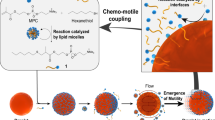
Abstract
Motility is the capacity for living organisms to move autonomously and with purpose, and is essential to life. The transition from abiotic chemistry into motile cellular compartments has yet to be understood, but motile behaviour likely followed chemical evolution because primeval cell survival depended on scouting for resources effectively. Minimalistic motile systems provide an experimental framework to delineate the emergence mechanisms of such an evolutionary asset. In this Review, we discuss frontier developments in controlling the movement of droplets in lipid systems, in particular, chemotactic behaviours driven by fluctuations in interfacial tension, because of its simple mechanism and prebiotic relevance. Although most efforts have focused on designing oil droplet motility in lipid-rich aqueous solutions, we highlight that water droplets can also move in lipid-enriched oils. First, we describe how droplets evolve chemotactic motility in lipid systems. Next, we review how these oil droplets can adapt their movement to illumination conditions. Finally, we discuss examples where chemical reactivity brings complexity to motility. This work contributes to systems chemistry, where chemical reactions combined with physicochemical phenomena can yield new functions, such that a limited set of molecules can promote complex movement at larger functional scales by following the rules of molecular chemistry.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
“Il moto è causa d’ogni vita”. Extract from the Codex Trivulzianus, Leonardo da Vinci (1487).
Baym, M. et al. Spatiotemporal microbial evolution on antibiotic landscapes. Science 353, 1147–1152 (2016).
Melkikh, A. V. & Chesnokova, O. I. Origin of the directed movement of protocells in the early stages of the evolution of life. Orig. Life Evol. Biosph. 42, 317–331 (2012).
Jarrell, K. F. & McBride, M. J. The surprisingly diverse ways that prokaryotes move. Nat. Rev. Microbiol. 6, 466–476 (2008).
Lu, M. et al. Bacteria-specific phototoxic reactions triggered by blue light and phytochemical carvacrol. Sci. Transl. Med. 13, eaba3571 (2021).
Melkikh, A. V. Paradoxes of early stages of evolution of life and biological complexity. Orig. Life Evol. Biosph. 45, 163–171 (2015).
Ramaswamy, S. The mechanics and statistics of active matter. Annu. Rev. Condens. Matter Phys. 1, 323–345 (2010).
Segré, D., Ben-Eli, D., Deamer, D. W. & Lancet, D. The lipid world. Orig. Life Evol. Biosph. 31, 119–145 (2001).
Kahana, A. & Lancet, D. Self-reproducing catalytic micelles as nanoscopic protocell precursors. Nat. Rev. Chem. 5, 870–878 (2021).
Oparin, A. I. Origin and evolution of metabolism. Comp. Biochem. Physiol. 4, 371–377 (1962).
Ghosh, B., Bose, R. & Tang, T. Y. D. Can coacervation unify disparate hypotheses in the origin of cellular life? Curr. Opin. Colloid Interface Sci. 52, 101415 (2021).
Rubio-Sánchez, R. et al. Thermally driven membrane phase transitions enable content reshuffling in primitive cells. J. Am. Chem. Soc. 143, 16589–16598 (2021).
Nilson, F. P. R. Possible impact of a primordial oil slick on atmospheric and chemical evolution. Orig. Life Evol. Biosph. 32, 247–253 (2002).
Oró, J. & Nooner, D. Aliphatic hydrocarbons in meteorites. Nature 213, 1085–1087 (1967).
Proskurowski, G. et al. Abiogenic hydrocarbon production at Lost City hydrothermal field. Science 319, 604–607 (2008).
Deamer, D. W. in Self-Production of Supramolecular Structures: From Synthetic Structures to Models of Minimal Living Systems (eds Fleischaker, G. R., Colonna, S. & Luisi, P. L.) 217–229 (Springer, 1994).
Yamamoto, T. & Sano, M. Chirality-induced helical self-propulsion of cholesteric liquid crystal droplets. Soft Matter 13, 3328–3333 (2017).
Cheon, S. I., Batista Capaverde Silva, L., Khair, A. & Zarzar, L. Interfacially-adsorbed particles enhance the self-propulsion of oil droplets in aqueous surfactant. Soft Matter 17, 6742–6750 (2021).
Dwivedi, P., Si, B. R., Pillai, D. & Mangal, R. Solute induced jittery motion of self-propelled droplets. Phys. Fluids 33, 022103 (2021).
Lohse, D. & Zhang, X. Physicochemical hydrodynamics of droplets out of equilibrium. Nat. Rev. Phys. 2, 426–443 (2020).
Yang, Z., Wei, J., Sobolev, Y. I. & Grzybowski, B. A. Systems of mechanized and reactive droplets powered by multi-responsive surfactants. Nature 553, 313–318 (2018).
Singh, D. P. et al. Interface-mediated spontaneous symmetry breaking and mutual communication between drops containing chemically active particles. Nat. Commun. 11, 2210 (2020).
Dreher, Y., Jahnke, K., Schröter, M. & Göpfrich, K. Light-triggered cargo loading and division of DNA-containing giant unilamellar lipid vesicles. Nano Lett. 21, 5952–5957 (2021).
Taylor, J. W. et al. Autonomous model protocell division driven by molecular replication. Nat. Commun. 8, 237 (2017).
Ebbens, S. J. & Howse, J. R. In pursuit of propulsion at the nanoscale. Soft Matter 6, 726–738 (2010).
Rao, K. J. et al. A force to be reckoned with: a review of synthetic microswimmers powered by ultrasound. Small 11, 2836–2846 (2015).
Moran, J. & Posner, J. Microswimmers with no moving parts. Phys. Today 72, 44–50 (2019).
Walther, A. & Müller, A. H. E. Janus particles. Soft Matter 4, 663–668 (2008).
Wang, W., Lv, X., Moran, J. L., Duan, S. & Zhou, C. A practical guide to active colloids: choosing synthetic model systems for soft matter physics research. Soft Matter 16, 3846–3868 (2020).
Zhang, Y. & Hess, H. Chemically-powered swimming and diffusion in the microscopic world. Nat. Rev. Chem. 5, 500–510 (2021).
Taro, T., Hironori, S., Soichiro, H., Hiroaki, I. & Hiroyuki, K. Chemically artificial rovers based on self-propelled droplets in micrometer-scale environment. Curr. Opin. Colloid Interface Sci. 49, 60–68 (2020).
Lauga, E. & Davis, A. M. J. Viscous Marangoni propulsion. J. Fluid Mech. 705, 120–133 (2012).
Schmitt, M. & Stark, H. Swimming active droplet: A theoretical analysis. Europhys. Lett. 101, 44008 (2013).
Rednikov, A. Y., Ryazantsev, Y. S. & Velárde, M. G. Drop motion with surfactant transfer in an inhomogeneous medium. Int. J. Heat Mass Transf. 37, 361–374 (1994).
Kovalchuk, N. M. & Vollhardt, D. Marangoni instability and spontaneous non-linear oscillations produced at liquid interfaces by surfactant transfer. Adv. Colloid Interface Sci. 120, 1–31 (2006).
Sterling, C. V. & Scriven, L. E. Interfacial turbulence: hydrodynamic instability and the Marangoni effect. Angew. Chem. Int. Ed. 5, 514–523 (1959).
Hanczyc, M. M. Metabolism and motility in prebiotic structures. Philos. Trans. R. Soc. B Biol. Sci. 366, 2885–2893 (2011).
van der Weijden, A. et al. Autonomous mesoscale positioning emerging from myelin filament self-organization and Marangoni flows. Nat. Commun. 11, 4800 (2020).
Maass, C. C., Krüger, C., Herminghaus, S. & Bahr, C. Swimming droplets. Annu. Rev. Condens. Matter Phys. 7, 171–193 (2016).
Morozov, M. Adsorption inhibition by swollen micelles may cause multistability in active droplets. Soft Matter 16, 5624–5632 (2020).
Schmitt, M. & Stark, H. Marangoni flow at droplet interfaces: Three-dimensional solution and applications. Phys. Fluids 28, 012106 (2016).
Herminghaus, S. et al. Interfacial mechanisms in active emulsions. Soft Matter 10, 7008–7022 (2014).
Izri, Z., Van Der Linden, M. N., Michelin, S. & Dauchot, O. Self-propulsion of pure water droplets by spontaneous Marangoni-stress-driven motion. Phys. Rev. Lett. 113, 248302 (2014).
Suematsu, N. J., Saikusa, K., Nagata, T. & Izumi, S. Interfacial dynamics in the spontaneous motion of an aqueous droplet. Langmuir 35, 11601–11607 (2019).
Suematsu, N. J., Mori, Y., Amemiya, T. & Nakata, S. Oscillation of speed of a self-propelled Belousov–Zhabotinsky droplet. J. Phys. Chem. Lett. 7, 3424–3428 (2016).
Zhang, S. et al. Engineering motile aqueous phase-separated droplets via liposome stabilisation. Nat. Commun. 12, 1673 (2021).
Li, M., Brinkmann, M., Pagonabarraga, I., Seemann, R. & Fleury, J.-B. Spatiotemporal control of cargo delivery performed by programmable self-propelled Janus droplets. Commun. Phys. 1, 23 (2018).
Haller, B. et al. Autonomous directional motion of actin-containing cell-sized droplets. Adv. Intell. Syst. 3, 2000190 (2021).
Thutupalli, S., Seemann, R. & Herminghaus, S. Swarming behavior of simple model squirmers. New J. Phys. 13, 073021 (2011). A pioneering example of a system in which a chemical reaction occurring at the water–oil interface is used to impart motility to droplets.
Yewdall, N. A., André, A. A. M., Lu, T. & Spruijt, E. Coacervates as models of membraneless organelles. Curr. Opin. Colloid Interface Sci. 52, 101416 (2021).
Zhang, R., Mozaffari, A. & de Pablo, J. J. Autonomous materials systems from active liquid crystals. Nat. Rev. Mater. 6, 437–453 (2021).
Krüger, C., Klös, G., Bahr, C. & Maass, C. C. Curling liquid crystal microswimmers: A cascade of spontaneous symmetry breaking. Phys. Rev. Lett. 117, 048003 (2016). Droplets of nematic liquid crystals follow curved trajectories, even in the absence of chiral molecules.
Jin, C., Kruger, C. & Maass, C. C. Chemotaxis and autochemotaxis of self-propelling droplet swimmers. Proc. Natl Acad. Sci. USA 114, 5089–5094 (2017).
Lancia, F. et al. Reorientation behavior in the helical motility of light-responsive spiral droplets. Nat. Commun. 10, 5238 (2019). The molecular-scale operation of artificial molecular motors was used to impart helical motility to droplets and to reorient their swim under illumination.
Werner, M. & Simmons, L. W. Insect sperm motility. Biol. Rev. 83, 191–208 (2008).
Hyams, J. S. & Borisy, G. G. Isolated flagellar apparatus of Chlamydomonas: characterization of forward swimming and alteration of waveform and reversal of motion by calcium ions in vitro. J. Cell Sci. 33, 235–253 (1978).
Kitzerow, H.-S. & Bahr, C. Chirality in Liquid Crystals (Springer, 2001).
Lancia, F., Ryabchun, A. & Katsonis, N. Life-like motion driven by artificial molecular machines. Nat. Rev. Chem. 3, 536–551 (2019).
Rhutesh, K. S. et al. Designer emulsions using microfluidics. Mater. Today 11, 18–27 (2008).
Engl, W., Backov, R. & Panizza, P. Controlled production of emulsions and particles by milli- and microfluidic techniques. Curr. Opin. Colloid Interface Sci. 13, 206–216 (2008).
Hokmabad, B. V., Baldwin, K. A., Krüger, C., Bahr, C. & Maass, C. C. Topological stabilization and dynamics of self-propelling nematic shells. Phys. Rev. Lett. 123, 178003 (2019).
Meredith, C. H. et al. Predator–prey interactions between droplets driven by non-reciprocal oil exchange. Nat. Chem. 12, 1136–1142 (2020). Predator–prey behaviour emerges in a binary system of motile droplets, with key system parameters being the composition of the oil and the chemical structure of the lipids.
Meredith, C. H. et al. Chemical design of self-propelled Janus droplets. Matter 5, 616–633 (2022).
Morstein, J., Impastato, A. C. & Trauner, D. Photoswitchable lipids. ChemBioChem 22, 73–83 (2021).
Suzuki, K. & Sugawara, T. Phototaxis of oil droplets comprising a caged fatty acid tightly linked to internal convection. ChemPhysChem 1192, 2300–2303 (2016).
Xiao, Y. et al. Photocontrolled directional transport using water-in-oil droplets. New J. Chem. 45, 1172–1175 (2021).
Sakai, Y., Sohn, W. Y. & Katayama, K. Optical motion control of liquid crystalline droplets by host–guest molecular interaction. Soft Matter 15, 7159–7165 (2019).
Xiao, Y. et al. Moving droplets in 3D using light. Adv. Mater. 30, 1801821 (2018).
Russew, M.-M. & Hecht, S. Photoswitches: From molecules to materials. Adv. Mater. 22, 3348–3360 (2010).
Natali, M. & Giordani, S. Molecular switches as photocontrollable “smart” receptors. Chem. Soc. Rev. 41, 4010–4029 (2012).
Kortekaas, L. & Browne, W. R. The evolution of spiropyran: fundamentals and progress of an extraordinarily versatile photochrome. Chem. Soc. Rev. 48, 3406 (2019).
Klajn, R. Spiropyran-based dynamic materials. Chem. Soc. Rev. 43, 148 (2014).
Mahimwalla, Z. et al. Azobenzene photomechanics: prospects and potential applications. Polym. Bull. 69, 967–1006 (2012).
Zhang, D. et al. A phototactic liquid micromotor. J. Mater. Chem. C 6, 12234–12239 (2018).
Wilde, A. & Mullineaux, C. W. Light-controlled motility in prokaryotes and the problem of directional light perception. FEMS Microbiol. Rev. 41, 900–922 (2017).
Santer, S. Remote control of soft nano-objects by light using azobenzene containing surfactants. J. Phys. D Appl. Phys. 51, 013002 (2018).
Diguet, A. et al. Photomanipulation of a droplet by the chromocapillary effect. Angew. Chem. Int. Ed. 48, 9281–9284 (2009).
Cunjing, L., Varanakkottu, S. N., Baier, T. & Hardt, S. Controlling the trajectories of nano/micro particles using light-actuated Marangoni flow. Nano Lett. 18, 6924–6930 (2018).
Feldmann, D. et al. Manipulation of small particles at solid liquid interface: light driven diffusioosmosis. Sci. Rep. 6, 36443 (2016).
Kaneko, S., Asakura, K. & Banno, T. Phototactic behavior of self-propelled micrometer-sized oil droplets in a surfactant solution. Chem. Commun. 53, 2237–2240 (2017).
Ryabchun, A., Babu, D., Movilli, J., Plamont, R. & Katsonis, N. Run-and-halt behavior of motile droplets in response to light. ChemRxiv https://doi.org/10.26434/chemrxiv.14417552.v1 (2021). Run-and-halt and photokinetic behaviour emerge in systems of motile droplets, when micelles of photoactive lipids are used as fuel for the motion.
Hanczyc, M. M., Toyota, T., Ikegami, T., Packard, N. & Sugawara, T. Fatty acid chemistry at the oil–water interface: Self-propelled oil droplets. J. Am. Chem. Soc. 129, 9386–9391 (2007).
Miura, S. et al. pH-induced motion control of self-propelled oil droplets using a hydrolyzable gemini cationic surfactant. Langmuir 30, 7977–7985 (2014).
Menger, F. M. & Keiper, J. S. Gemini surfactants. Angew. Chem. Int. Ed. 39, 1906–1920 (2000).
Toyota, Taro et al. Listeria-like motion of oil droplets. Chem. Lett. 35, 708–709 (2006).
Banno, T., Kuroha, R. & Toyota, T. pH-sensitive self-propelled motion of oil droplets in the presence of cationic surfactants containing hydrolyzable ester linkages. Langmuir 28, 1190–1195 (2012).
Kasuo, Y. et al. Start of micrometer-sized oil droplet motion through generation of surfactants. Langmuir 35, 13351–13355 (2019).
Ban, T., Yamagami, T., Nakata, H. & Okano, Y. pH-dependent motion of self-propelled droplets due to Marangoni effect at neutral pH. Langmuir 29, 2554–2561 (2013).
Banno, T., Tanaka, Y., Asakura, K. & Toyota, T. Self-propelled oil droplets and their morphological change to giant vesicles induced by a surfactant solution at low pH. Langmuir 32, 9591–9597 (2016).
Banno, T. et al. Deformable self-propelled micro-object comprising underwater oil droplets. Sci. Rep. 6, 31292 (2016).
Epstein, I. R. & Showalter, K. Nonlinear chemical dynamics: oscillations, patterns, and chaos. J. Phys. Chem. 100, 13132–13147 (1996).
Babu, D. et al. Acceleration of lipid reproduction by emergence of microscopic motion. Nat. Commun. 12, 2959 (2021). First demonstration that a feedback loop can be established between a chemical reaction (at the nanoscale) and droplet motility (at the macroscale).
Cholakova, D. et al. Rechargeable self-assembled droplet microswimmers driven by surface phase transitions. Nat. Phys. 17, 1050–1055 (2021). As an alternative to inducing Marangoni flows, changes in interfacial tension can also be used to form elastic ‘flagella-like’ filaments and, thus, to set liquid droplets in motion.
Denkov, N., Tcholakova, S., Lesov, I., Cholakova, D. & Smoukov, S. K. Self-shaping of oil droplets via the formation of intermediate rotator phases upon cooling. Nature 528, 392–395 (2015).
Ueno, N. et al. Self-propelled motion of monodisperse underwater oil droplets formed by a microfluidic device. Langmuir 33, 5393–5397 (2017).
Seč, D., Čopar, S. & Žumer, S. Topological zoo of free-standing knots in confined chiral nematic fluids. Nat. Commun. 5, 3057 (2014).
Israelachvili, J. N. & Mitchell, D. J. A model for the packing of lipids in bilayer membranes. Biochim. Biophys. Acta 389, 13–19 (1975).
Stuart, M. C. A. & Boekema, E. J. Two distinct mechanisms of vesicle-to-micelle and micelle-to-vesicle transition are mediated by the packing parameter of phospholipid–detergent systems. Biochim. Biophys. Acta 1768, 2681–2689 (2007).
Howse, J. R. et al. Self-motile colloidal particles: From directed propulsion to random walk. Phys. Rev. Lett. 99, 048102 (2007).
Volpe, G., Buttinoni, I., Vogt, D., Kümmerer, H. J. & Bechinger, C. Microswimmers in patterned environments. Soft Matter 7, 8810–8815 (2011).
Jiang, H. R., Yoshinaga, N. & Sano, M. Active motion of a Janus particle by self-thermophoresis in a defocused laser beam. Phys. Rev. Lett. 105, 268302 (2010).
Golestanian, R., Liverpool, T. B. & Ajdari, A. Designing phoretic micro- and nano-swimmers. New J. Phys. 9, 126 (2007).
Gonzalez-Rodriguez, D. & Lauga, E. Reciprocal locomotion of dense swimmers in Stokes flow. J. Phys. Condens. Matter 21, 204103 (2009).
Lauga, E. Bacterial hydrodynamics. Annu. Rev. Fluid Mech. 48, 105–130 (2016).
Berg, H. C. The rotary motor of bacterial flagellae. Annu. Rev. Biochem. 72, 19–54 (2003).
Chattopadhyay, S., Moldovan, R., Yeung, C. & Wu, X. L. Swimming efficiency of bacterium Escherichia coli. Proc. Natl Acad. Sci. USA 103, 13712–13717 (2006).
Purcell, E. M. Life at low Reynolds number. Am. J. Phys. 45, 3–11 (1977).
Lauga, E. & Powers, T. R. The hydrodynamics of swimming microorganisms. Rep. Prog. Phys. 72, 096601 (2009).
Childress, S. & Dudley, R. Transition from ciliary to flapping mode in a swimming mollusc: Flapping flight as a bifurcation in Reω. J. Fluid Mech. 498, 257–288 (2004).
Hilfinger, A. & Jülicher, F. The chirality of ciliary beats. Phys. Biol. 5, 016003 (2008).
Acknowledgements
N.K. thanks the European Research Council for funding (Consolidator Grant 772564). This work was supported by the Dutch Ministry of Education, Culture and Science (Gravity Program FMS 024.001.035). The authors thank the Animation Studio of the Institute for Complex Molecular Systems and Koen Pieterse (University of Eindhoven) for help with the illustrations.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, contributed substantially to discussion of the content and to the writing of the article. N.K. and D.B. finalized the article text. All authors reviewed and commented on the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Chemistry thanks K. Göpfrich and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Protocells
-
Primitive, abiotic ‘original cells’. In chemistry, refers to chemical assemblies that display minimalistic functions or life-like characteristics, as seen in cells or microorganisms.
- Lipids
-
Substances that are soluble in non-polar organic solvents and are usually insoluble in water. Lipids have possibly aided the emergence of life, because they form compartments in which catalytic networks can be formed. In this manuscript, we focus on lipids that contain a hydrophilic head and a hydrophobic tail.
- Coacervate droplets
-
Membrane-free droplets formed through liquid–liquid phase separation.
- Interfacial tension
-
Interfacial tension is the tendency of liquid interfaces at rest to shrink into the minimum surface area possible. Interfacial tension plays a key role when two non-miscible liquids are involved in a heterogeneous system.
- Advecting
-
Transporting of molecules due to the bulk movement of liquid.
- Reverse micelles
-
Micelles formed in oils with their hydrophilic head aligned inwards and hydrophobic tail outwards, encapsulating water inside the core.
- Liquid crystals
-
Anisotropic molecules that exhibit a liquid crystalline phase in specific conditions of temperature (thermotropic liquid crystals) or dilution (lyotropic liquid crystals). The liquid crystal phase is characterized by fluidity as found in liquids, combined with long-range molecular orientation as found in crystals.
- Nematic liquid crystal
-
A class of liquid crystals in which the molecules exhibit long-range order in molecular orientation but no positional order. The lack of periodic ordering makes them flow and, therefore, they can form droplets.
- Topological defect
-
In liquid crystals, a topological defect is a point where the long-range ordering is absent or disrupted. The liquid crystalline order can be disrupted because of conflicting boundary conditions, in response to external stimuli or by phase transitions.
- Isotropic transition temperature
-
Temperature of phase transition at which nematic liquid crystals transition into the isotropic state.
- Cholesteric liquid crystal
-
A liquid crystal in which the molecules are organized into a helix, because a chiral component is present. Cholesteric liquid crystals are also known as chiral nematic liquid crystals.
- Artificial molecular motors
-
Man-made molecules or molecular systems that perform useful tasks by converting energy into mechanically relevant motion.
- Core–shell droplets
-
A microscopic compartment where one droplet is encapsulated inside another droplet dispersed in a liquid. The inner droplet is called the core and the droplet encapsulating it is called the shell.
- Critical aggregation concentration
-
The concentration of lipids above which aggregates are formed. The aggregate types that form depends on the lipid structure.
- Critical propulsion concentration
-
The minimum concentration of lipids that is required to initiate motility of droplets through solubilization by lipid aggregates. This concentration is larger than the critical aggregation concentration of lipids.
- Belousov–Zhabotinsky (BZ) reaction
-
An oscillating chemical reaction where transition metal ions catalyse the oxidation of organic reductants in an acidic solution. The oscillatory cycles mediate the formation of complex chemical patterns in time and space.
Rights and permissions
About this article
Cite this article
Babu, D., Katsonis, N., Lancia, F. et al. Motile behaviour of droplets in lipid systems. Nat Rev Chem 6, 377–388 (2022). https://doi.org/10.1038/s41570-022-00392-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-022-00392-8