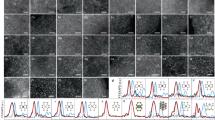
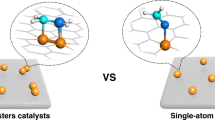
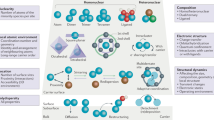
Abstract
Heterogeneous single-cluster catalysts (SCCs) comprising atomically precise and isolated metal clusters stabilized on appropriately chosen supports offer exciting prospects for enabling novel chemical reactions owing to their broad structural diversity with unparalled opportunities for engineering their properties. Although the pioneering work revealed intriguing performance trends of size-selected metal clusters deposited on supports, synthetic and analytical challenges hindered a thorough understanding of surface chemistry under realistic conditions. This Review underscores the importance of considering the cluster environment in SCCs, encompassing the development of robust metal–support interactions, precise control over the ligand sphere, the influence of reaction media and dynamic behaviour, to uncover new reactivities. Through examples, we illustrate the criticality of tailoring the entire catalytic ensemble in SCCs to achieve stable and selective performance with practically relevant metal coverages. This expansion in application scope transcends from model reactions to complex and technically relevant reactions. Furthermore, we provide a perspective on the opportunities and future directions for SCC design within this rapidly evolving field.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Mistry, H., Varela, A. S., Kühl, S., Strasser, P. & Cuenya, B. R. Nanostructured electrocatalysts with tunable activity and selectivity. Nat. Rev. Mater. 1, 16009 (2016).
Liu, L. & Corma, A. Metal catalysts for heterogeneous catalysis: from single atoms to nanoclusters and nanoparticles. Chem. Rev. 118, 4981–5079 (2018).
Mitchell, S., Qin, R., Zheng, N. & Pérez-Ramírez, J. Nanoscale engineering of catalytic materials for sustainable technologies. Nat. Nanotechnol. 16, 129–139 (2021). This paper examines how nanoscale engineering can enhance the selectivity and stability of heterogeneous catalysts.
Zhao, Z.-J. et al. Theory-guided design of catalytic materials using scaling relationships and reactivity descriptors. Nat. Rev. Mater. 4, 792–804 (2019).
Pérez-Ramírez, J. & López, N. Strategies to break linear scaling relationships. Nat. Catal. 2, 971–976 (2019).
Zhang, J., Yang, H. B., Zhou, D. & Liu, B. Adsorption energy in oxygen electrocatalysis. Chem. Rev. 122, 17028–17072 (2022).
Seh, Z. W. et al. Combining theory and experiment in electrocatalysis: insights into materials design. Science 355, eaad4998 (2017).
Zhao, X., Levell, Z. H., Yu, S. & Liu, Y. Atomistic understanding of two-dimensional electrocatalysts from first principles. Chem. Rev. 122, 10675–10709 (2022).
Li, X. et al. Ordered clustering of single atomic Te vacancies in atomically thin PtTe2 promotes hydrogen evolution catalysis. Nat. Commun. 12, 2351 (2021).
Guo, Y., Wang, M., Zhu, Q., Xiao, D. & Ma, D. Ensemble effect for single-atom, small cluster and nanoparticle catalysts. Nat. Catal. 5, 766–776 (2022).
Liu, L. & Corma, A. Confining isolated atoms and clusters in crystalline porous materials for catalysis. Nat. Rev. Mater. 6, 244–263 (2021).
Yang, H. et al. Catalytically active atomically thin cuprate with periodic Cu single sites. Natl Sci. Rev. 10, nwac100 (2023).
Heiz, U. & Schneider, W.-D. Size-selected clusters on solid surfaces. Crit. Rev. Solid State Mater. Sci. 26, 251–290 (2001).
Lei, Y. et al. Increased silver activity for direct propylene epoxidation via subnanometer size effects. Science 328, 224–228 (2010).
Vajda, S. & White, M. G. Catalysis applications of size-selected cluster deposition. ACS Catal. 5, 7152–7176 (2015).
Valden, M., Lai, X. & Goodman, D. W. Onset of catalytic activity of gold clusters on titania with the appearance of nonmetallic properties. Science 281, 1647–1650 (1998).
Yoon, B. et al. Charging effects on bonding and catalyzed oxidation of CO on Au8 clusters on MgO. Science 307, 403–407 (2005).
Mitchell, S. & Pérez-Ramírez, J. Atomically precise control in the design of low-nuclearity supported metal catalysts. Nat. Rev. Mater. 6, 969–985 (2021).
Sun, L., Reddu, V. & Wang, X. Multi-atom cluster catalysts for efficient electrocatalysis. Chem. Soc. Rev. 51, 8923–8956 (2022).
Tyo, E. C. & Vajda, S. Catalysis by clusters with precise numbers of atoms. Nat. Nanotechnol. 10, 577–588 (2015). This paper summarizes work on size-selected supported clusters to provide a mechanistic understanding of their catalytic properties.
Liu, J.-C. et al. Heterogeneous Fe3 single-cluster catalyst for ammonia synthesis via an associative mechanism. Nat. Commun. 9, 1610 (2018). This paper is one of the earliest papers using the term of SCCs.
Ma, X.-L., Liu, J.-C., Xiao, H. & Li, J. Surface single-cluster catalyst for N2-to-NH3 thermal conversion. J. Am. Chem. Soc. 140, 46–49 (2018). This paper first introduces the term SCC.
Xing, D.-H., Xu, C.-Q., Wang, Y.-G. & Li, J. Heterogeneous single-cluster catalysts for selective semihydrogenation of acetylene with graphdiyne-supported triatomic clusters. J. Phys. Chem. C 123, 10494–10500 (2019).
Yao, C. et al. Atomically-precise dopant-controlled single cluster catalysis for electrochemical nitrogen reduction. Nat. Commun. 11, 4389 (2020).
Rong, H., Ji, S., Zhang, J., Wang, D. & Li, Y. Synthetic strategies of supported atomic clusters for heterogeneous catalysis. Nat. Commun. 11, 5884 (2020). This review presents the progress of the synthesis of supported atomic clusters and highlights how the structure affects catalytic properties.
Han, A. et al. Construction of Co4 atomic clusters to enable Fe-N4 motifs with highly active and durable oxygen reduction performance. Angew. Chem. Int. Ed. Engl. 135, e202303185 (2023).
Tian, S. et al. Dual-atom Pt heterogeneous catalyst with excellent catalytic performances for the selective hydrogenation and epoxidation. Nat. Commun. 12, 3181 (2021).
Zhang, N. et al. A supported Pd2 dual-atom site catalyst for efficient electrochemical CO2 reduction. Angew. Chem. Int. Ed. Engl. 60, 13388–13393 (2021).
Wang, C. et al. Co and Pt dual-single-atoms with oxygen-coordinated Co-O-Pt dimer sites for ultrahigh photocatalytic hydrogen evolution efficiency. Adv. Mater. 33, 2003327 (2021). This paper is a representative paper about dual-atom catalysts, which can be seen as the simplest form of SCCs.
Gu, J. et al. Synergizing metal–support interactions and spatial confinement boosts dynamics of atomic nickel for hydrogenations. Nat. Nanotechnol. 16, 1141–1149 (2021). This paper reports the enhanced stability of SCCs compared with SACs.
Yang, Y. et al. O-coordinated W-Mo dual-atom catalyst for pH-universal electrocatalytic hydrogen evolution. Sci. Adv. 6, eaba6586 (2020).
Zhang, L. et al. Atomic layer deposited Pt-Ru dual-metal dimers and identifying their active sites for hydrogen evolution reaction. Nat. Commun. 10, 4936 (2019).
Ye, W. et al. Precisely tuning the number of Fe atoms in clusters on N-doped carbon toward acidic oxygen reduction reaction. Chem 5, 2865–2878 (2019).
Ren, W. et al. Isolated diatomic Ni-Fe metal-nitrogen sites for synergistic electroreduction of CO2. Angew. Chem. Int. Ed. Engl. 58, 6972–6976 (2019).
Jiao, J. et al. Copper atom-pair catalyst anchored on alloy nanowires for selective and efficient electrochemical reduction of CO2. Nat. Chem. 11, 222–228 (2019).
Bai, L., Hsu, C.-S., Alexander, D. T. L., Chen, H. M. & Hu, X. A cobalt-iron double-atom catalyst for the oxygen evolution reaction. J. Am. Chem. Soc. 141, 14190–14199 (2019).
Imaoka, T. et al. Platinum clusters with precise numbers of atoms for preparative-scale catalysis. Nat. Commun. 8, 688 (2017).
Wei, Y. S. et al. Fabricating dual-atom iron catalysts for efficient oxygen evolution reaction: a heteroatom modulator approach. Angew. Chem. Int. Ed. Engl. 59, 16013–16022 (2020).
Zhou, Y. et al. Revealing of active sites and catalytic mechanism in N-coordinated Fe, Ni dual-doped carbon with superior acidic oxygen reduction than single-atom catalyst. J. Phys. Chem. Lett. 11, 1404–1410 (2020).
Dai, S. et al. Platinum-trimer decorated cobalt–palladium core–shell nanocatalyst with promising performance for oxygen reduction reaction. Nat. Commun. 10, 440 (2019).
Wang, J. et al. Design of N-coordinated dual-metal sites: a stable and active Pt-free catalyst for acidic oxygen reduction reaction. J. Am. Chem. Soc. 139, 17281–17284 (2017).
Xie, C., Niu, Z., Kim, D., Li, M. & Yang, P. Surface and interface control in nanoparticle catalysis. Chem. Rev. 120, 1184–1249 (2020).
Xia, Y., Yang, H. & Campbell, T. C. Nanoparticles for catalysis. Acc. Chem. Res. 46, 1671–1672 (2013).
Yan, H. et al. Bottom-up precise synthesis of stable platinum dimers on graphene. Nat. Commun. 8, 1070 (2017).
Ji, S. et al. Confined pyrolysis within metal–organic frameworks to form uniform Ru3 clusters for efficient oxidation of alcohols. J. Am. Chem. Soc. 139, 9795–9798 (2017).
Tian, S. et al. Carbon nitride supported Fe2 cluster catalysts with superior performance for alkene epoxidation. Nat. Commun. 9, 2353 (2018).
Zhao, Y. et al. Stable iridium dinuclear heterogeneous catalysts supported on metal-oxide substrate for solar water oxidation. Proc. Natl Acad. Sci. USA 115, 2902 (2018).
Ji, S. et al. Atomically dispersed ruthenium species inside metal–organic frameworks: combining the high activity of atomic sites and the molecular sieving effect of MOFs. Angew. Chem. Int. Ed. Engl. 58, 4271–4275 (2019).
Li, X. et al. Microenvironment modulation of single-atom catalysts and their roles in electrochemical energy conversion. Sci. Adv. 6, eabb6833 (2020).
Zhang, L., Ren, Y., Liu, W., Wang, A. & Zhang, T. Single-atom catalyst: a rising star for green synthesis of fine chemicals. Natl Sci. Rev. 5, 653–672 (2018).
Fortea-Pérez, F. R. et al. The MOF-driven synthesis of supported palladium clusters with catalytic activity for carbene-mediated chemistry. Nat. Mater. 16, 760–766 (2017).
Lu, Z. Y. et al. An isolated zinc–cobalt atomic pair for highly active and durable oxygen reduction. Angew. Chem. Int. Ed. 58, 2622–2626 (2019).
Liu, J., Cao, D., Xu, H. & Cheng, D. From double-atom catalysts to single-cluster catalysts: a new frontier in heterogeneous catalysis. Nano Sel. 2, 251–270 (2021).
Pei, W. et al. Immobilized trimeric metal clusters: a family of the smallest catalysts for selective CO2 reduction toward multi-carbon products. Nano Energy 76, 105049 (2020).
Liu, J.-C., Xiao, H. & Li, J. Constructing high-loading single-atom/cluster catalysts via an electrochemical potential window strategy. J. Am. Chem. Soc. 142, 3375–3383 (2020).
Wang, Z. et al. Atomically dispersed Co2-N6 and Fe-N4 costructures boost oxygen reduction reaction in both alkaline and acidic media. Adv. Mater. 33, 2104718 (2021).
Wang, Y. et al. Precisely constructing orbital coupling-modulated dual-atom Fe pair sites for synergistic CO2 electroreduction. ACS Energy Lett. 7, 640–649 (2022).
Ling, C. et al. Atomic-layered Cu5 nanoclusters on FeS2 with dual catalytic sites for efficient and selective H2O2 activation. Angew. Chem. Int. Ed. Engl. 134, e202200670 (2022).
Yan, X. et al. Atomically dispersed Co2MnN8 triatomic sites anchored in N-doped carbon enabling efficient oxygen reduction reaction. Adv. Mater. https://doi.org/10.1002/adma.202210975 (2023).
Sun, X. et al. Palladium dimer supported on Mo2Co2(Mxene) for direct methane to methanol conversion. Adv. Theory Simul. 2, 1800158 (2019).
Zhang, S. et al. Catalysis on singly dispersed bimetallic sites. Nat. Commun. 6, 7938 (2015).
Li, X. et al. Atomically precise single metal oxide cluster catalyst with oxygen-controlled activity. Adv. Funct. Mater. 32, 2200933 (2022).
Baxter, E. T., Ha, M.-A., Cass, A. C., Alexandrova, A. N. & Anderson, S. L. Ethylene dehydrogenation on Pt4,7,8 clusters on Al2O3: strong cluster size dependence linked to preferred catalyst morphologies. ACS Catal. 7, 3322–3335 (2017).
Ren, Y., Yang, Y., Zhao, Y.-X. & He, S.-G. Conversion of methane with oxygen to produce hydrogen catalyzed by triatomic Rh3−cluster anion. JACS Au 2, 197–203 (2021).
Watanabe, Y. Atomically precise cluster catalysis towards quantum controlled catalysts. Sci. Technol. Adv. Mater. 15, 063501 (2014).
Jimenez-Izal, E. & Alexandrova, A. N. Computational design of clusters for catalysis. Annu. Rev. Phys. Chem. 69, 377–400 (2018).
Li, H. et al. Synergetic interaction between neighbouring platinum monomers in CO2 hydrogenation. Nat. Nanotechnol. 13, 411–417 (2018).
Kwon, G. et al. Size-dependent subnanometer Pd cluster (Pd4, Pd6, and Pd17) water oxidation electrocatalysis. ACS Nano 7, 5808–5817 (2013).
Halder, A. et al. Water oxidation catalysis via size-selected iridium clusters. J. Phys. Chem. C 122, 9965–9972 (2018).
Xiao, M. et al. Identification of binuclear Co2N5 active sites for oxygen reduction reaction with more than one magnitude higher activity than single atom CoN4 site. Nano Energy 46, 396–403 (2018).
Liu, D. et al. Distinguished Zn,Co-Nx-C-Sy active sites confined in dentric carbon for highly efficient oxygen reduction reaction and flexible Zn-air batteries. Nano Energy 58, 277–283 (2019).
Wang, J. et al. Synergistic effect of well-defined dual sites boosting the oxygen reduction reaction. Energy Environ. Sci. 11, 3375–3379 (2018).
Jiang, M. et al. Rationalization on high-loading iron and cobalt dual metal single atoms and mechanistic insight into the oxygen reduction reaction. Nano Energy 93, 106793 (2022).
Zhang, T. et al. Dual-atom nickel moieties of Ni(II)2N4(µ2-N)2 anchored on alfalfa-derived developed porous N-doped carbon for high-performance Li-S battery. Small 18, 2201996 (2022).
Zeng, X. et al. Single-atom to single-atom grafting of Pt1 onto Fe-N4 center: Pt1@Fe-N-C multifunctional electrocatalyst with significantly enhanced properties. Adv. Energy Mater. 8, 1701345 (2018).
Jin, R., Zeng, C., Zhou, M. & Chen, Y. Atomically precise colloidal metal nanoclusters and nanoparticles: fundamentals and opportunities. Chem. Rev. 116, 10346–10413 (2016).
Wang, X. et al. Confined Fe-Cu clusters as sub-nanometer reactors for efficiently regulating the electrochemical nitrogen reduction reaction. Adv. Mater. 32, 2004382 (2020).
Kaden, W. E., Wu, T., Kunkel, W. A. & Anderson, S. L. Electronic structure controls reactivity of size-selected Pd clusters adsorbed on TiO2 surfaces. Science 326, 826 (2009).
Lu, J. et al. Effect of the size-selective silver clusters on lithium peroxide morphology in lithium-oxygen batteries. Nat. Commun. 5, 4895 (2014).
Liu, C. et al. Carbon dioxide conversion to methanol over size-selected Cu4 clusters at low pressures. J. Am. Chem. Soc. 137, 8676–8679 (2015).
Yang, B. et al. Copper cluster size effect in methanol synthesis from CO2. J. Phys. Chem. C 121, 10406–10412 (2017).
Taketoshi, A. & Haruta, M. Size- and structure-specificity in catalysis by gold clusters. Chem. Lett. 43, 380–387 (2014).
Judai, K., Abbet, S., Wörz, A. S., Heiz, U. & Henry, C. R. Low-temperature cluster catalysis. J. Am. Chem. Soc. 126, 2732–2737 (2004).
Rötzer, M. D. et al. Nanotuning via local work function control: ethylene hydrogenation on supported Pt nanoclusters. ACS Catal. 10, 1799–1809 (2020).
Vorobyeva, E. et al. Atom-by-atom resolution of structure–function relations over low-nuclearity metal catalysts. Angew. Chem. Int. Ed. Engl. 58, 8724–8729 (2019).
Zhang, G. et al. A general route via formamide condensation to prepare atomically dispersed metal–nitrogen–carbon electrocatalysts for energy technologies. Energy Environ. Sci. 12, 1317–1325 (2019).
Han, X. et al. Atomically dispersed binary Co-Ni sites in nitrogen-doped hollow carbon nanocubes for reversible oxygen reduction and evolution. Adv. Mater. 31, 1905622 (2019).
Zhu, X. et al. Harnessing the interplay of Fe-Ni atom pairs embedded in nitrogen-doped carbon for bifunctional oxygen electrocatalysis. Nano Energy 71, 104597 (2020).
George, S. M. Atomic layer deposition: an overview. Chem. Rev. 110, 111–131 (2010).
Qian, H., Zhu, M., Wu, Z. & Jin, R. Quantum sized gold nanoclusters with atomic precision. Acc. Chem. Res. 45, 1470–1479 (2012).
Ni, B. & Wang, X. Chemistry and properties at a sub-nanometer scale. Chem. Sci. 7, 3978–3991 (2016).
Park, H., Shin, D. J. & Yu, J. Categorization of quantum dots, clusters, nanoclusters, and nanodots. J. Chem. Educ. 98, 703–709 (2021).
Taylor, K. J., Pettiette-Hall, C. L., Cheshnovsky, O. & Smalley, R. E. Ultraviolet photoelectron spectra of coinage metal clusters. J. Chem. Phys. 96, 3319–3329 (1992).
Prats, H. & Stamatakis, M. Atomistic and electronic structure of metal clusters supported on transition metal carbides: implications for catalysis. J. Mater. Chem. A 10, 1522–1534 (2022).
Zhao, W. et al. Triggering Pt active sites in basal plane of van der Waals PtTe2 materials by amorphization engineering for hydrogen evolution. Adv. Mater. 35, 2301593 (2023).
Ding, T. et al. Atomically precise dinuclear site active toward electrocatalytic CO2 reduction. J. Am. Chem. Soc. 143, 11317–11324 (2021).
Nørskov, J. K., Bligaard, T., Rossmeisl, J. & Christensen, C. H. Towards the computational design of solid catalysts. Nat. Chem. 1, 37–46 (2009).
Montemore, M. M., van Spronsen, M. A., Madix, R. J. & Friend, C. M. O2 activation by metal surfaces: implications for bonding and reactivity on heterogeneous catalysts. Chem. Rev. 118, 2816–2862 (2018).
Du, Y., Sheng, H., Astruc, D. & Zhu, M. Atomically precise noble metal nanoclusters as efficient catalysts: a bridge between structure and properties. Chem. Rev. 120, 526–622 (2020).
Medford, A. J. et al. From the Sabatier principle to a predictive theory of transition-metal heterogeneous catalysis. J. Catal. 328, 36–42 (2015).
Xie, C. et al. Insight into the design of defect electrocatalysts: from electronic structure to adsorption energy. Mater. Today 31, 47–68 (2019).
Kress, P. L. et al. A priori design of dual-atom alloy sites and experimental demonstration of ethanol dehydrogenation and dehydration on PtCrAg. J. Am. Chem. Soc. 145, 8401–8407 (2023).
Zhang, S., Sykes, E. C. H. & Montemore, M. M. Tuning reactivity in trimetallic dual-atom alloys: molecular-like electronic states and ensemble effects. Chem. Sci. 13, 14070–14079 (2022).
Zhou, P. et al. Synergetic interaction between neighboring platinum and ruthenium monomers boosts CO oxidation. Chem. Sci. 10, 5898–5905 (2019).
Liu, M. et al. Tuning the site-to-site interaction in Ru-M (M = Co, Fe, Ni) diatomic electrocatalysts to climb up the volcano plot of oxygen electroreduction. ACS Nano 16, 10657–10666 (2022).
Shi, H. et al. Atomically dispersed indium-copper dual-metal active sites promoting C-C coupling for CO2 photoreduction to ethanol. Angew. Chem. Int. Ed. Engl. 61, e202208908 (2022).
Li, F., Liu, X. & Chen, Z. 1 + 1’ > 2: Heteronuclear biatom catalyst outperforms its homonuclear counterparts for CO oxidation. Small Methods 3, 1800480 (2019).
Giulimondi, V. et al. Controlled formation of dimers and spatially isolated atoms in bimetallic Au-Ru catalysts via carbon-host functionalization. Small 18, 2200224 (2022).
Wang, J. et al. N-coordinated dual-metal single-site catalyst for low-temperature CO oxidation. ACS Catal. 10, 2754–2761 (2020).
Xiao, M. et al. Climbing the apex of the ORR volcano plot via binuclear site construction: electronic and geometric engineering. J. Am. Chem. Soc. 141, 17763–17770 (2019).
Sarkar, S. et al. Unravelling the role of Fe-Mn binary active sites electrocatalyst for efficient oxygen reduction reaction and rechargeable Zn-air batteries. Inorg. Chem. 59, 5194–5205 (2020).
Jia, G. et al. Asymmetric coupled dual-atom sites for selective photoreduction of carbon dioxide to acetic acid. Adv. Funct. Mater. 32, 2206817 (2022).
Zhu, J. et al. Quasi-covalently coupled Ni-Cu atomic pair for synergistic electroreduction of CO2. J. Am. Chem. Soc. 144, 9661–9671 (2022).
Wan, X.-K., Wang, J.-Q., Nan, Z.-A. & Wang, Q.-M. Ligand effects in catalysis by atomically precise gold nanoclusters. Sci. Adv. 3, e1701823 (2017).
DeRosha, D. E. et al. Planar three-coordinate iron sulfide in a synthetic [4Fe-3S] cluster with biomimetic reactivity. Nat. Chem. 11, 1019–1025 (2019).
Watanabe, Y., Wu, X., Hirata, H. & Isomura, N. Size-dependent catalytic activity and geometries of size-selected Pt clusters on TiO2(110) surfaces. Catal. Sci. Technol. 1, 1490–1495 (2011).
Yang, C.-T., Wood, B. C., Bhethanabotla, V. R. & Joseph, B. The effect of the morphology of supported subnanometer Pt clusters on the first and key step of CO2 photoreduction. Phys. Chem. Chem. Phys. 17, 25379–25392 (2015).
Wang, J., Wang, G. & Zhao, J. Density-functional study of Aun (n = 2–20) clusters: lowest-energy structures and electronic properties. Phys. Rev. B 66, 035418 (2002).
Li, X., Zhong, W., Cui, P., Li, J. & Jiang, J. Design of efficient catalysts with double transition metal atoms on C2N layer. J. Phys. Chem. Lett. 7, 1750–1755 (2016).
Zhao, J., Zhao, J., Li, F. & Chen, Z. Copper dimer supported on a C2N layer as an efficient electrocatalyst for CO2 reduction reaction: a computational study. J. Phys. Chem. C 122, 19712–19721 (2018).
Li, Y., Su, H., Chan, S. H. & Sun, Q. CO2 electroreduction performance of transition metal dimers supported on graphene: a theoretical study. ACS Catal. 5, 6658–6664 (2015).
Kumar, A. et al. Moving beyond bimetallic-alloy to single-atom dimer atomic-interface for all-pH hydrogen evolution. Nat. Commun. 12, 6766 (2021).
Shen, L., Dadras, J. & Alexandrova, A. N. Pure and Zn-doped Pt clusters go flat and upright on MgO(100). Phys. Chem. Chem. Phys. 16, 26436–26442 (2014).
Dadras, J., Shen, L. & Alexandrova, A. Pt-Zn clusters on stoichiometric MgO(100) and TiO2(110): dramatically different sintering behavior. J. Phys. Chem. C 119, 6047–6055 (2015).
Parkinson, G. S. et al. Carbon monoxide-induced adatom sintering in a Pd-Fe3O4 model catalyst. Nat. Mater. 12, 724–728 (2013).
Corma, A. et al. Exceptional oxidation activity with size-controlled supported gold clusters of low atomicity. Nat. Chem. 5, 775–781 (2013).
Yang, F., Chen, M. S. & Goodman, D. W. Sintering of Au particles supported on TiO2(110) during CO oxidation. J. Phys. Chem. C 113, 254–260 (2009).
Moliner, M. et al. Reversible transformation of Pt nanoparticles into single atoms inside high-silica chabazite zeolite. J. Am. Chem. Soc. 138, 15743–15750 (2016).
Wang, Y.-G., Mei, D., Glezakou, V.-A., Li, J. & Rousseau, R. Dynamic formation of single-atom catalytic active sites on ceria-supported gold nanoparticles. Nat. Commun. 6, 6511 (2015).
Liu, J.-C., Wang, Y.-G. & Li, J. Toward rational design of oxide-supported single-atom catalysts: atomic dispersion of gold on ceria. J. Am. Chem. Soc. 139, 6190–6199 (2017).
Chavez, S. et al. Studying, promoting, exploiting, and predicting catalyst dynamics: the next frontier in heterogeneous catalysis. J. Phys. Chem. C 127, 2127–2146 (2023). This review summarizes the doping, alloying, cluster sintering, adsorbate and support influence on the stability of catalysts.
Bullock, R. M. et al. Using nature’s blueprint to expand catalysis with earth-abundant metals. Science 369, eabc3183 (2020).
Wagner, T., Ermler, U. & Shima, S. The methanogenic CO2 reducing-and-fixing enzyme is bifunctional and contains 46 [4Fe-4S] clusters. Science 354, 114–117 (2016).
Chan, S. I. et al. Redox potentiometry studies of particulate methane monooxygenase: support for a trinuclear copper cluster active site. Angew. Chem. Int. Ed. Engl. 46, 1992–1994 (2007).
Wagner, T., Koch, J., Ermler, U. & Shima, S. Methanogenic heterodisulfide reductase (HdrABC-MvhAGD) uses two noncubane [4Fe-4S] clusters for reduction. Science 357, 699–703 (2017).
Stanley, P. M. et al. Photocatalytic CO2-to-syngas evolution with molecular catalyst metal-organic framework nanozymes. Adv. Mater. 35, 2207380 (2023).
Chen, J.-C. et al. Heterogeneous two-atom single-cluster catalysts for the nitrogen electroreduction reaction. J. Phys. Chem. C 125, 19821–19830 (2021).
Liu, N., Ma, X.-L., Li, J. & Xiao, H. Singly dispersed bimetallic sites as stable and efficient single-cluster catalysts for activating N2 and CO2. J. Phys. Chem. C 125, 27192–27198 (2021).
Liu, J.-C. et al. Computational prediction of graphdiyne-supported three-atom single-cluster catalysts. CCS Chem. 5, 152–163 (2022).
Liu, J.-C. et al. Metal affinity of support dictates sintering of gold catalysts. J. Am. Chem. Soc. 144, 20601–20609 (2022).
Xu, J., Xie, W., Han, Y. & Hu, P. Atomistic insights into the oxidation of flat and stepped platinum surfaces using large-scale machine learning potential-based grand-canonical Monte Carlo. ACS Catal. 12, 14812–14824 (2022).
Kitchin, J. R. Machine learning in catalysis. Nat. Catal. 1, 230–232 (2018).
Esterhuizen, J. A., Goldsmith, B. R. & Linic, S. Interpretable machine learning for knowledge generation in heterogeneous catalysis. Nat. Catal. 5, 175–184 (2022).
Ma, S. & Liu, Z.-P. Machine learning for atomic simulation and activity prediction in heterogeneous catalysis: current status and future. ACS Catal. 10, 13213–13226 (2020).
Wang, Y.-G., Yoon, Y., Glezakou, V.-A., Li, J. & Rousseau, R. The role of reducible oxide-metal cluster charge transfer in catalytic processes: new insights on the catalytic mechanism of CO oxidation on Au/TiO2 from ab initio molecular dynamics. J. Am. Chem. Soc. 135, 10673–10683 (2013).
Mekkering, M. J. et al. Dry reforming of methane over single-atom Rh/Al2O3 catalysts prepared by exsolution. Catal. Sci. Technol. 13, 2255–2260 (2023).
Shrestha, S., Liu, Y. & Mustain, W. E. Electrocatalytic activity and stability of Pt clusters on state-of-the-art supports: a review. Catal. Rev. Sci. Eng. 53, 256–336 (2011).
Zandkarimi, B., Poths, P. & Alexandrova, A. N. When fluxionality beats size selection: acceleration of Ostwald ripening of sub-nano clusters. Angew. Chem. 133, 12080–12089 (2021).
Munarriz, J., Zhang, Z., Sautet, P. & Alexandrova, A. N. Graphite-supported Ptn cluster electrocatalysts: major change of active sites as a function of the applied potential. ACS Catal. 12, 14517–14526 (2022).
Häkkinen, H., Abbet, S., Sanchez, A., Heiz, U. & Landman, U. Structural, electronic, and impurity-doping effects in nanoscale chemistry: supported gold nanoclusters. Angew. Chem. Int. Ed. Engl. 42, 1297–1300 (2003). This paper reveals that clusters can occupy a dynamic ensemble of states with varying reactivities.
Sun, T. et al. Ferromagnetic single-atom spin catalyst for boosting water splitting. Nat. Nanotechnol. 18, 763–771 (2023).
Li, X. et al. Single cobalt atoms anchored on porous N-doped graphene with dual reaction sites for efficient fenton-like catalysis. J. Am. Chem. Soc. 140, 12469–12475 (2018).
Boyes, E. D. & Gai, P. L. Visualizing reacting single atoms in chemical reactions: advancing the frontiers of materials research. MRS Bull. 40, 600–609 (2015).
Gai, P. L. & Boyes, E. D. Advances in atomic resolution in situ environmental transmission electron microscopy and 1 Å aberration corrected in situ electron microscopy. Microsc. Res. Tech. 72, 153–164 (2009).
Gai, P. L., Lari, L., Ward, M. R. & Boyes, E. D. Visualisation of single atom dynamics and their role in nanocatalysts under controlled reaction environments. Chem. Phys. Lett. 592, 355–359 (2014).
Mitchell, S. et al. Automated image analysis for single-atom detection in catalytic materials by transmission electron microscopy. J. Am. Chem. Soc. 144, 8018–8029 (2022).
Han, L. et al. Modulating single-atom palladium sites with copper for enhanced ambient ammonia electrosynthesis. Angew. Chem. Int. Ed. Engl. 60, 345–350 (2021).
Hai, X. et al. Geminal-atom catalysis for cross-coupling. Nature https://doi.org/10.1038/s41586-023-06529-z (2023).
Acknowledgements
J.Lu acknowledges the support from National Research Foundation, CRP programme (CRP29-2022-0038) and from Agency for Science, Technology and Research (A*STAR) under its MTC IRG Grant (M22K2c0082). S.M. and J.P.-R. acknowledge NCCR Catalysis (No. 180544), a National Centre of Competence in Research funded by the Swiss National Science Foundation. X.L. acknowledges the support from the Natural Science Foundation of Shaanxi Province (No. 2023-JC-QN-0136), ‘Young Talent Support Plan’ of Xi’an Jiaotong University (No. ND6J026) and Qinchuangyuan High-Level Talent Project (No. QCYRCXM-2022-344). J.Li. acknowledges the financial support from the National Natural Science Foundation of China (Grant No. 22033005), NSFC Center for Single-Atom Catalysis, the National Key Research and Development Project (Grant Nos 2022YFA1503900 and 2022YFA1503000) and the Guangdong Provincial Key Laboratory of Catalysis (No. 2020B121201002). Y.F. acknowledges the financial support from the National Natural Science Foundation of China (No. 22005244) and Fundamental Research Funds for the Central Universities (No. D5000230127).
Author information
Authors and Affiliations
Contributions
X.L. and S.M. contributed equally. All authors contributed substantially to the discussion of the content and structure, investigated the literature, wrote, reviewed and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Chemistry thanks Aiqin Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, X., Mitchell, S., Fang, Y. et al. Advances in heterogeneous single-cluster catalysis. Nat Rev Chem 7, 754–767 (2023). https://doi.org/10.1038/s41570-023-00540-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-023-00540-8
This article is cited by
-
A possibility to infer frustrations of supported catalytic clusters from macro-scale observations
Scientific Reports (2024)
-
Electrochemical hydrogenation and oxidation of organic species involving water
Nature Reviews Chemistry (2024)
-
Ultimate structures in catalysis: Single atoms, subnano-clusters, and electrons
Science China Materials (2023)