Abstract
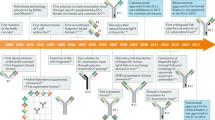
Bispecific antibodies (bsAbs) target two different epitopes. These are an up-and-coming class of biologics, with two such therapeutics (emicizumab and blinatumomab) FDA approved and on the market, and many more in clinical trials. While the first reported bsAbs were constructed by chemical methods, this approach has fallen out of favour with the advent of modern genetic engineering techniques and, nowadays, the vast majority of bsAbs are produced by protein engineering. However, in recent years, relying on innovations in the fields of bioconjugation and bioorthogonal click chemistry, new chemical methods have appeared that have the potential to be competitive with protein engineering techniques and, indeed, hold some advantages. These approaches offer modularity, reproducibility and batch-to-batch consistency, as well as the integration of handles, whereby additional cargo molecules can be attached easily, e.g. to generate bispecific antibody–drug conjugates. The linker between the antibodies/antibody fragments can also be easily varied, and new formats (types, defined by structural properties or by construction methodology) can be generated rapidly. These attributes offer the potential to revolutionize the field. Here, we review chemical methods for the generation of bsAbs, showing that the newest examples of these techniques are worthy competitors to the industry-standard expression-based strategies.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Overland, R. et al. Antitumor activity of a novel bispecific antibody that targets the ErbB2/ErbB3 oncogenic unit and inhibits heregulin-induced activation of ErbB3. Mol. Cancer Ther. 11, 582–593 (2012).
Schuurman, J. et al. A novel bispecific antibody targeting EGFR and cMet is effective against EGFR inhibitor–resistant lung tumors. Cancer Res. 76, 3942–3953 (2016).
Schanzer, J. M. et al. A novel glycoengineered bispecific antibody format for targeted inhibition of epidermal growth factor receptor (EGFR) and insulin-like growth factor receptor type I (IGF-1R) demonstrating unique molecular properties. J. Biol. Chem. 289, 18693–18706 (2014).
Huang, S. et al. Dual targeting of EGFR and HER3 with MEHD7945A overcomes acquired resistance to EGFR inhibitors and radiation. Cancer Res. 73, 824–833 (2012).
Slaga, D. et al. Avidity-based binding to HER2 results in selective killing of HER2-overexpressing cells by anti-HER2/CD3. Sci. Transl. Med. 10, eaat5775 (2018).
Thom, G. et al. Isolation of blood-brain barrier-crossing antibodies from a phage display library by competitive elution and their ability to penetrate the central nervous system. MAbs 10, 304–314 (2017).
Pardridge, W. M. Re-engineering therapeutic antibodies for Alzheimer’s disease as blood-brain barrier penetrating bi-specific antibodies. Expert Opin. Biol. Ther. 16, 1455–1468 (2016).
Andreev, J. et al. Bispecific antibodies and antibody–drug conjugates (ADCs) bridging HER2 and prolactin receptor improve efficacy of HER2 ADCs. Mol. Cancer Ther. 16, 681–693 (2017).
Shima, M. et al. Factor VIII–mimetic function of humanized bispecific antibody in hemophilia A. N. Engl. J. Med. 374, 2044–2053 (2016).
Lee, N.-K. et al. Cell-type specific potent Wnt signaling blockade by bispecific antibody. Sci. Rep. 8, 766 (2018).
Efimov, G. A. et al. Cell-type–restricted anti-cytokine therapy: TNF inhibition from one pathogenic source. Proc. Natl Acad. Sci. USA 113, 3006–3011 (2016).
Rairdan, X. Y. et al. Sustained brown fat stimulation and insulin sensitization by a humanized bispecific antibody agonist for fibroblast growth factor receptor 1/βKlotho complex. EBioMedicine 2, 730–743 (2015).
Kitazawa, T. et al. A bispecific antibody to factors IXa and X restores factor VIII hemostatic activity in a hemophilia A model. Nat. Med. 18, 1570–1574 (2012).
Husain, B. & Ellerman, D. Expanding the boundaries of biotherapeutics with bispecific antibodies. BioDrugs 32, 441–464 (2018).
Blair, H. A. Emicizumab: A review in haemophilia A. Drugs 79, 1697–1707 (2019).
Topp, M. S. et al. Targeted therapy with the T-cell–engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J. Clin. Oncol. 29, 2493–2498 (2011).
Labrijn, A. F., Janmaat, M. L., Reichert, J. M. & Parren, P. W. H. I. Bispecific antibodies: a mechanistic review of the pipeline. Nat. Rev. Drug Discov. 18, 585–608 (2019).
Nisonoff, A. & Rivers, M. M. Recombination antibody of a mixture of univalent fragments of different specificity. Arch. Biochem. Biophys. 93, 460–462 (1961).
Milstein, C. & Cuello, A. C. Hybrid hybridomas and their use in immunohistochemistry. Nature 305, 537–540 (1983).
Kufer, P., Lutterbüse, R. & Baeuerle, P. A. A revival of bispecific antibodies. Trends Biotechnol. 22, 238–244 (2004).
Maruani, A. Bispecifics and antibody–drug conjugates: A positive synergy. Drug Discov. Today: Technol. 30, 55–61 (2018).
Staerz, U. D., Kanagawa, O. & Bevan, M. J. Hybrid antibodies can target sites for attack by T cells. Nature 314, 628–631 (1985).
Brinkmann, U. & Kontermann, R. E. The making of bispecific antibodies. MAbs 9, 182–212 (2017).
Merchant, A. M. et al. An efficient route to human bispecific IgG. Nat. Biotechnol. 16, 677–681 (1998).
Holliger, P., Prospero, T. & Winter, G. “Diabodies”: small bivalent and bispecific antibody fragments. Proc. Natl Acad. Sci. USA 90, 6444–6448 (2006).
Walsh, C. T., Garneau-Tsodikova, S. & Gatto, G. J. Protein posttranslational modifications: the chemistry of proteome diversifications. Angew. Chem. Int. Ed. 44, 7342–7372 (2005).
Badescu, G. et al. A new reagent for stable thiol-specific conjugation. Bioconjugate Chem. 25, 460–469 (2014).
Verma, S. et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 367, 1783–1791 (2012).
Jackson, D. Y. Processes for constructing homogeneous antibody drug conjugates. Org. Process Res. Dev. 20, 852–866 (2016).
Sau, S., Alsaab, H. O., Kashaw, S. K., Tatiparti, K. & Iyer, A. K. Advances in antibody–drug conjugates: a new era of targeted cancer therapy. Drug Discov. Today 22, 1547–1556 (2017).
Chudasama, V., Maruani, A. & Caddick, S. Recent advances in the construction of antibody–drug conjugates. Nat. Chem. 8, 114–119 (2016).
Perez, H. L. et al. Antibody–drug conjugates: current status and future directions. Drug Discov. Today 19, 869–881 (2014).
Lamb, Y. N. Inotuzumab ozogamicin: first global approval. Drugs 77, 1603–1610 (2017).
Godwin, C. D., Gale, R. P. & Walter, R. B. Gemtuzumab ozogamicin in acute myeloid leukemia. Leukemia 31, 1855–1868 (2017).
Moura, A., Savageau, M. A. & Alves, R. Relative amino acid composition signatures of organisms and environments. PLoS ONE 8, e77319 (2013).
van de Donk, N. W. C. J. & Dhimolea, E. Brentuximab vedotin. MAbs 4, 458–465 (2012).
Challita-Eid, P. M. et al. Enfortumab vedotin antibody–drug conjugate targeting nectin-4 is a highly potent therapeutic agent in multiple preclinical cancer models. Cancer Res. 76, 3003–3013 (2016).
Nakada, T. et al. Novel antibody drug conjugates containing exatecan derivative-based cytotoxic payloads. Bioorg. Med. Chem. Lett. 26, 1542–1545 (2016).
Polson, A. G. et al. Antibody-drug conjugates targeted to CD79 for the treatment of non-Hodgkin lymphoma. Blood 110, 616–623 (2007).
Brotzel, F. & Mayr, H. Nucleophilicities of amino acids and peptides. Org. Biomol. Chem. 5, 3814–3820 (2007).
Moon, S. et al. Antibody conjugates of 7-ethyl-10-hydroxycamptothecin (SN-38) for targeted cancer chemotherapy. J. Med. Chem. 51, 6916–6926 (2009).
Cardillo, T. M., Govindan, S. V., Sharkey, R. M., Trisal, P. & Goldenberg, D. M. Humanized anti-trop-2 IgG-SN-38 conjugate for effective treatment of diverse epithelial cancers: preclinical studies in human cancer xenograft models and monkeys. Clin. Cancer Res. 17, 3157–3169 (2011).
Ogitani, Y. et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin. Cancer Res. 22, 5097–5108 (2016).
LoRusso, P. M., Weiss, D., Guardino, E., Girish, S. & Sliwkowski, M. X. Trastuzumab emtansine: a unique antibody-drug conjugate in development for human epidermal growth factor receptor 2–positive cancer. Clin. Cancer Res. 17, 6437–6447 (2011).
Shen, B. Q. et al. Conjugation site modulates the in vivo stability and therapeutic activity of antibody-drug conjugates. Nat. Biotechnol. 30, 184–189 (2012).
Alley, S. C. et al. Contribution of linker stability to the activities of anticancer immunoconjugates. Bioconjugate Chem. 19, 759–765 (2008).
Baldwin, A. D. & Kiick, K. L. Tunable degradation of maleimide–thiol adducts in reducing environments. Bioconjugate Chem. 22, 1946–1953 (2011).
Badescu, G. et al. Bridging disulfides for stable and defined antibody drug conjugates. Bioconjugate Chem. 25, 1124–1136 (2014).
Bernardes, G. J. L., Steiner, M., Hartmann, I., Neri, D. & Casi, G. Site-specific chemical modification of antibody fragments using traceless cleavable linkers. Nat. Protoc. 8, 2079–2089 (2013).
Toda, N., Asano, S. & Barbas, C. F. Rapid, stable, chemoselective labeling of thiols with Julia–Kocieński-like reagents: a serum-stable alternative to maleimide-based protein conjugation. Angew. Chem. Int. Ed. 52, 12592–12596 (2013).
Koniev, O. et al. Selective irreversible chemical tagging of cysteine with 3-arylpropiolonitriles. Bioconjugate Chem. 25, 202–206 (2014).
Smith, M. E. B. et al. A platform for efficient, thiol-stable conjugation to albumin’s native single accessible cysteine. Org. Biomol. Chem. 13, 7946–7949 (2015).
Kalia, D., Malekar, P. V. & Parthasarathy, M. Exocyclic olefinic maleimides: synthesis and application for stable and thiol-selective bioconjugation. Angew. Chem. Int. Ed. 55, 1432–1435 (2016).
Zhang, Y. et al. Cysteine-specific protein multi-functionalization and disulfide bridging using 3-bromo-5-methylene pyrrolones. Nat. Commun. 11, 1015 (2020).
Tumey, L. N. et al. Mild method for succinimide hydrolysis on ADCs: impact on ADC potency, stability, exposure, and efficacy. Bioconjugate Chem. 25, 1871–1880 (2014).
Lyon, R. P. et al. Self-hydrolyzing maleimides improve the stability and pharmacological properties of antibody-drug conjugates. Nat. Biotechnol. 32, 1059–1062 (2014).
Fontaine, S. D., Reid, R., Robinson, L., Ashley, G. W. & Santi, D. V. Long-term stabilization of maleimide–thiol conjugates. Bioconjugate Chem. 26, 145–152 (2015).
Christie, R. J. et al. Stabilization of cysteine-linked antibody drug conjugates with N-aryl maleimides. J. Control. Rel. 220, 660–670 (2015).
Kalia, D., Pawar, S. P. & Thopate, J. S. Stable and rapid thiol bioconjugation by light-triggered thiomaleimide ring hydrolysis. Angew. Chem. Int. Ed. 56, 1885–1889 (2017).
Ponte, J. F. et al. Understanding how the stability of the thiol-maleimide linkage impacts the pharmacokinetics of lysine-linked antibody–maytansinoid conjugates. Bioconjugate Chem. 27, 1588–1598 (2016).
Dovgan, I., Kolodych, S., Koniev, O. & Wagner, A. 2-(Maleimidomethyl)-1,3-dioxanes (MD): A serum-stable self-hydrolysable hydrophilic alternative to classical maleimide conjugation. Sci. Rep. 6, 30835 (2016).
Tobaldi, E., Dovgan, I., Mosser, M., Becht, J. M. & Wagner, A. Structural investigation of cyclo-dioxo maleimide cross-linkers for acid and serum stability. Org. Biomol. Chem. 15, 9305–9310 (2017).
Szijj, P. A., Bahou, C. & Chudasama, V. Minireview: Addressing the retro-Michael instability of maleimide bioconjugates. Drug Discov. Today Technol. 30, 27–34 (2018).
Forte, N. et al. Tuning the hydrolytic stability of next generation maleimide cross-linkers enables access to albumin-antibody fragment conjugates and tri-scFvs. Bioconjugate Chem. 29, 486–492 (2018).
Morais, M. et al. Optimisation of the dibromomaleimide (DBM) platform for native antibody conjugation by accelerated post-conjugation hydrolysis. Org. Biomol. Chem. 15, 2947–2952 (2017).
Lee, M. T. W., Maruani, A. & Chudasama, V. The use of 3,6-pyridazinediones in organic synthesis and chemical biology. J. Chem. Res. 40, 1–9 (2016).
Bahou, C. et al. Highly homogeneous antibody modification through optimisation of the synthesis and conjugation of functionalised dibromopyridazinediones. Org. Biomol. Chem. 16, 1359–1366 (2018).
Robinson, E. et al. Pyridazinediones deliver potent, stable, targeted and efficacious antibody–drug conjugates (ADCs) with a controlled loading of 4 drugs per antibody. RSC Adv. 7, 9073–9077 (2017).
Lee, M. T. W., Maruani, A., Baker, J. R., Caddick, S. & Chudasama, V. Next-generation disulfide stapling: reduction and functional re-bridging all in one. Chem. Sci. 7, 799–802 (2016).
Maruani, A. et al. A mild TCEP-based para-azidobenzyl cleavage strategy to transform reversible cysteine thiol labelling reagents into irreversible conjugates. Chem. Commun. 51, 5279–5282 (2015).
Brennan, M., Davison, P. F. & Paulus, H. Preparation of bispecific antibodies by chemical recombination of monoclonal immunoglobulin G1 fragments. Science 229, 81–83 (1985).
Glennie, M. J., McBride, H. M., Worth, A. T. & Stevenson, G. T. Preparation and performance of bispecific F(ab′ gamma)2 antibody containing thioether-linked Fab′ gamma fragments. J. Immunol. 139, 2367–2375 (1987).
Shalaby, M. R. Development of humanized bispecific antibodies reactive with cytotoxic lymphocytes and tumor cells overexpressing the HER2 protooncogene. J. Exp. Med. 175, 217–225 (2004).
Patke, S. et al. bisFabs: Tools for rapidly screening hybridoma IgGs for their activities as bispecific antibodies. MAbs 9, 430–437 (2017).
Segal, D. M. & Bast, B. J. E. G. Production of bispecific antibodies. Curr. Protoc. Immunol. 14, 2.13.1–2.13.16 (1995).
Reusch, U. et al. Anti-CD3 × anti-epidermal growth factor receptor (EGFR) bispecific antibody redirects T-cell cytolytic activity to EGFR-positive cancers in vitro and in an animal model. Clin. Cancer Res. 12, 183–190 (2006).
Lee, R. J. et al. Antibody targeting of stem cells to infarcted myocardium. Stem Cell 25, 712–717 (2007).
Barbas, C. F. III et al. Immune versus natural selection: antibody aldolases with enzymic rates but broader scope. Science 278, 2085–2092 (1997).
Rader, C., Lerner, R. A., Barbas, C. F., Popkov, M. & Sinha, S. C. Chemically programmed monoclonal antibodies for cancer therapy: adaptor immunotherapy based on a covalent antibody catalyst. Proc. Natl Acad. Sci. USA 100, 5396–5400 (2003).
Gavrilyuk, J. I. et al. An efficient chemical approach to bispecific antibodies and antibodies of high valency. Bioorg. Med. Chem. Lett. 19, 3716–3720 (2009).
Walseng, E. et al. Chemically programmed bispecific antibodies in diabody format. J. Biol. Chem. 291, 19661–19673 (2016).
Doppalapudi, V. R. et al. Chemical generation of bispecific antibodies. Proc. Natl Acad. Sci. USA 107, 22611–22616 (2010).
Junutula, J. R. et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat. Biotechnol. 26, 925–932 (2008).
Scheer, J. M. et al. Reorienting the Fab domains of trastuzumab results in potent HER2 activators. PLoS ONE 7, 51817 (2012).
Bundy, B. C. & Swartz, J. R. Site-specific incorporation of p-propargyloxyphenylalanine in a cell-free environment for direct protein–protein click conjugation. Bioconjugate Chem. 21, 255–263 (2010).
Kim, C. H. et al. Synthesis of bispecific antibodies using genetically encoded unnatural amino acids. J. Am. Chem. Soc. 134, 9918–9921 (2012).
Cao, Y. et al. Multiformat T-cell-engaging bispecific antibodies targeting human breast cancers. Angew. Chem. Int. Ed. 54, 7022–7027 (2015).
Kazane, S. A. et al. Self-assembled antibody multimers through peptide nucleic acid conjugation. J. Am. Chem. Soc. 135, 340–346 (2013).
Wagner, K. et al. Bispecific antibody generated with sortase and click chemistry has broad antiinfluenza virus activity. Proc. Natl Acad. Sci. USA 111, 16820–16825 (2014).
Bartels, L., Ploegh, H. L., Spits, H. & Wagner, K. Preparation of bispecific antibody-protein adducts by site-specific chemo-enzymatic conjugation. Methods 154, 93–101 (2019).
Baalmann, M. et al. A bioorthogonal click chemistry toolbox for targeted synthesis of branched and well-defined protein–protein conjugates. Angew. Chem. Int. Ed. 59, 12885–12893 (2020).
Maruani, A. et al. A plug-and-play approach for the de novo generation of dually functionalized bispecifics. Bioconjugate Chem. 31, 520–529 (2020).
Khalili, H. et al. Fab-PEG-Fab as a potential antibody mimetic. Bioconjugate Chem. 24, 1870–1882 (2013).
Hull, E. A. et al. Homogeneous bispecifics by disulfide bridging. Bioconjugate Chem. 25, 1395–1401 (2014).
Patterson, J. T. et al. PSMA-targeted bispecific Fab conjugates that engage T cells. Bioorg. Med. Chem. Lett. 27, 5490–5495 (2017).
Patterson, J. T. et al. Chemically generated IgG2 bispecific antibodies through disulfide bridging. Bioorg. Med. Chem. Lett. 27, 3647–3652 (2017).
McCafferty, J., Griffiths, A. D., Winter, G. & Chiswellt, D. J. Phage antibodies: filamentous phage displaying antibody variable domains. Nature 348, 552–554 (1990).
Autio, K. A., Boni, V., Humphrey, R. W. & Naing, A. Probody therapeutics: an emerging class of therapies designed to enhance on-target effects with reduced off-tumor toxicity for use in immuno-oncology. Clin. Cancer Res. 26, 984–989 (2020).
Bahou, C. et al. Disulfide modified IgG1: an investigation of biophysical profile and clinically relevant Fc interactions. Bioconjugate Chem. 30, 1048–1054 (2019).
Acknowledgements
P.S. would like to thank the Wellcome Trust for their generous funding.
Author information
Authors and Affiliations
Contributions
P.S. and V.C. co-wrote the Review and co-analysed the literature.
Corresponding author
Ethics declarations
Competing interests
V.C. is a co-founder and director of the company ThioLogics. P.S. has no competing interests to declare.
Additional information
Peer review information
Nature Reviews Chemistry thanks D. Neri, T. Pillow and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Szijj, P., Chudasama, V. The renaissance of chemically generated bispecific antibodies. Nat Rev Chem 5, 78–92 (2021). https://doi.org/10.1038/s41570-020-00241-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-020-00241-6
This article is cited by
-
SpyMask enables combinatorial assembly of bispecific binders
Nature Communications (2024)
-
Chemical generation of checkpoint inhibitory T cell engagers for the treatment of cancer
Nature Chemistry (2023)
-
Antibody–drug conjugates and bispecific antibodies targeting cancers: applications of click chemistry
Archives of Pharmacal Research (2023)
-
Optical image-guided therapy of pancreatic cancer with an ultra-small bispecific protein
European Journal of Nuclear Medicine and Molecular Imaging (2023)
-
Nanotechnology-based strategies against SARS-CoV-2 variants
Nature Nanotechnology (2022)