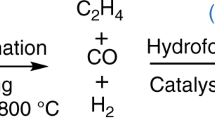
Abstract
A greenhouse gas and mild oxidant, CO2 can effect the oxidative dehydrogenation (CO2-ODH) of light alkanes over heterogeneous catalysts. These catalysts are bifunctional in that they mediate CO2 reduction while oxidizing the alkanes, most notably the C2–C4 components in shale gas. In this way, one obtains CO and alkenes as value-added products. Although desirable, this transformation has proven challenging in terms of catalyst design, with most catalysts for the CO2-ODH being metal oxides that typically undergo rapid deactivation. More recently, bimetallic catalysts have been identified as promising systems to activate alkanes by either selectively cleaving C–H bonds to produce alkenes or breaking all the C–C and C–H bonds to produce the dry reforming products CO and H2. This Review describes general trends in the CO2-ODH of light alkanes. We will also outline how to use a combined approach involving flow reactor experiments, in operando characterization and density functional theory to determine whether a catalyst is intrinsically active for CO2-ODH or dry reforming.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Speight, J. G. in Handbook of Alternative Fuel Technologies 2nd edn (eds. Lee, S., Speight, J. G. & Loyalka, S. K.) 162 (Taylor and Francis Group, 2015).
Tang, P., Zhu, Q., Wu, Z. & Ma, D. Methane activation: the past and future. Energy Environ. Sci. 7, 2580–2591 (2014).
Kawi, S. & Kathiraser, Y. CO2 as an oxidant for high-temperature reactions. Front. Energy Res. 3, 13 (2015). A review of catalysts for high-temperature CO 2 reactions, including the CO 2 -ODH of light alkanes, the conversion of ethylbenzene into styrene and CO 2 reforming of CH 4 and alcohols.
Plotkin, J. S. The propylene gap: how can it be filled? ACS News Industrial Chemistry and Engineering https://www.acs.org/content/acs/en/pressroom/cutting-edge-chemistry/the-propylene-gap-how-can-it-be-filled.html (2015).
Tian, P., Wei, Y., Ye, M. & Liu, Z. Methanol to olefins (MTO): from fundamentals to commercialization. ACS Catal. 5, 1922–1938 (2015).
Li, Y. et al. Hierarchical SAPO-34/18 zeolite with low acid site density for converting methanol to olefins. Catal. Today 233, 2–7 (2014).
Amghizar, I., Vandewalle, L. A., Geem, K. M. Van & Marin, G. B. New trends in olefin production. Engineering 3, 171–178 (2017).
Zhai, P. et al. Highly tunable selectivity for syngas-derived alkenes over zinc and sodium-modulated Fe5C2 catalyst. Angew. Chem. Int. Ed. 55, 9902–9907 (2016).
IHS Markit. Propylene, Ethylene, Butadiene — Chemical Economics Handbook. (2018).
Hasanbeigi, A. Chemical industry’s energy use and emissions. Global Efficiency Intelligence https://www.globalefficiencyintel.com/new-blog/2018/chemical-industrys-energy-use-emissions (2018).
U.S. Energy Information Administration. What is the volume of world natural gas reserves?. https://www.eia.gov/tools/faqs/faq.php?id=52&t=8 (2019).
Mukherjee, D., Park, S.-E. & Reddy, B. M. CO2 as a soft oxidant for oxidative dehydrogenation reaction: an eco benign process for industry. J. CO 2 Util. 16, 301–312 (2016). This review describes the advantages and challenges of using CO 2 as a soft oxidant, with a focus on CO 2 activation and factors such as promoters, surface acid–base residues, redox activity and lattice oxygen, and the influence of supports.
Aresta, M., Dibenedetto, A. & Quaranta, E. State of the art and perspectives in catalytic processes for CO2 conversion into chemicals and fuels: the distinctive contribution of chemical catalysis and biotechnology. J. Catal. 343, 2–45 (2016).
Álvarez, A. et al. CO2 activation over catalytic surfaces. ChemPhysChem 18, 3135–3141 (2017).
van Santen, R. A., Tranca, I. & Hensen, E. J. M. Theory of surface chemistry and reactivity of reducible oxides. Catal. Today 244, 63–84 (2015).
Xu, B., Zheng, B., Hua, W., Yue, Y. & Gao, Z. Support effect in dehydrogenation of propane in the presence of CO2 over supported gallium oxide catalysts. J. Catal. 239, 470–477 (2006).
Osaki, T. & Mori, T. Kinetics of the reverse-Boudouard reaction over supported nickel catalysts. React. Kinet. Catal. Lett. 89, 333–339 (2006).
Wang, S. & Zhu, Z. H. Catalytic conversion of alkanes to olefins by carbon dioxide oxidative dehydrogenation — a review. Energy Fuels 18, 1126–1139 (2004). A useful review of catalysts to utilize CO 2 to accept H 2 from light alkanes, CH 4 or ethylbenzene. At the time, these studies mainly consisted of activity trends and ex situ characterization.
Gärtner, C. A., van Veen, A. C. & Lercher, J. A. Oxidative dehydrogenation of ethane: common principles and mechanistic aspects. ChemCatChem 5, 3196–3217 (2013).
Védrine, J. C. Heterogeneous partial (amm)oxidation and oxidative dehydrogenation catalysis on mixed metal oxides. Catalysts 6, 22 (2016).
Grabowski, R. Kinetics of oxidative dehydrogenation of C2–C3 alkanes on oxide catalysts. Catal. Rev. 48, 199–268 (2006).
Cavani, F. & Trifirò, F. The oxidative dehydrogenation of ethane and propane as an alternative way for the production of light olefins. Catal. Today 24, 307–313 (1995).
Ansari, M. B. & Park, S.-E. Carbon dioxide utilization as a soft oxidant and promoter in catalysis. Energy Environ. Sci. 5, 9419–9437 (2012). A useful background on CO 2 activation, describing catalyst properties for the CO 2 -ODH reaction and other chemistries for which CO 2 may act as a soft oxidant, including ethylbenzene to styrene.
Atanga, M. A., Rezaei, F., Jawad, A., Fitch, M. & Rownaghi, A. A. Oxidative dehydrogenation of propane to propylene with carbon dioxide. Appl. Catal. B Environ. 220, 429–445 (2018). This review focuses on the CO 2 -ODH of propane over zeolite and metal-oxide catalysts, and suggests that future work should be geared towards catalyst design platforms that address the operating-temperature constraints for CO 2 -ODH.
Du, X. et al. Catalytic dehydrogenation of propane by carbon dioxide: a medium-temperature thermochemical process for carbon dioxide utilisation. Faraday Discuss. 183, 161–176 (2015).
Zangeneh, F. T., Taeb, A., Gholivand, K. & Sahebdelfar, S. Thermodynamic equilibrium analysis of propane dehydrogenation with carbon dioxide and side reactions. Chem. Eng. Commun. 203, 557–565 (2016).
Michorczyk, P., Zéczak, K., Niekurzak, R. & Ogonowski, J. Dehydrogenation of propane with CO2 — a new green process for propene and synthesis gas production. Polish J. Chem. Technol. 14, 77–82 (2012).
Krylov, O. V., Mamedov, A. Kh & Mirzabekova, S. R. The regularities in the interaction of alkanes with CO2 on oxide catalysts. Catal. Today 24, 371–375 (1995).
Mimura, N., Takahara, I., Inaba, M., Okamoto, M. & Murata, K. High-performance Cr/H-ZSM-5 catalysts for oxidative dehydrogenation of ethane to ethylene with CO2 as an oxidant. Catal. Commun. 3, 257–262 (2002).
Mimura, N., Okamoto, M., Yamashita, H., Oyama, S. T. & Murata, K. Oxidative dehydrogenation of ethane over Cr/ZSM-5 catalysts using CO2 as an oxidant. J. Phys. Chem. B 110, 21764–21770 (2006).
Deng, S., Li, H., Li, S. & Zhang, Y. Activity and characterization of modified Cr2O3/ZrO2 nano-composite catalysts for oxidative dehydrogenation of ethane to ethylene with CO2. J. Mol. Catal. A Chem. 268, 169–175 (2007).
Raju, G., Reddy, B. M. & Park, S.-E. Utilization of carbon dioxide in oxidative dehydrogenation reactions. Indian J. Chem. Sect. A 51, 1315–1324 (2012).
Wei, C. et al. Dehydrogenation of isobutane with carbon dioxide over SBA-15-supported vanadium oxide catalysts. Catalysts 6, 171 (2016).
Sun, G. et al. Vanadium oxide supported on MSU-1 as a highly active catalyst for dehydrogenation of isobutane with CO2. Catalysts 6, 41 (2016).
Shen, Z. et al. Dehydrogenation of ethane to ethylene over a highly efficient Ga2O3/HZSM-5 catalyst in the presence of CO2. Appl. Catal. A General 356, 148–153 (2009).
Koirala, R., Buechel, R., Krumeich, F., Pratsinis, S. E. & Baiker, A. Oxidative dehydrogenation of ethane with CO2 over flame-made Ga-loaded TiO2. ACS Catal. 5, 690–702 (2015).
Chen, M. et al. Dehydrogenation of propane over In2O3–Al2O3 mixed oxide in the presence of carbon dioxide. J. Catal. 272, 101–108 (2010).
Chen, M. et al. Study in support effect of In2O3/MOx (M=Al, Si, Zr) catalysts for dehydrogenation of propane in the presence of CO2. Appl. Catal. A General 407, 20–28 (2011).
Zhang, X., Ye, Q., Xu, B. & He, D. Oxidative dehydrogenation of ethane over Co–BaCO3 catalysts using CO2 as oxidant: effects of Co promoter. Catal. Lett. 117, 140–145 (2007).
Koirala, R., Buechel, R., Pratsinis, S. E. & Baiker, A. Silica is preferred over various single and mixed oxides as support for CO2-assisted cobalt-catalyzed oxidative dehydrogenation of ethane. Appl. Catal. A General 527, 96–108 (2016).
Zhu, J. et al. Na2WO4/Mn/SiO2 catalyst for oxidative dehydrogenation of ethane using CO2 as oxidant. Catal. Today 148, 310–315 (2009).
Baidya, T., van Vegten, N. & Baiker, A. Selective conversion of ethane to ethene via oxidative dehydrogenation over Ca-doped ThO2 using CO2 as oxidant. Top. Catal. 54, 881–887 (2011).
Valenzuela, R. X., Bueno, G., Cortés Corberán, V., Xu, Y. & Chen, C. Selective oxidehydrogenation of ethane with CO2 over CeO2-based catalysts. Catal. Today 61, 43–48 (2000).
Talati, A., Haghighi, M. & Rahmani, F. Oxidative dehydrogenation of ethane to ethylene by carbon dioxide over Cr/TiO2–ZrO2 nanocatalyst: effect of active phase and support composition on catalytic properties and performance. Adv. Powder Technol. 27, 1195–1206 (2016).
Ramesh, Y. et al. Oxidative dehydrogenation of ethane to ethylene on Cr2O3/Al2O3–ZrO2 catalysts: the influence of oxidizing agent on ethylene selectivity. Appl. Petrochem. Res. 4, 247–252 (2014).
Bugrova, T. A., Dutov, V. V., Svetlichnyi, V. A., Cortés Corberán, V. & Mamontov, G. V. Oxidative dehydrogenation of ethane with CO2 over CrOx catalysts supported on Al2O3, ZrO2, CeO2 and CexZr1–xO2. Catal. Today 333, 71–80 (2018).
Michorczyk, P., Ogonowski, J. & Gajek, T. Dehydrogenation of light alkanes over mesoporous-siliceous supported chromium, vanadium, gallium and iron oxides in presence of carbon dioxide. Przem. Chem. 91, 84–88 (2012).
Zhao, X. & Wang, X. Oxidative dehydrogenation of ethane to ethylene by carbon dioxide over Cr/TS-1 catalysts. Catal. Commun. 7, 633–638 (2006).
Aresta, M., Dibenedetto, A. & Quaranta, E. Reaction Mechanisms in Carbon Dioxide Conversion (Springer, 2016).
Kocoń, M., Michorczyk, P. & Ogonowski, J. Effect of supports on catalytic activity of chromium oxide-based catalysts in the dehydrogenation of propane with CO2. Catal. Lett. 101, 53–57 (2005).
Wang, S., Murata, K., Hayakawa, T., Hamakawa, S. & Suzuki, K. Dehydrogenation of ethane with carbon dioxide over supported chromium oxide catalysts. Appl. Catal. A General 196, 1–8 (2000).
Jin, L. et al. Studies on dehydrogenation of ethane in the presence of CO2 over octahedral molecular sieve (OMS-2) catalysts. ChemCatChem 1, 441–444 (2009).
Shishido, T., Shimamura, K., Teramura, K. & Tanaka, T. Role of CO2 in dehydrogenation of propane over Cr-based catalysts. Catal. Today 185, 151–156 (2012).
Liu, L., Li, H. & Zhang, Y. A comparative study on catalytic performances of chromium incorporated and supported mesoporous MSU-x catalysts for the oxidehydrogenation of ethane to ethylene with carbon dioxide. Catal. Today 115, 235–241 (2006).
Takehira, K. et al. Behavior of active sites on Cr-MCM-41 catalysts during the dehydrogenation of propane with CO2. J. Catal. 224, 404–416 (2004).
Liu, L., Li, H. & Zhang, Y. Mesoporous silica-supported chromium catalyst: characterization and excellent performance in dehydrogenation of propane to propylene with carbon dioxide. Catal. Commun. 8, 565–570 (2007).
Takahara, I. & Saito, M. Promoting effects of carbon dioxide on dehydrogenation of propane over a SiO2-supported Cr2O3 catalyst. Chem. Lett. 25, 973–974 (1998).
Baek, J., Yun, H. J., Yun, D., Choi, Y. & Yi, J. Preparation of highly dispersed chromium oxide catalysts supported on mesoporous silica for the oxidative dehydrogenation of propane using CO2: insight into the nature of catalytically active chromium sites. ACS Catal. 2, 1893–1903 (2012).
Nakagawa, K., Okamura, M., Ikenaga, N., Suzuki, T. & Kobayashi, T. Dehydrogenation of ethane over gallium oxide in the presence of carbon dioxide. Chem. Commun. 9, 1025–1026 (1998).
Zhang, F. et al. Chromium oxide supported on ZSM-5 as a novel efficient catalyst for dehydrogenation of propane with CO2. Microporous Mesoporous Mater. 145, 194–199 (2011).
Asghari, E., Haghighi, M. & Rahmani, F. CO2-oxidative dehydrogenation of ethane to ethylene over Cr/MCM-41 nanocatalyst synthesized via hydrothermal/impregnation methods: influence of chromium content on catalytic properties and performance. J. Mol. Catal. A Chem. 418–419, 115–124 (2016).
Botavina, M. A. et al. Oxidative dehydrogenation of C3–C4 paraffins in the presence of CO2 over CrOx/SiO2 catalysts. Appl. Catal. A Gen. 347, 126–132 (2008).
Deng, S., Li, S., Li, H. & Zhang, Y. Oxidative dehydrogenation of ethane to ethylene with CO2 over Fe–Cr/ZrO2 catalysts. Ind. Eng. Chem. Res. 48, 7561–7566 (2009).
Yun, D. et al. Promotional effect of Ni on a CrOx catalyst supported on silica in the oxidative dehydrogenation of propane with CO2. ChemCatChem 4, 1952–1959 (2012).
Ajayi, B. P., Rabindran Jermy, B., Abussaud, B. A. & Al-Khattaf, S. Oxidative dehydrogenation of n-butane over bimetallic mesoporous and microporous zeolites with CO2 as mild oxidant. J. Porous Mater. 20, 1257–1270 (2013).
Ascoop, I. et al. The role of CO2 in the dehydrogenation of propane over WOx–VOx/SiO2. J. Catal. 335, 1–10 (2016).
Cavani, F., Ballarini, N. & Cericola, A. Oxidative dehydrogenation of ethane and propane: how far from commercial implementation? Catal. Today 127, 113–131 (2007).
Chen, K., Bell, A. T. & Iglesia, E. The relationship between the electronic and redox properties of dispersed metal oxides and their turnover rates in oxidative dehydrogenation reactions. J. Catal. 209, 35–42 (2002).
Raju, G., Reddy, B. M. & Park, S.-E. CO2 promoted oxidative dehydrogenation of n-butane over VOx/MO2–ZrO2 (M=Ce or Ti) catalysts. J. CO 2 Util. 5, 41–46 (2014).
Raju, G., Reddy, B. M., Abhishek, B., Mo, Y.-H. & Park, S.-E. Synthesis of C4 olefins from n-butane over a novel VOx/SnO2–ZrO2 catalyst using CO2 as soft oxidant. Appl. Catal. A General 423–424, 168–175 (2012).
Wang, X. et al. Synthesis of V-MCM-41 catalysts and their application in CO2-assisted isobutane dehydrogenation. Chem. Eng. Technol. 41, 563–572 (2018).
Han, Z.-F., Xue, X.-L., Wu, J.-M., Lang, W.-Z. & Guo, Y.-J. Preparation and catalytic properties of mesoporous nV-MCM-41 for propane oxidative dehydrogenation in the presence of CO2. Chin. J. Catal. 39, 1099–1109 (2018).
Taghavinezhad, P., Haghighi, M. & Alizadeh, R. CO2/O2-oxidative dehydrogenation of ethane to ethylene over highly dispersed vanadium oxide on MgO-promoted sulfated-zirconia nanocatalyst: effect of sulfation on catalytic properties and performance. Korean J. Chem. Eng. 34, 1346–1357 (2017).
Rozanska, X., Fortrie, R. & Sauer, J. Size-dependent catalytic activity of supported vanadium oxide species: oxidative dehydrogenation of propane. J. Am. Chem. Soc. 136, 7751–7761 (2014).
Xue, X.-L., Lang, W.-Z., Yan, X. & Guo, Y.-J. Dispersed vanadium in three-dimensional dendritic mesoporous silica nanospheres: active and stable catalysts for the oxidative dehydrogenation of propane in the presence of CO2. ACS Appl. Mater. Interfaces 9, 15408–15423 (2017).
Ye, J., Liu, C. & Ge, Q. DFT study of CO2 adsorption and hydrogenation on the In2O3 surface. J. Phys. Chem. C 116, 7817–7825 (2012).
Chen, M. et al. Supported indium oxide as novel efficient catalysts for dehydrogenation of propane with carbon dioxide. Appl. Catal. A General 377, 35–41 (2010).
Wang, W. et al. Reverse water gas shift over In2O3–CeO2 catalysts. Catal. Today 259, 402–408 (2016).
Pan, Y.-x., Liu, C.-j., Mei, D. & Ge, Q. Effects of hydration and oxygen vacancy on CO2 adsorption and activation on β-Ga2O3(100). Langmuir 26, 5551–5558 (2010).
Liu, Y., Li, Z. H., Lu, J. & Fan, K.-N. Periodic density functional theory study of propane dehydrogenation over perfect Ga2O3(100) surface. J. Phys. Chem. C 112, 20382–20392 (2008).
Michorczyk, P. & Ogonowski, J. Dehydrogenation of propane in the presence of carbon dioxide over oxide-based catalysts. React. Kinet. Catal. Lett. 78, 41–47 (2003).
Chen, J. G. Carbide and nitride overlayers on early transition metal surfaces: preparation, characterization, and reactivities. Chem. Rev. 96, 1477–1498 (1996).
Yu, W., Porosoff, M. D. & Chen, J. G. Review of Pt-based bimetallic catalysis: from model surfaces to supported catalysts. Chem. Rev. 112, 5780–5817 (2012).
Porosoff, M. D., Yu, W. & Chen, J. G. Challenges and opportunities in correlating bimetallic model surfaces and supported catalysts. J. Catal. 308, 2–10 (2013).
Porosoff, M. D., Yan, B. & Chen, J. G. Catalytic reduction of CO2 by H2 for synthesis of CO, methanol and hydrocarbons: challenges and opportunities. Energy Environ. Sci. 9, 62–73 (2016).
Trovarelli, A. Catalytic properties of ceria and CeO2-containing materials. Catal. Rev. 38, 439–520 (1996).
Cheng, Z., Sherman, B. J. & Lo, C. S. Carbon dioxide activation and dissociation on ceria (110): a density functional theory study. J. Chem. Phys. 138, 014702 (2013).
Appel, L. G., Eon, J. G. & Schmal, M. The CO2–CeO2 interaction and its role in the CeO2 reactivity. Catal. Lett. 56, 199–202 (1998).
Chueh, W. C. & Haile, S. M. A thermochemical study of ceria: exploiting an old material for new modes of energy conversion and CO2 mitigation. Philos. Trans. R. Soc. A 368, 3269–3294 (2010).
Bueno-López, A., Krishna, K. & Makkee, M. Oxygen exchange mechanism between isotopic CO2 and Pt/CeO2. Appl. Catal. A General 342, 144–149 (2008).
Demoulin, O., Navez, M., Mugabo, J.-L. & Ruiz, P. The oxidizing role of CO2 at mild temperature on ceria-based catalysts. Appl. Catal. B Environ. 70, 284–293 (2007).
Myint, M. N. Z., Yan, B., Wan, J., Zhao, S. & Chen, J. G. Reforming and oxidative dehydrogenation of ethane with CO2 as a soft oxidant over bimetallic catalysts. J. Catal. 343, 168–177 (2016).
Yan, B. et al. Active sites for tandem reactions of CO2 reduction and ethane dehydrogenation. Proc. Natl Acad. Sci. USA 115, 8278–8283 (2018).
Solymosi, F. & Németh, R. The oxidative dehydrogenation of ethane with CO2 over Mo2C/SiO2 catalyst. Catal. Lett. 62, 197–200 (1999).
Solymosi, F., Németh, R., Óvári, L. & Egri, L. Reactions of propane on supported Mo2C catalysts. J. Catal. 195, 316–325 (2000).
Neylon, M. K., Choi, S., Kwon, H., Curry, K. E. & Thompson, L. T. Catalytic properties of early transition metal nitrides and carbides: n-butane hydrogenolysis, dehydrogenation and isomerization. Appl. Catal. A General 183, 253–263 (1999).
Porosoff, M. D., Yang, X., Boscoboinik, J. A. & Chen, J. G. Molybdenum carbide as alternative catalysts to precious metals for highly selective reduction of CO2 to CO. Angew. Chem. Int. Ed. 53, 6705–6709 (2014).
Zhang, X. et al. Highly dispersed copper over β-Mo2C as an efficient and stable catalyst for the reverse water gas shift (RWGS) reaction. ACS Catal. 7, 912–918 (2017).
Porosoff, M. D. et al. Identifying different types of catalysts for CO2 reduction by ethane through dry reforming and oxidative dehydrogenation. Angew. Chem. Int. Ed. 54, 15501–15505 (2015).
Yao, S. et al. Combining CO2 reduction with ethane oxidative dehydrogenation by oxygen-modification of molybdenum carbide. ACS Catal. 8, 5374–5381 (2018).
Gomez, E., Xie, Z. & Chen, J. G. The effects of bimetallic interactions for CO2-assisted oxidative dehydrogenation and dry reforming of propane. AIChE J. 65, e16670 (2019).
Gomez, E. et al. Combining CO2 reduction with propane oxidative dehydrogenation over bimetallic catalysts. Nat. Commun. 9, 1398 (2018).
Li, X. et al. Oxidative dehydrogenation and dry reforming of n-butane with CO2 over NiFe bimetallic catalysts. Appl. Catal. B Environ. 231, 213–223 (2018).
Kainulainen, T. A., Niemelä, M. K. & Krause, A. O. I. Rh/C catalysts in ethene hydroformylation: the effect of different supports and pretreatments. J. Mol. Catal. A Chem. 140, 173–184 (1999).
Ahlers, S. J., Pohl, M.-M., Holena, M., Linke, D. & Kondratenko, E. V. Direct propanol synthesis from CO2, C2H4, and H2 over Cs–Au/TiO2 rutile: effect of promoter loading, temperature and feed composition. Catal. Sci. Technol. 6, 2171–2180 (2016).
Navidi, N., Thybaut, J. W. & Marin, G. B. Experimental investigation of ethylene hydroformylation to propanal on Rh and Co based catalysts. Appl. Catal. A General 469, 357–366 (2014).
Acknowledgements
The work is sponsored by the U.S. Department of Energy (DOE), Office of Science, Office of Basic Energy Sciences, Division of Chemical Sciences, Biosciences, and Geosciences, under contract no. DE-SC0012704. E.G. acknowledges the U.S. National Science Foundation Graduate Research Fellowship Program: DGE-16-44869 and the Gates Millennium Scholarship Foundation.
Author information
Authors and Affiliations
Contributions
E.G. and J.G.C. prepared the manuscript with contributions from Y.B.H and S.K. E.G. conducted thermodynamic calculations with contributions from Y.B.H.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gomez, E., Yan, B., Kattel, S. et al. Carbon dioxide reduction in tandem with light-alkane dehydrogenation. Nat Rev Chem 3, 638–649 (2019). https://doi.org/10.1038/s41570-019-0128-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-019-0128-9
This article is cited by
-
Atomically synergistic Zn-Cr catalyst for iso-stoichiometric co-conversion of ethane and CO2 to ethylene and CO
Nature Communications (2024)
-
Selective synthesis of butane from carbon monoxide using cascade electrolysis and thermocatalysis at ambient conditions
Nature Catalysis (2023)
-
Economically viable electrocatalytic ethylene production with high yield and selectivity
Nature Sustainability (2023)
-
Porous nitrogen-doped carbon supported MoO2/Mo2C hybrid catalyst for efficient oxidative coupling of primary amines to imines
Journal of Porous Materials (2023)
-
Promotional nature of Sn on Pt/CeO2 for the oxidative dehydrogenation of propane with carbon dioxide
Nano Research (2023)