated twin room temperature phosphorescence from pyromellitic diimides. Angew. Chem. Int. Ed. Engl. 60, 12323–12327 (2021).
Xie, Z. et al. Wide-range lifetime-tunable and responsive ultralong organic phosphorescent multi-host/guest system. Nat. Commun. 12, 3522 (2021).
Li, W. et al. A dish-like molecular architecture for dynamic ultralong room-temperature phosphorescence through reversible guest accommodation. Nat. Commun. 13, 7423 (2022).
Liu, X. Q. et al. Monochromophore-based phosphorescence and fluorescence from pure organic assemblies for ratiometric hypoxia detection. Angew. Chem. Int. Ed. Engl. 59, 23456–23460 (2020).
He, Z. et al. Achieving persistent, efficient, and robust room-temperature phosphorescence from pure organics for versatile applications. Adv. Mater. 31, e1807222 (2019).
Ma, Y. J., Fang, X., Xiao, G. & Yan, D. Dynamic manipulating space-resolved persistent luminescence in core-shell MOFs heterostructures via reversible photochromism. Angew. Chem. Int. Ed. 61, e202114100 (2022).
Wang, Z. et al. Four-in-one stimulus-responsive long-lived luminescent systems based on pyrene-doped amorphous polymers. Angew. Chem. Int. Ed. 61, e202203254 (2022).
Yang, Y. et al. Tunable photoresponsive behaviors based on triphenylamine derivatives: the pivotal role of π-conjugated structure and corresponding application. Adv. Mater. 33, e2104002 (2021).
Cai, S. et al. Ultralong organic phosphorescent foams with high mechanical strength. J. Am. Chem. Soc. 143, 16256–16263 (2021).
Wang, T. et al. Aggregation-induced dual-phosphorescence from organic molecules for nondoped light-emitting diodes. Adv. Mater. 31, 1904273 (2019).
Gong, Y. et al. Achieving persistent room temperature phosphorescence and remarkable mechanochromism from pure organic luminogens. Adv. Mater. 27, 6195–6201 (2015).
Wang, H. et al. Visualization and manipulation of solid-state molecular motions in cocrystallization processes. J. Am. Chem. Soc. 143, 9468–9477 (2021).
Wang, X. F. et al. Pure organic room temperature phosphorescence from unique micelle-assisted assembly of nanocrystals in water. Adv. Funct. Mater. 30, 1907282 (2020).
Yang, J. et al. The influence of the molecular packing on the room temperature phosphorescence of purely organic luminogens. Nat. Commun. 9, 840 (2018).
Bolton, O., Lee, K., Kim, H. J., Lin, K. Y. & Kim, J. Activating efficient phosphorescence from purely organic materials by crystal design. Nat. Chem. 3, 205–210 (2011).
Yuan, W. Z. et al. Crystallization-induced phosphorescence of pure organic luminogens at room temperature. J. Phys. Chem. C 114, 6090–6099 (2010).
Gu, L. et al. Color-tunable ultralong organic room temperature phosphorescence from a multicomponent copolymer. Nat. Commun. 11, 944 (2020).
Gu, L. et al. Circularly polarized organic room temperature phosphorescence from amorphous copolymers. J. Am. Chem. Soc. 143, 18527–18535 (2021).
Zhang, Z. Y. et al. A synergistic enhancement strategy for realizing ultralong and efficient room-temperature phosphorescence. Angew. Chem. Int. Ed. Engl. 59, 18748–18754 (2020).
Wu, H. et al. Molecular phosphorescence in polymer matrix with reversible sensitivity. ACS Appl. Mater. Interfaces 12, 20765–20774 (2020).
Zhang, Y. et al. Ultraviolet irradiation-responsive dynamic ultralong organic phosphorescence in polymeric systems. Nat. Commun. 12, 2297 (2021).
Su, Y. et al. Excitation-dependent long-life luminescent polymeric systems under ambient conditions. Angew. Chem. Int. Ed. Engl. 59, 9967–9971 (2020).
Wang, Z. et al. Accessing excitation- and time-responsive afterglows from aqueous processable amorphous polymer films through doping and energy transfer. Adv. Mater. 34, 2202182 (2022).
Wu, H. et al. Tailoring noncovalent interactions to activate persistent room-temperature phosphorescence from doped polyacrylonitrile films. Adv. Funct. Mater. 31, 2101656 (2021).
Ding, B. et al. Engendering persistent organic room temperature phosphorescence by trace ingredient incorporation. Sci. Adv. 7, eabf9668 (2021).
Zhou, W. L. et al. Ultralong purely organic aqueous phosphorescence supramolecular polymer for targeted tumor cell imaging. Nat. Commun. 11, 4655 (2020).
Zhu, W., Xing, H., Li, E., Zhu, H. & Huang, F. Room-temperature phosphorescence in the amorphous state enhanced by copolymerization and host–guest complexation. Macromolecules 55, 9802–9809 (2022).
Wang, J., Huang, Z., Ma, X. & Tian, H. Visible-light-excited room-temperature phosphorescence in water by cucurbit[8]uril-mediated supramolecular assembly. Angew. Chem. Int. Ed. Engl. 59, 9928–9933 (2020).
Zhang, X. et al. Ultralong UV/mechano-excited room temperature phosphorescence from purely organic cluster excitons. Nat. Commun. 10, 5161 (2019).
Li, D. et al. Amorphous metal-free room-temperature phosphorescent small molecules with multicolor photoluminescence via a host-guest and dual-emission strategy. J. Am. Chem. Soc. 140, 1916–1923 (2018).
Zhang, Z. Y., Chen, Y. & Liu, Y. Efficient room-temperature phosphorescence of a solid-state supramolecule enhanced by cucurbit[6]uril. Angew. Chem. Int. Ed. Engl. 58, 6028–6032 (2019).
Yu, X. et al. Room-temperature phosphorescent gamma-cyclodextrin-cucurbit[6]uril-cowheeled [4]rotaxanes for specific sensing of tryptophan. Chem. Commun. 55, 3156–3159 (2019).
Dou, X. et al. Color-tunable, excitation-dependent, and time-dependent afterglows from pure organic amorphous polymers. Adv. Mater. 32, e2004768 (2020).
Li, D., Yang, J., Fang, M., Tang, B. Z. & Li, Z. Stimulus-responsive room temperature phosphorescence materials with full-color tunability from pure organic amorphous polymers. Sci. Adv. 8, eabl8392 (2022).
Fan, Y. et al. Mobile phone flashlight-excited red afterglow bioimaging. Adv. Mater. 34, 2201280 (2022).
Sun, S., Ma, L., Wang, J., Ma, X. & Tian, H. Red-light excited efficient metal-free near-infrared room-temperature phosphorescent films. Natl Sci. Rev. 9, nwab085 (2022).
Xiao, F. et al. Guest-host doped strategy for constructing ultralong-lifetime near-infrared organic phosphorescence materials for bioimaging. Nat. Commun. 13, 186 (2022).
Pan, Y. et al. Highly efficient TADF-type organic afterglow of long emission wavelengths. Adv. Funct. Mater. 32, 2110207 (2022).
Li, Z., Han, Y. & Wang, F. Compartmentalization-induced phosphorescent emission enhancement and triplet energy transfer in aqueous medium. Nat. Commun. 10, 3735 (2019).
Katsurada, Y., Hirata, S., Totani, K., Watanabe, T. & Vacha, M. Photoreversible on-off recording of persistent room-temperature phosphorescence. Adv. Opt. Mater. 3, 1726–1737 (2015).
Hoshi, M., Nishiyabu, R., Hayashi, Y., Yagi, S. & Kubo, Y. Room-temperature phosphorescence-active boronate particles: characterization and ratiometric afterglow-sensing behavior by surface grafting of Rhodamine B. Chem. Asian J. 15, 787–795 (2020).
Ning, Y. et al. Ultralong organic room-temperature phosphorescence of electron-donating and commercially available host and guest molecules through efficient Förster resonance energy transfer. Sci. China Chem. 64, 739–744 (2021).
Mu, Y. et al. Reversible and continuous color-tunable persistent luminescence of metal-free organic materials by “self”-interface energy transfer. ACS Appl. Mater. Interfaces 12, 5073–5080 (2020).
Peng, H. Q. et al. Biological applications of supramolecular assemblies designed for excitation energy transfer. Chem. Rev. 115, 7502–7542 (2015).
Bennett, R. G. Radiationless intermolecular energy transfer. I. Singlet→singlet transfer. J. Chem. Phys. 41, 3037–3040 (1964).
Scholes, G. D. Long-range resonance energy transfer in molecular systems. Annu. Rev. Phys. Chem. 54, 57–87 (2003).
Főrster, T. 10th Spiers Memorial Lecture. Transfer mechanisms of electronic excitation. Discuss. Faraday Soc. 27, 7–17 (1959).
Gao, R., Mei, X., Yan, D., Liang, R. & Wei, M. Nano-photosensitizer based on layered double hydroxide and isophthalic acid for singlet oxygenation and photodynamic therapy. Nat. Commun. 9, 2798 (2018).
Tanner, P. A., Zhou, L., Duan, C. & Wong, K. L. Misconceptions in electronic energy transfer: bridging the gap between chemistry and physics. Chem. Soc. Rev. 47, 5234–5265 (2018).
Zhao, W. et al. Boosting the efficiency of organic persistent room-temperature phosphorescence by intramolecular triplet-triplet energy transfer. Nat. Commun. 10, 1595 (2019).
Li, G. et al. Organic supramolecular zippers with ultralong organic phosphorescence by a Dexter energy transfer mechanism. Angew. Chem. Int. Ed. Engl. 61, e202113425 (2022).
Kislov, D. A. & Kucherenko, M. G. Nonradiative triplet-singlet transfer of electronic excitation energy between dye molecules in the vicinity of the silver-film surface. Opt. Spectrosc. 117, 784–791 (2014).
Ermolaev, V. L. Energy transfer in organic systems involving the triplet state III. Rigid solutions and crystals. Sov. Phys. Uspekhi 6, 333–358 (1963).
Wasserberg, D., Meskers, S. J. & Janssen, R. J. Phosphorescent resonant energy transfer between iridium complexes. J. Phys. Chem. A 111, 1381–1388 (2007).
Volyniuk, D. et al. Highly efficient blue organic light-emitting diodes based on intermolecular triplet–singlet energy transfer. J. Phys. Chem. C 117, 22538–22544 (2013).
Ma, X., Wang, J. & Tian, H. Assembling-induced emission: an efficient approach for amorphous metal-free organic emitting materials with room-temperature phosphorescence. Acc. Chem. Res. 52, 738–748 (2019).
Ma, X. K. & Liu, Y. Supramolecular purely organic room-temperature phosphorescence. Acc. Chem. Res. 54, 3403–3414 (2021).
Zhu, X., Wang, J.-X., Niu, L.-Y. & Yang, Q.-Z. Aggregation-induced emission materials with narrowed emission band by light-harvesting strategy: fluorescence and chemiluminescence imaging. Chem. Mater. 31, 3573–3581 (2019).
Guo, S., Song, Y., He, Y., Hu, X. Y. & Wang, L. Highly efficient artificial light-harvesting systems constructed in aqueous solution based on supramolecular self-assembly. Angew. Chem. Int. Ed. Engl. 57, 3163–3167 (2018).
Hao, M. et al. A supramolecular artificial light-harvesting system with two-step sequential energy transfer for photochemical catalysis. Angew. Chem. Int. Ed. Engl. 59, 10095–10100 (2020).
Song, Q. et al. Efficient artificial light-harvesting system based on supramolecular peptide nanotubes in water. J. Am. Chem. Soc. 143, 382–389 (2021).
Zhao, W., He, Z. & Tang, B. Z. Room-temperature phosphorescence from organic aggregates. Nat. Rev. Mater. 5, 869–885 (2020).
Barman, D. et al. Review on recent trends and prospects in π-conjugated luminescent aggregates for biomedical applications. Aggregate 3, e172 (2022).
Börjesson, K. et al. Multiplicity conversion based on intramolecular triplet-to-singlet energy transfer. Sci. Adv. 5, eaaw5978 (2019).
Cravcenco, A., Ye, C., Grafenstein, J. & Borjesson, K. Interplay between Förster and Dexter energy transfer rates in isomeric donor-bridge-acceptor systems. J. Phys. Chem. A 124, 7219–7227 (2020).
Maliwal, B. P., Gryczynski, Z. & Lakowicz, J. R. Long-wavelength long-lifetime luminophores. Anal. Chem. 73, 4277–4285 (2001).
D’Andrade, B. W. et al. High-efficiency yellow double-doped organic light-emitting devices based on phosphor-sensitized fluorescence. Appl. Phys. Lett. 79, 1045–1047 (2001).
Chidirala, S. et al. Pyrene-oxadiazoles for organic light-emitting diodes: triplet to singlet energy transfer and role of hole-injection/hole-blocking materials. J. Org. Chem. 81, 603–614 (2016).
Sun, Y. et al. Management of singlet and triplet excitons for efficient white organic light-emitting devices. Nature 440, 908–912 (2006).
Tavares, I. G., Enkvist, E., Kaimre, J., Uri, A. & Dias, F. B. Intramolecular interchromophore singlet-singlet and triplet-singlet energy transfer in a metal-free donor-acceptor emitter. J. Lumin. 237, 118183 (2021).
Ibrayev, N., Seliverstova, E., Temirbayeva, D. & Ishchenko, A. Plasmon effect on simultaneous singlet-singlet and triplet-singlet energy transfer. J. Lumin. 251, 119203 (2022).
Baldo, M. A., Thompson, M. E. & Forrest, S. R. High-efficiency fluorescent organic light-emitting devices using a phosphorescent sensitizer. Nature 403, 750–753 (2000).
Xia, D. et al. Functional supramolecular polymeric networks: the marriage of covalent polymers and macrocycle-based host-guest interactions. Chem. Rev. 120, 6070–6123 (2020).
Liu, Z. & Liu, Y. Multicharged cyclodextrin supramolecular assemblies. Chem. Soc. Rev. 51, 4786–4827 (2022).
Nie, H., Wei, Z., Ni, X. L. & Liu, Y. Assembly and applications of macrocyclic-confinement-derived supramolecular organic luminescent emissions from cucurbiturils. Chem. Rev. 122, 9032–9077 (2022).
Gao, R., Yan, D. & Duan, X. Layered double hydroxides-based smart luminescent materials and the tuning of their excited states. Cell Rep. Phys. Sci. 2, 100536 (2021).
Yan, X. et al. Recent advances on host-guest material systems toward organic room temperature phosphorescence. Small 18, e2104073 (2022).
Zhou, W. L., Lin, W., Chen, Y. & Liu, Y. Supramolecular assembly confined purely organic room temperature phosphorescence and its biological imaging. Chem. Sci. 13, 7976–7989 (2022).
Guo, S. et al. Recent progress in pure organic room temperature phosphorescence of small molecular host–guest systems. ACS Mater. Lett. 3, 379–397 (2021).
Sun, H., Shen, S. & Zhu, L. Photo-stimuli-responsive organic room-temperature phosphorescent materials. ACS Mater. Lett. 4, 1599–1615 (2022).
Sun, H. & Zhu, L. Achieving purely organic room temperature phosphorescence in aqueous solution. Aggregate 4, e253 (2022).
Zhang, Y. et al. Cross-linked polyphosphazene nanospheres boosting long-lived organic room-temperature phosphorescence. J. Am. Chem. Soc. 144, 6107–6117 (2022).
Li, J. J., Chen, Y., Yu, J., Cheng, N. & Liu, Y. A supramolecular artificial light-harvesting system with an ultrahigh antenna effect. Adv. Mater. 29, 1701905 (2017).
Su, Y. et al. Ultralong room temperature phosphorescence from amorphous organic materials toward confidential information encryption and decryption. Sci. Adv. 4, eaas9732 (2018).
Wu, H. et al. Achieving amorphous ultralong room temperature phosphorescence by coassembling planar small organic molecules with polyvinyl alcohol. Adv. Funct. Mater. 29, 1807243 (2019).
Zhang, Y. et al. Large-area, flexible, transparent, and long-lived polymer-based phosphorescence films. J. Am. Chem. Soc. 143, 13675–13685 (2021).
Kuila, S. & George, S. J. Phosphorescence energy transfer: ambient afterglow fluorescence from water-processable and purely organic dyes via delayed sensitization. Angew. Chem. Int. Ed. Engl. 59, 9393–9397 (2020). This work reports the ambient red afterglow in water-processable and flexible poly(vinylalcohol) films via a delayed sensitization process.
Wang, D. et al. Achieving color-tunable and time-dependent organic long persistent luminescence via phosphorescence energy transfer for advanced anti-counterfeiting. Adv. Funct. Mater. 33, 2208895 (2023). This study establishes the structure–property relationship between similar isomers and phosphorescence performance, and presents tunable solid-state long persistent luminescence via phosphorescence energy transfer.
Sun, W., Shi, B., Xia, Z. & Lv, C. Visible-light-excited long-lived organic room-temperature phosphorescence of phenanthroline derivatives in PVA matrix by H-bonding interaction for security applications. Mater. Today Chem. 27, 101297 (2023).
Xiong, S. et al. Achieving tunable organic afterglow and UV irradiation-responsive ultralong room-temperature phosphorescence from pyridine-substituted triphenylamine derivatives. Adv. Mater. 35, 2301874 (2023).
Wei, P. et al. New wine in old bottles: prolonging room-temperature phosphorescence of crown ethers by supramolecular interactions. Angew. Chem. Int. Ed. Engl. 59, 9293–9298 (2020).
Wang, H. J. et al. Noncovalent bridged bis(coumarin-24-crown-8) phosphorescent supramolecular switch. Adv. Opt. Mater. 10, 2201903 (2022).
Kanakubo, M., Yamamoto, Y. & Kubo, Y. Room-temperature phosphorescence of thiophene boronate ester-cross linked polyvinyl alcohol; a triplet-to-singlet FRET-induced multi-color afterglow luminescence with Sulforhodamine B. Bull. Chem. Soc. Jpn 94, 1204–1209 (2021).
Tian, R. et al. Large-scale preparation for efficient polymer-based room-temperature phosphorescence via click chemistry. Sci. Adv. 6, eaaz6107 (2020).
Li, D. et al. Completely aqueous processable stimulus responsive organic room temperature phosphorescence materials with tunable afterglow color. Nat. Commun. 13, 347 (2022). This work introduces B–O covalent bond into poly(vinylalcohol) matrix to facilitate phosphorescence emission and realizes stimulus-responsive tunable afterglow.
Lin, F. et al. Stepwise energy transfer: near-infrared persistent luminescence from doped polymeric systems. Adv. Mater. 34, e2108333 (2022). This paper describes a universal method to realize near-infrared persistent luminescence via stepwise energy transfer.
Kirch, A., Gmelch, M. & Reineke, S. Simultaneous singlet-singlet and triplet-singlet Förster resonance energy transfer from a single donor material. J. Phys. Chem. Lett. 10, 310–315 (2019). This work reports the simultaneous singlet-to-singlet and triplet-to-singlet Förster resonance energy transfer from a biluminescent donor molecule in an amorphous polymeric film.
Wang, X. et al. Reversible photoswitching between fluorescence and room temperature phosphorescence by manipulating excited state dynamics in molecular aggregates. Angew. Chem. Int. Ed. Engl. 61, e202114264 (2022). This work demonstrates a photoreversible fluorescence and room-temperature phosphorescence switching based on a photo-controlled triplet-to-singlet Förster resonance energy transfer.
Zhao, Y. et al. Visible light activated organic room-temperature phosphorescence based on triplet-to-singlet Förster-resonance energy transfer. Adv. Opt. Mater. 10, 2102701 (2022).
Zheng, X. et al. A processable, scalable, and stable full-color ultralong afterglow system based on heteroatom-free hydrocarbon doped polymers. Mater. Horiz. 10, 197–208 (2023).
Wang, C. et al. Photo-induced dynamic room temperature phosphorescence based on triphenyl phosphonium containing polymers. Adv. Funct. Mater. 32, 2111941 (2022).
Wu, M. et al. Two-component design strategy: achieving intense organic afterglow and diverse functions in coronene-matrix systems. J. Phys. Chem. C 125, 26986–26998 (2021).
Hayashi, K., Fukasawa, K., Yamashita, T. & Hirata, S. Rational design of a triplet afterglow sensitizer allowing for bright long-wavelength afterglow room-temperature emission. Chem. Mater. 34, 1627–1637 (2022).
Gao, R. & Yan, D. Layered host-guest long-afterglow ultrathin nanosheets: high-efficiency phosphorescence energy transfer at 2D confined interface. Chem. Sci. 8, 590–599 (2017).
Ma, X., Xu, C., Wang, J. & Tian, H. Amorphous pure organic polymers for heavy-atom-free efficient room-temperature phosphorescence emission. Angew. Chem. Int. Ed. Engl. 57, 10854–10858 (2018).
Lin, X., Wang, J., Ding, B., Ma, X. & Tian, H. Tunable-emission amorphous room-temperature phosphorescent polymers based on thermoreversible dynamic covalent bonds. Angew. Chem. Int. Ed. Engl. 60, 3459–3463 (2021).
Peng, H. et al. On-demand modulating afterglow color of water-soluble polymers through phosphorescence FRET for multicolor security printing. Sci. Adv. 8, eabk2925 (2022). This study develops a full-colour organic ultralong phosphorescence materials with multicolour-emitting afterglow which are successfully used for multicolour security printing.
Zhang, X. et al. Multicolor hyperafterglow from isolated fluorescence chromophores. Nat. Commun. 14, 475 (2023).
Xu, W. W. et al. Tunable second-level room-temperature phosphorescence of solid supramolecules between acrylamide-phenylpyridium copolymers and cucurbit[7]uril. Angew. Chem. Int. Ed. Engl. 61, e202115265 (2022). This work reports tunable second-level phosphorescence based on acrylamide copolymerization and host–guest interaction.
Gui, H., Huang, Z., Yuan, Z. & Ma, X. Ambient white-light afterglow emission based on triplet-to-singlet Förster resonance energy transfer. CCS Chem. 4, 173–181 (2022). This work reports the tunable luminescent copolymers based on two simple single-benzene-based molecules which show multicolour afterglow including ambient white afterglow.
Gu, F., Ding, B., Ma, X. & Tian, H. Tunable fluorescence and room-temperature phosphorescence from multiresponsive pure organic copolymers. Ind. Eng. Chem. Res. 59, 1578–1583 (2020).
Hu, Y. Y., Dai, X. Y., Dong, X., Huo, M. & Liu, Y. Generation of tunable ultrastrong white-light emission by activation of a solid supramolecule through bromonaphthylpyridinium polymerization. Angew. Chem. Int. Ed. Engl. 61, e202213097 (2022).
Ma, L. & Ma, X. Recent advances in room-temperature phosphorescent materials by manipulating intermolecular interactions. Sci. China Chem. 66, 304–314 (2022).
Huang, Q., Lin, Z. & Yan, D. Tuning organic room-temperature phosphorescence through the confinement effect of inorganic micro/nanostructures. Small Struct. 2, 2100044 (2021).
Ye, W. et al. Confining isolated chromophores for highly efficient blue phosphorescence. Nat. Mater. 20, 1539–1544 (2021).
Wang, C., Liu, Y. H. & Liu, Y. Near-infrared phosphorescent switch of diarylethene phenylpyridinium derivative and cucurbit[8]uril for cell imaging. Small 18, e2201821 (2022).
Yu, H. J. et al. A tunable full-color lanthanide noncovalent polymer based on cucurbituril-mediated supramolecular dimerization. Chem. Sci. 13, 8187–8192 (2022).
Ma, X. K., Zhou, X., Wu, J., Shen, F. F. & Liu, Y. Two-photon excited near-infrared phosphorescence based on secondary supramolecular confinement. Adv. Sci. 9, e2201182 (2022).
Huo, M., Dai, X. Y. & Liu, Y. Uncommon supramolecular phosphorescence-capturing assembly based on cucurbit[8]uril-mediated molecular folding for near-infrared lysosome imaging. Small 18, e2104514 (2022).
Huo, M., Dai, X. Y. & Liu, Y. Ultrahigh supramolecular cascaded room-temperature phosphorescence capturing system. Angew. Chem. Int. Ed. Engl. 60, 27171–27177 (2021). This study describes a cascaded phosphorescence-capturing system in aqueous medium using cucurbit[7]uril and sulfonatocalix[4]arene.
Huo, M., Dai, X. Y. & Liu, Y. Ultralarge Stokes shift phosphorescence artificial harvesting supramolecular system with near-infrared emission. Adv. Sci. 9, e2201523 (2022).
Dai, X. Y. et al. A highly efficient phosphorescence/fluorescence supramolecular switch based on a bromoisoquinoline cascaded assembly in aqueous solution. Adv. Sci. 9, e2200524 (2022).
Wang, C. et al. Highly reversible supramolecular light switch for NIR phosphorescence resonance energy transfer. Adv. Sci. 9, e2103041 (2022).
Wang, H. J., Xing, W. W., Zhang, H. Y., Xu, W. W. & Liu, Y. Cucurbit[8]uril confined 6–bromoisoquinoline derivative dicationic phosphorescent energy transfer supramolecular switch for lysosome targeted imaging. Adv. Opt. Mater. 10, 2201178 (2022).
Dang, Q. et al. Room-temperature phosphorescence resonance energy transfer for construction of near-infrared afterglow imaging agents. Adv. Mater. 32, e2006752 (2020).
Liu, Z., Lin, W. & Liu, Y. Macrocyclic supramolecular assemblies based on hyaluronic acid and their biological applications. Acc. Chem. Res. 55, 3417–3429 (2022).
Shen, F. F. et al. Purely organic light-harvesting phosphorescence energy transfer by β-cyclodextrin pseudorotaxane for mitochondria targeted imaging. Chem. Sci. 12, 1851–1857 (2020).
Dai, X. Y., Huo, M., Dong, X., Hu, Y. Y. & Liu, Y. Noncovalent polymerization-activated ultrastrong near-infrared room-temperature phosphorescence energy transfer assembly in aqueous solution. Adv. Mater. 34, e2203534 (2022). This work reports the noncovalent polymerization-activated near-infrared phosphorescence-harvesting system in aqueous solution for targeted tumour cell imaging.
Liu, Y. H. & Liu, Y. Highly efficient discrimination of cancer cells based on in situ-activated phosphorescence energy transfer for targeted cell imaging. J. Mater. Chem. B 10, 8058–8063 (2022).
Zhou, W. L. et al. Multivalent supramolecular assembly with ultralong organic room temperature phosphorescence, high transfer efficiency and ultrahigh antenna effect in water. Chem. Sci. 13, 573–579 (2022).
Shi, H., Wu, Y., Xu, J., Shi, H. & An, Z. Recent advances of carbon dots with afterglow emission. Small 19, 2207104 (2023).
Teng, X., Sun, X., Pan, W., Song, Z. & Wang, J. Carbon dots confined in silica nanoparticles for triplet-to-singlet Föster resonance energy-transfer-induced delayed fluorescence. ACS Appl. Nano Mater. 5, 5168–5175 (2022).
Liang, Y. C. et al. Phosphorescent carbon-nanodots-assisted Forster resonant energy transfer for achieving red afterglow in an aqueous solution. ACS Nano 15, 16242–16254 (2021).
Mo, L. et al. Cascade resonance energy transfer for the construction of nanoparticles with multicolor long afterglow in aqueous solutions for information encryption and bioimaging. Adv. Opt. Mater. 10, 2102666 (2022).
Li, T. et al. Phosphorescent carbon dots as long-lived donors to develop an energy transfer-based sensing platform. Anal. Chem. 95, 2445–2451 (2023).
Voorhaar, L. & Hoogenboom, R. Supramolecular polymer networks: hydrogels and bulk materials. Chem. Soc. Rev. 45, 4013–4031 (2016).
Lu, W., Le, X., Zhang, J., Huang, Y. & Chen, T. Supramolecular shape memory hydrogels: a new bridge between stimuli-responsive polymers and supramolecular chemistry. Chem. Soc. Rev. 46, 1284–1294 (2017).
Chen, H., Ma, X., Wu, S. & Tian, H. A rapidly self-healing supramolecular polymer hydrogel with photostimulated room-temperature phosphorescence responsiveness. Angew. Chem. Int. Ed. Engl. 53, 14149–14152 (2014).
Yuan, J. et al. Tunable dual emission of fluorescence-phosphorescence at room temperature based on pure organic supramolecular gels. Dye. Pigment. 181, 108506 (2020).
Wang, H., Wang, H., Yang, X., Wang, Q. & Yang, Y. Ion-unquenchable and thermally “on-off” reversible room temperature phosphorescence of 3-bromoquinoline induced by supramolecular gels. Langmuir 31, 486–491 (2015).
Chen, H., Xu, L., Ma, X. & Tian, H. Room temperature phosphorescence of 4-bromo-1,8-naphthalic anhydride derivative-based polyacrylamide copolymer with photo-stimulated responsiveness. Polym. Chem. 7, 3989–3992 (2016).
Sun, X. R. et al. Supramolecular room-temperature phosphorescent hydrogel based on hexamethyl cucurbit[5]uril for cell imaging. ACS Appl. Mater. Interfaces 15, 4668–4676 (2023).
Wei, L. et al. Triplet-triplet annihilation upconversion in LAPONITE®/PVP nanocomposites: absolute quantum yields of up to 23.8% in the solid state and application to anti-counterfeiting. Mater. Horiz. 9, 3048–3056 (2022).
Kuila, S. et al. Aqueous phase phosphorescence: ambient triplet harvesting of purely organic phosphors via supramolecular scaffolding. Angew. Chem. Int. Ed. Engl. 57, 17115–17119 (2018).
Garain, S., Garain, B. C., Eswaramoorthy, M., Pati, S. K. & George, S. J. Light-harvesting supramolecular phosphors: highly efficient room temperature phosphorescence in solution and hydrogels. Angew. Chem. Int. Ed. Engl. 60, 19720–19724 (2021). This work reports the high quantum yield solution-state phosphorescence which acts as light-harvesting scaffold to achieve delayed fluorescence in solution.
Li, J. J., Zhang, H. Y., Zhang, Y., Zhou, W. L. & Liu, Y. Room-temperature phosphorescence and reversible white light switch based on a cyclodextrin polypseudorotaxane xerogel. Adv. Opt. Mater. 7, 1900589 (2019).
Zhang, Y. et al. Photo-controlled reversible multicolor room-temperature phosphorescent solid supramolecular pseudopolyrotaxane. Adv. Opt. Mater. 10, 2102169 (2022).
Zhou, Y. et al. Cucurbit[8]uril mediated ultralong purely organic phosphorescence and excellent mechanical strength performance in double-network supramolecular hydrogels. Dye. Pigment. 195, 109725 (2021).
Zhang, T., Ma, X. & Tian, H. A facile way to obtain near-infrared room-temperature phosphorescent soft materials based on Bodipy dyes. Chem. Sci. 11, 482–487 (2020).
Yu, J., Wang, H. & Liu, Y. Double-network confined supramolecular phosphorescence light-harvesting boosting photocatalysis. Adv. Opt. Mater. 10, 2201761 (2022).
Hamzehpoor, E. et al. Efficient room-temperature phosphorescence of covalent organic frameworks through covalent halogen doping. Nat. Chem. 15, 83–90 (2023).
Li, Y., Sui, J., Cui, L. S. & Jiang, H. L. Hydrogen bonding regulated flexibility and disorder in hydrazone-linked covalent organic frameworks. J. Am. Chem. Soc. 145, 1359–1366 (2023).
Acknowledgements
We thank National Natural Science Foundation of China (22131008) for the financial support.
Author information
Authors and Affiliations
Contributions
X.-Y.D. and M.H. contributed equally to this work. Y.L. revised the manuscript and conceived the overall orientation of the manuscript. All authors contributed to the discussion and editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dai, XY., Huo, M. & Liu, Y. Phosphorescence resonance energy transfer from purely organic supramolecular assembly. Nat Rev Chem 7, 854–874 (2023). https://doi.org/10.1038/s41570-023-00555-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-023-00555-1
This article is cited by
-
On-demand molecular design for efficient blue phosphorescence
Science China Chemistry (2024)