Abstract
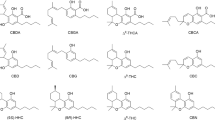
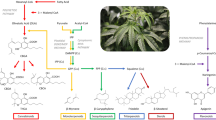
The cannabis plant has had a tumultuous past. Once revered for its medicinal properties, it then became a banned narcotic and now the perceived medical benefits of cannabis see it receiving renewed attention. The active ingredients in cannabis plant extracts — phytocannabinoids — are now being investigated, both as formulations and in isolation, for pharmaceutical applications. The most abundant phytocannabinoid is (−)-trans-Δ9-tetrahydrocannabinol, a compound readily extracted from Cannabis sativa. There are over 100 known phytocannabinoids, some of which are present in such low concentrations that chemical syntheses are necessary to advance their medicinal potential. In this Review, we examine phytocannabinoids in terms of their mode of action, biosynthesis, and various total syntheses and derivatizations. Finally, we describe the policy issues surrounding the possession, use and control of phytocannabinoids.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Farnsworth, N. R. Pharmacognosy and chemistry of ‘Cannabis sativa’. J. Am. Pharm. Assoc. 9, 410–440 (1969).
Russo, E. B. in Handbook of Cannabis (ed. Pertwee, R. G. ) 23–43 (Oxford Scholarship Online, 2014).
Doyle, E. & Spence, A. A. Cannabis as a medicine? Br. J. Anaesth. 74, 359–361 (1995).
Crean, R. D., Crane, N. A. & Mason, B. J. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J. Addict. Med. 5, 1–8 (2011).
Devane, W. A., Dysarz, F. A., Johnson, M. R., Melvin, L. S. & Howlett, A. C. Determination and characterization of a cannabinoid receptor in rat brain. Mol. Pharmacol. 34, 605–613 (1988).
Munro, S., Thomas, K. L. & Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 365, 61–65 (1993).
Devane, W. A. et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258, 1946–1949 (1992).
Sugiura, T. et al. 2-Arachidonoylgylcerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 215, 89–97 (1995).
Hua, T. et al. Crystal structure of the human cannabinoid receptor CB1 . Cell 167, 750–762.e14 (2016).
Shao, Z. et al. High-resolution crystal structure of the human CB1 cannabinoid receptor. Nature 540, 602–606 (2016).
Hua, T. et al. Crystal structures of agonist-bound human cannabinoid receptor CB1 . Nature 547, 468–471 (2017).
Gaoni, Y. & Mechoulam, R. Isolation, structure, and partial synthesis of an active constituent of hashish. J. Am. Chem. Soc. 86, 1646–1647 (1964).
Mechoulam, R. & Shvo, Y. Hashish. I. The structure of cannabidiol. Tetrahedron 19, 2073–2078 (1963).
Aizpurua-Olaizola, O. et al. Evolution of the cannabinoid and terpene content during the growth of Cannabis sativa plants from different chemotypes. J. Nat. Prod. 79, 324–331 (2016).
Ahmed, S. A. et al. Minor oxygenated cannabinoids from high potency Cannabis sativa L. Phytochemistry 117, 194–199 (2015).
ElSohly, M. A., Radwan, M. M., Gul, W., Chandra, S. & Galal, A. Phytochemistry of Cannabis sativa L. Prog. Chem. Org. Nat. Prod. 103, 1–36 (2017).
Englund, A. et al. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J. Psychopharmacol. 27, 19–27 (2013).
Sastre-Garriga, J., Vila, C., Clissold, S. & Montalban, X. THC and CBD oromucosal spray (Sativex®) in the management of spasticity associated with multiple sclerosis. Expert Rev. Neurother. 11, 627–637 (2011).
Morgan, C. J. A., Schafer, G., Freeman, T. P. & Curran, H. V. Impact of cannabidiol on the acute memory and psychotomimetic effects of smoked cannabis: naturalistic study. Br. J. Psychiatry 197, 285–290 (2010).
Karniol, I. G., Shirakawa, I., Kasinski, N., Pfeferman, A. & Carlini, E. A. Cannabidiol interferes with the effects of Δ9-tetrahydrocannabinol in man. Eur. J. Pharmacol. 28, 172–177 (1974).
Hanuš, L. O., Meyer, S. M., Muñoz, E., Taglialatela-Scafati, O. & Appendino, G. Phytocannabinoids: a unified critical inventory. Nat. Prod. Rep. 33, 1357–1392 (2016).
Shoyama, Y., Yagi, M., Nishioka, I. & Yamauchi, T. Biosynthesis of cannabinoid acids. Phytochemistry 14, 2189–2192 (1975).
Taura, F., Morimoto, S., Shoyama, Y. & Mechoulam, R. First direct evidence for the mechanism of Δ1-tetrahydrocannabinolic acid biosynthesis. J. Am. Chem. Soc. 117, 9766–9767 (1995).
Chemical Abstracts Service. SciFinder. American Chemical Societyhttps://scifinder.cas.org (accessed 29 Oct 2017).
Petrzilka, T., Haefliger, W. & Sikemeier, C. Synthese von Haschisch-Inhaltsstoffen. 4. Mitteilung. Helv. Chim. Acta 52, 1102–1134 (1969).
Wilkinson, S. M., Price, J. & Kassiou, M. Improved accessibility to the desoxy analogues of Δ9-tetrahydrocannabinol and cannabidiol. Tetrahedron Lett. 54, 52–54 (2013).
Rickards, R. W. & Watson, W. P. Conversion of (+)-(R )-Limonene into (+)-(1S, 4R)-p-mentha-2,8-dien-1-ol, an intermediate in the synthesis of tetrahydrocannabinoids. Aust. J. Chem. 33, 451–454 (1980).
Schenck, G. O., Gollnick, K., Buchwald, G., Schroeter, S. & Ohloff, G. Zur chemischen und sterischen Selektivität der photosensibilisierten O2-Übertragung auf (+)-Limonen und (+)-Carvomenthen [German]. Justus Liebigs Ann. Chem. 674, 93–117 (1964).
Razdan, R. K., Dalzell, H. C. & Handrick, G. R. Hashish. X. Simple one-step synthesis of (−)-Δ1-tetrahydrocannabinol (THC) from p-mentha-2,8-dien-1-ol and olivetol. J. Am. Chem. Soc. 96, 5860–5865 (1974).
Petrzilka, T., Haefliger, W., Sikemeier, C., Ohloff, G. & Eschenmoser, A. Synthese und Chiralität des (−)-Cannabidiols Vorläufige Mitteilung. Helv. Chim. Acta 50, 719–723 (1967).
Baek, S.-H., Srebnik, M. & Mechoulam, R. Boron triflouride etherate on alimina — a modified Lewis acid reagent. Tetrahedron Lett. 26, 1083–1086 (1985).
Rickards, R. W. & Roenneberg, H. Synthesis of (−)-Δ9-6a, 10a-trans-tetrahydrocannabinol. Boron trifluoride catalyzed arylation by a homocuprate. J. Org. Chem. 49, 572–573 (1984).
Stoss, P. & Merrath, P. A useful approach towards Δ9-tetrahydrocannabinol. Synlett 1991, 553–554 (1991).
Razdan, R. K. & Handrick, G. R. Hashish. V. A stereospecific synthesis of (−)-Δ1- and (−)-Δ1(6)-tetrahydrocannabinols. J. Am. Chem. Soc. 92, 6061–6062 (1970).
William, A. D. & Kobayashi, Y. A method to accomplish a 1,4-addition reaction of bulky nucleophiles to enones and subsequent formation of reactive enolates. Org. Lett. 3, 2017–2020 (2001).
William, A. D. & Kobayashi, Y. Synthesis of tetrahydrocannabinols based on an indirect 1,4-addition strategy. J. Org. Chem. 67, 8771–8782 (2002).
Kobayashi, Y., Takeuchi, A. & Wang, Y.-G. Synthesis of cannabidiols via alkenylation of cyclohexenyl monoacetate. Org. Lett. 8, 2699–2702 (2006).
Cheng, L.-J., Xie, J.-H., Chen, Y., Wang, L.-X. & Zhou, Q.-L. Enantioselective total synthesis of (−)-Δ8-THC and (−)-Δ9-THC via catalytic asymmetric hydrogenation and SNAr cyclization. Org. Lett. 15, 764–767 (2013).
Petrzilka, T. & Sikemeier, C. Über Inhaltsstoffe des Haschisch. 3., vorläufige Mitteilung. Umwandlung von (−)-Δ6,1-3,4-trans-tetrahydrocannabinol in (−)-Δ1,2-3,4-trans tetrahydrocannabinol. Helv. Chim. Acta 50, 2111–2113 (1967).
Evans, D. A., Shaughnessy, E. A. & Barnes, D. M. Cationic bis(oxazoline)Cu(II) Lewis acid catalysts. Application to the asymmetric synthesis of ent-Δ1-tetrahydrocannabinol. Tetrahedron Lett. 38, 3193–3194 (1997).
Evans, D. A. et al. Bis(oxazoline) and bis(oxazolinyl)pyridine copper complexes as enantioselective Diels–Alder catalysts: reaction scope and synthetic applications. J. Am. Chem. Soc. 121, 7582–7594 (1999).
Pearson, E. L., Kanizaj, N., Willis, A. C., Paddon-Row, M. N. & Sherburn, M. S. Experimental and computational studies into an ATPH-promoted exo-selective IMDA reaction: a short total synthesis of Δ9-THC. Chem. Eur. J. 16, 8280–8284 (2010).
Trost, B. M. & Dogra, K. Synthesis of (−)-Δ9-trans-tetrahydrocannabinol: stereocontrol via Mo-catalyzed asymmetric allylic alkylation reaction. Org. Lett. 9, 861–863 (2007).
Schafroth, M. A., Zuccarello, G., Krautwald, S., Sarlah, D. & Carreira, E. M. Stereodivergent total synthesis of Δ9-tetrahydrocannabinols. Angew. Chem. Int. Ed. 53, 13898–13901 (2014).
Krautwald, S., Sarlah, D., Schafroth, M. A. & Carreira, E. M. Enantio- and diastereodivergent dual catalysis: α-allylation of branched aldehydes. Science 340, 1065–1068 (2013).
Krautwald, S., Schafroth, M. A., Sarlah, D. & Carreira, E. M. Stereodivergent α-allylation of linear aldehydes with dual iridium and amine catalysis. J. Am. Chem. Soc. 136, 3020–3023 (2014).
Shani, A. & Mechoulam, R. Cannabielsoic acids: isolation and synthesis by a novel oxidative cyclization. Tetrahedron 30, 2437–2446 (1974).
Yamauchi, T., Shoyama, Y., Aramaki, H., Azuma, T. & Nishioka, I. Tetrahydrocannabinolic acid, a genuine substance of tetrahydrocannabinol. Chem. Pharm. Bull. 15, 1075–1076 (1967).
Crombie, L. & Crombie, W. M. L. Cannabinoid acids and esters: miniaturized synthesis and chromatographic study. Phytochemistry 16, 1413–1420 (1977).
Mechoulam, R. & Ben-Zvi, Z. Carboxylation of resorcinols with methylmagnesium carbonate. Synthesis of cannabinoid acids. J. Chem. Soc. D 343–344 (1969).
Winnicki, R. & Donsky, M. Biosynthesis of cannabinoids. Patent WO2014134281 A1 (2014).
Roth, N., Wohlfarth, A., Müller, M. & Auwärter, V. Regioselective synthesis of isotopically labeled Δ9-tetrahydrocannabinolic acid A (THCA-A-D3) by reaction of Δ9-tetrahydrocannabinol-D3 with magnesium methyl carbonate. Forensic Sci. Int. 222, 368–372 (2012).
Cardillo, G., Cricchio, R. & Merlini, L. Synthesis of d, l-cannabichromene, franklinone and other natural chromenes. Tetrahedron 24, 4825–4831 (1968).
Lee, Y. R. & Wang, X. Concise synthesis of biologically interesting (±)-cannabichromene, (±)-cannabichromenic acid, and (±)-daurichromenic acid. Bull. Kor. Chem. Soc. 26, 1933–1936 (2005).
Lange, K., Schmid, A. & Julsing, M. K. Δ9-Tetrahydrocannabinolic acid synthase: the application of a plant secondary metabolite enzyme in biocatalytic chemical synthesis. J. Biotechnol. 233, 42–48 (2016).
Appendino, G. et al. Antibacterial cannabinoids from Cannabis sativa: a structure–activity study. J. Nat. Prod. 71, 1427–1430 (2008).
Ghosh, R., Todd, A. R. & Wilkinson, S. Cannabis indica. Part V. The synthesis of cannabinol. J. Chem. Soc. 1393–1396 (1940).
Mahadevan, A. et al. Novel cannabinol probes for CB1 and CB2 cannabinoid receptors. J. Med. Chem. 43, 3778–3785 (2000).
Bastola, K. P., Hazekamp, A. & Verpoorte, R. Synthesis and spectroscopic characterization of cannabinolic acid. Planta Med. 73, 273–275 (2007).
Nandaluru, P. R. & Bodwell, G. J. Multicomponent synthesis of 6H-dibenzo[b. d]pyran-6-ones and a total synthesis of cannabinol. Org. Lett. 14, 310–313 (2012).
Fan, F. et al. An intramolecular pyranone Diels–Alder cycloaddition approach to cannabinol. Adv. Synth. Catal. 356, 1337–1342 (2014).
Mou, C. et al. Green and rapid access to benzocoumarins via direct benzene construction through base-mediated formal [4 + 2] reaction and air oxidation. Adv. Synth. Catal. 358, 707–712 (2016).
Tetsutaro, H., Takatsugu, S., Noriyuki, H., Nobuyuki, K. & Sotaro, M. Convenient synthesis of biphenyl-2-carboxylic acids via the nucleophilic aromatic substitution reaction of 2-methoxybenzoates by aryl Grignard reagents. Bull. Chem. Soc. Jpn 66, 3034–3040 (1993).
Nüllen, M. P. & Göttlich, R. Synthesis of cannabinol by a modified Ullmann–Ziegler cross-coupling. Synlett 24, 1109–1112 (2013).
Li, Y., Ding, Y.-J., Wang, J.-Y., Su, Y.-M. & Wang, X.-S. Pd-catalyzed C–H lactonization for expedient synthesis of biaryl lactones and total synthesis of cannabinol. Org. Lett. 15, 2574–2577 (2013).
Teske, J. A. & Deiters, A. A cyclotrimerization route to cannabinoids. Org. Lett. 10, 2195–2198 (2008).
Mechoulam, R. & Gaoni, Y. A total synthesis of dl-Δ1-tetrahydrocannabinol, the active constituent of hashish. J. Am. Chem. Soc. 87, 3273–3275 (1965).
Mechoulam, R., Braun, P. & Gaoni, Y. Syntheses of Δ1-tetrahydrocannabinol and related cannabinoids. J. Am. Chem. Soc. 94, 6159–6165 (1972).
Gaoni, Y. & Mechoulam, R. Cannabichromene, a new active principle in hashish. Chem. Commun. 0, 20–21 (1966).
Claussen, U., Spulak, F.v. & Korte, F. Zur chemischen klassifizierung von pflanzen—XXXI, haschisch—X: cannabichromen, ein neuer haschisch-inhalts-stoff [German]. Tetrahedron 22, 1477–1479 (1966).
Mechoulam, R., Yagnitinsky, B. & Gaoni, Y. Hashish. XII. Stereoelectronic factor in the chloranil dehydrogenation of cannabinoids. Total synthesis of dl-cannabichromene. J. Am. Chem. Soc. 90, 2418–2420 (1968).
Yamaguchi, S., Shouji, N. & Kuroda, K. A new approach to dl-cannabichromene. Bull. Chem. Soc. Jpn 68, 305–308 (1995).
Saimoto, H. et al. Effect of calcium reagents on aldol reactions of phenolic enolates with aldehydes in alcohol. J. Org. Chem. 61, 6768–6769 (1996).
Yeom, H.-S., Li, H., Tang, Y. & Hsung, R. P. Total syntheses of cannabicyclol, clusiacyclol A and B, iso-eriobrucinol A and B, and eriobrucinol. Org. Lett. 15, 3130–3133 (2013).
Li, X. & Lee, Y. R. Efficient and novel one-pot synthesis of polycycles bearing cyclols by FeCl3-promoted [2 + 2] cycloaddition: application to cannabicyclol, cannabicyclovarin, and ranhuadujuanine A. Org. Biomol. Chem. 12, 1250–1257 (2014).
Wall, M. E., Brine, D. R., Pitt, C. G. & Perez-Reyes, M. Identification of Δ9-tetrahydrocannabinol and metabolites in man. J. Am. Chem. Soc. 94, 8579–8581 (1972).
Mechoulam, R., McCallum, N. K. & Burstein, S. Recent advances in the chemistry and biochemistry of cannabis. Chem. Rev. 76, 75–112 (1976).
Lemberger, L. Tetrahydrocannabinol metabolism in man. Drug Metab. Dispos. 1, 461–468 (1973).
Woodhouse, E. J. Confirmation of the presence of 11-hydroxy- 9-tetrahydrocannabinol in the urine of marijuana smokers. Am. J. Public Health 62, 1394–1396 (1972).
Baek, S.-H., Szirmai, M. & Halldin, M. M. Synthesis of optically active (−)-11-Nor-Δ9-tetrahydrocannabinol-9-carboxylic acid. Pharmacol. Biochem. Behav. 40, 487–489 (1991).
Siegel, C. et al. Synthesis of racemic and optically active Δ9-tetrahydrocannabinol (THC) metabolites. J. Org. Chem. 56, 6865–6872 (1991).
Siegel, C., Gordon, P. M. & Razdan, R. K. An optically active terpenic synthon for Δ9-cannabinoids: synthesis of (−)-11-hydroxy-Δ9-tetrahydrocannabinol (THC) and its 1′,1′-dimethylheptyl analog. J. Org. Chem. 54, 5428–5430 (1989).
Tius, M. A., Gu, X.-q. & Kerr, M. A. A convenient synthesis of (−)-11-nor-Δ9-tetrahydrocannabinol-9-methanol. J. Chem. Soc. Chem. Commun. 62–63 (1989).
Archer, R. A. et al. Cannabinoids. 3. Synthetic approaches to 9-ketocannabinoids. Total synthesis of nabilone. J. Org. Chem. 42, 2277–2284 (1977).
Nikas, S. P. et al. A concise methodology for the synthesis of (−)-Δ9-tetrahydrocannabinol and (−)-Δ9-tetrahydrocannabivarin metabolites and their regiospecifically deuterated analogs. Tetrahedron 63, 8112–8123 (2007).
Kachensky, D. F. & Hui, R. A. H. F. Preparation of racemic, (−)- and (+)-11-Nor-Δ9-tetrahydrocannabinol- 9-carboxylic acid. J. Org. Chem. 62, 7065–7068 (1997).
Hanuš, L. O.et al. Enantiomeric cannabidiol derivatives: synthesis and binding to cannabinoid receptors. Org. Biomol. Chem. 3, 1116–1123 (2005).
Tchilibon, S. & Mechoulam, R. Synthesis of a primary metabolite of cannabidiol. Org. Lett. 2, 3301–3303 (2000).
Morales, P., Hurst, D. P. & Reggio, P. H. Molecular targets of the phytocannabinoids — a complex picture. Prog. Chem. Org. Nat. Prod. 103, 103–131 (2017).
Mackie, K., Devane, W. A. & Hille, B. Anandamide, an endogenous cannabinoid, inhibits calcium currents as a partial agonist in N18 neuroblastoma cells. Mol. Pharmacol. 44, 498–503 (1993).
Rosenthaler, S. et al. Differences in receptor binding affinity of several phytocannabinoids do not explain their effects on neural cell cultures. Neurotoxicol. Teratol. 46, 49–56 (2014).
Iwamura, H., Suzuki, H., Ueda, Y., Kaya, T. & Inaba, T. In vitro and in vivo pharmacological characterization of JTE-907, a novel selective ligand for cannabinoid CB2 receptor. J. Pharmacol. Exp. Ther. 296, 420–425 (2001).
Pertwee, R. G. Pharmacology of cannabinoid receptor ligands. Curr. Med. Chem. 6, 635–664 (1999).
Huffman, J. W. et al. 3-(1′,1′-Dimethylbutyl)-1-deoxy-Δ8-THC and related compounds: synthesis of selective ligands for the CB2 receptor. Biorg. Med. Chem. 7, 2905–2914 (1999).
Busch-Petersen, J. et al. Unsaturated side chain β-11-hydroxyhexahydrocannabinol analogs. J. Med. Chem. 39, 3790–3796 (1996).
MacLennan, S. J., Reynen, P. H., Kwan, J. & Bonhaus, D. W. Evidence for inverse agonism of SR141716A at human recombinant cannabinoid CB1 and CB2 receptors. Br. J. Pharmacol. 124, 619–622 (1998).
Rhee, M.-H. et al. Cannabinol derivatives: binding to cannabinoid receptors and inhibition of adenylylcyclase. J. Med. Chem. 40, 3228–3233 (1997).
Thomas, A. et al. Evidence that the plant cannabinoid Δ9-tetrahydrocannabivarin is a cannabinoid CB1 and CB2 receptor antagonist. Br. J. Pharmacol. 146, 917–926 (2005).
McPartland, J. M., Duncan, M., Di Marzo, V. & Pertwee, R. G. Are cannabidiol and Δ9-tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br. J. Pharmacol. 172, 737–753 (2015).
McPartland, J. M. et al. Affinity and efficacy studies of tetrahydrocannabinolic acid A at cannabinoid receptor types one and two. Cannabis Cannabinoid Res. 2, 87–95 (2017).
Thomas, A. et al. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br. J. Pharmacol. 150, 613–623 (2007).
Lynch, J. W. Molecular structure and function of the glycine receptor chloride channel. Physiol. Rev. 84, 1051–1095 (2004).
Vriens, J., Nilius, B. & Voets, T. Peripheral thermosensation in mammals. Nat. Rev. Neurosci. 15, 573–589 (2014).
Vassilatis, D. K. et al. The G protein-coupled receptor repertoires of human and mouse. Proc. Natl Acad. Sci. USA 100, 4903–4908 (2003).
Hoyer, D. et al. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (serotonin). Pharmacol. Rev. 46, 157–203 (1994).
Michalik, L. et al. International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol. Rev. 58, 726–741 (2006).
De Petrocellis, L. et al. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 163, 1479–1494 (2011).
McHugh, D. et al. N-arachidonoyl glycine, an abundant endogenous lipid, potently drives directed cellular migration through GPR18, the putative abnormal cannabidiol receptor. BMC Neurosci. 11, 44 (2010).
Brown, A. J. Novel cannabinoid receptors. Br. J. Pharmacol. 152, 567–575 (2007).
Overton, H. A. et al. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab. 3, 167–175 (2006).
Feigenbaum, J. J. et al. Nonpsychotropic cannabinoid acts as a functional N-methyl-d-aspartate receptor blocker. Proc. Natl Acad. Sci. USA 86, 9584–9587 (1989).
Cotter, J. Efficacy of crude marijuana and synthetic Δ9-tetrahydrocannabinol as treatment for chemotherapy-induced nausea and vomiting: a systematic literature review. Oncol. Nurs. Forum 36, 345–352 (2009).
Pertwee, R. G. et al. The psychoactive plant cannabinoid, Δ9-tetrahydrocannabinol, is antagonized by Δ8- and Δ9-tetrahydrocannabivarin in mice in vivo. Br. J. Pharmacol. 150, 586–594 (2007).
Rock, E. M. et al. Cannabidiol, a non-psychotropic component of cannabis, attenuates vomiting and nausea-like behaviour via indirect agonism of 5-HT1A somatodendritic autoreceptors in the dorsal raphe nucleus. Br. J. Pharmacol. 165, 2620–2634 (2012).
Rock, E. M. et al. Interaction between non-psychotropic cannabinoids in marihuana: effect of cannabigerol (CBG) on the anti-nausea or anti-emetic effects of cannabidiol (CBD) in rats and shrews. Psychopharmacology 215, 505–512 (2011).
Bolognini, D. et al. Cannabidiolic acid prevents vomiting in Suncus murinus and nausea-induced behaviour in rats by enhancing 5-HT1A receptor activation. Br. J. Pharmacol. 168, 1456–1470 (2013).
Rock, E. M., Kopstick, R. L., Limebeer, C. L. & Parker, L. A. Tetrahydrocannabinolic acid reduces nausea-induced conditioned gaping in rats and vomiting in Suncus murinus. Br. J. Pharmacol. 170, 641–648 (2013).
Farrimond, J. A., Whalley, B. J. & Williams, C. M. Cannabinol and cannabidiol exert opposing effects on rat feeding patterns. Psychopharmacology 223, 117–129 (2012).
Costa, B. On the pharmacological properties of Δ9-tetrahydrocannabinol (THC). Chem. Biodivers. 4, 1664–1677 (2007).
Costa, B., Trovato, A. E., Comelli, F., Giagnoni, G. & Colleoni, M. The non-psychoactive cannabis constituent cannabidiol is an orally effective therapeutic agent in rat chronic inflammatory and neuropathic pain. Eur. J. Pharmacol. 556, 75–83 (2007).
Cascio, M. G., Gauson, L. A., Stevenson, L. A., Ross, R. A. & Pertwee, R. G. Evidence that the plant cannabinoid cannabigerol is a highly potent α2-adrenoceptor agonist and moderately potent 5HT1A receptor antagonist. Br. J. Pharmacol. 159, 129–141 (2010).
DeLong, G. T., Wolf, C. E., Poklis, A. & Lichtman, A. H. Pharmacological evaluation of the natural constituent of Cannabis sativa, cannabichromene and its modulation by Δ9-tetrahydrocannabinol. Drug Alcohol Depend. 112, 126–133 (2010).
Patrik, R. & Ida, S. H. Antipsychotic-like effects of cannabidiol and rimonabant: systematic review of animal and human studies. Curr. Pharm. Des. 18, 5141–5155 (2012).
Esposito, G. et al. Cannabidiol in vivo blunts β-amyloid induced neuroinflammation by suppressing IL-1β and iNOS expression. Br. J. Pharmacol. 151, 1272–1279 (2007).
Martín-Moreno, A. M. et al. Cannabidiol and other cannabinoids reduce microglial activation in vitro and in vivo: relevance to Alzheimer's disease. Mol. Pharmacol. 79, 964–973 (2011).
Iuvone, T., Esposito, G., De Filippis, D., Scuderi, C. & Steardo, L. Cannabidiol: a promising drug for neurodegenerative disorders? CNS Neurosci. Ther. 15, 65–75 (2009).
García-Arencibia, M., García, C. & Fernández-Ruiz, J. Cannabinoids and Parkinson's disease. CNS Neurol. Disord. Drug Targets 8, 432–439 (2009).
Turkanis, S. A., Smiley, K. A., Borys, H. K., Olsen, D. M. & Karler, R. An electrophysiological analysis of the anticonvulsant action of cannabidiol on limbic seizures in conscious rats. Epilepsia 20, 351–363 (1979).
Jones, N. A. et al. Cannabidiol exerts anti-convulsant effects in animal models of temporal lobe and partial seizures. Seizure 21, 344–352 (2012).
Jones, N. A. et al. Cannabidiol displays antiepileptiform and antiseizure rroperties in vitro and in vivo. J. Pharm. Exp. Ther. 332, 569–577 (2010).
Devinsky, O. et al. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol. 15, 270–278 (2016).
Cunha, J. M. et al. Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology 21, 175–185 (1980).
Leweke, F. M. et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry 2, e94 (2012).
Hill, A. J. et al. Cannabidivarin is anticonvulsant in mouse and rat. Br. J. Pharmacol. 167, 1629–1642 (2012).
Hill, A. J. et al. Δ9-Tetrahydrocannabivarin suppresses in vitro epileptiform and in vivo seizure activity in adult rats. Epilepsia 51, 1522–1532 (2010).
Chesher, G. B. & Jackson, D. M. Anticonvulsant effects of cannabinoids in mice: drug interactions within cannabinoids and cannabinoid interactions with phenytoin. Psychopharmacologia 37, 255–264 (1974).
Karler, R. & Turkanis, S. A. Cannabis and epilepsy. Adv. Biosci. 22–23, 619–641 (1978).
United Nations. Article 1, Single Convention on Narcotic Drugs (UN, 1961).
Commission on Narcotic Drugs. Decision 50/2: Review of Dronabinol and its Stereoisomers (UNODC, 2007).
Fellermeier, M., Eisenreich, W., Bacher, A. & Zenk, M. H. Biosynthesis of cannabinoids: incorporation experiments with 13C-labeled glucoses. Eur. J. Biochem. 268, 1596–1604 (2001).
Hillig, K. W. & Mahlberg, P. G. A chemotaxonomic analysis of cannabinoid variation in Cannabis (Cannabaceae). Am. J. Bot. 91, 966–975 (2004).
McPartland, J. M., Glass, M. & Pertwee, R. G. Meta-analysis of cannabinoid ligand binding affinity and receptor distribution: interspecies differences. Br. J. Pharmacol. 152, 583–593 (2007).
Sugiura, T. et al. Evidence that 2-arachidonoylglycerol but not N-palmitoylethanolamine or anandamide is the physiological ligand for the cannabinoid CB2 receptor: comparison of the agonistic activities of various cannabinoid receptor ligands in HL-60 cells. J. Biol. Chem. 275, 605–612 (2000).
Bolognini, D., Cascio, M. G., Parolaro, D. & Pertwee, R. G. AM630 behaves as a protean ligand at the human cannabinoid CB2 receptor. Br. J. Pharmacol. 165, 2561–2574 (2012).
Bolognini, D. et al. The plant cannabinoid Δ9-tetrahydrocannabivarin can decrease signs of inflammation and inflammatory pain in mice. Br. J. Pharmacol. 160, 677–687 (2010).
Pertwee, R. G., Ross, R. A., Craib, S. J. & Thomas, A. (−)-Cannabidiol antagonizes cannabinoid receptor agonists and noradrenaline in the mouse vas deferens. Eur. J. Pharmacol. 456, 99–106 (2002).
Acknowledgements
The National Health and Medical Research Council (NHMRC)-EU collaborative grant and The Lambert Initiative for Cannabinoid Research, The University of Sydney, are acknowledged for funding.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the preparation of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Reekie, T., Scott, M. & Kassiou, M. The evolving science of phytocannabinoids. Nat Rev Chem 2, 0101 (2018). https://doi.org/10.1038/s41570-017-0101
Published:
DOI: https://doi.org/10.1038/s41570-017-0101
This article is cited by
-
Synthesis, characterization and stress-testing of a robust quillaja saponin stabilized oil-in-water phytocannabinoid nanoemulsion
Journal of Cannabis Research (2021)
-
The biosynthesis of the cannabinoids
Journal of Cannabis Research (2021)
-
A review on the syntheses of Dronabinol and Epidiolex as classical cannabinoids with various biological activities including those against SARS-COV2
Journal of the Iranian Chemical Society (2021)
-
One-flow synthesis of tetrahydrocannabinol and cannabidiol using homo- and heterogeneous Lewis acids
Journal of Flow Chemistry (2021)
-
Intralaboratory comparison of analytical methods for quantification of major phytocannabinoids
Analytical and Bioanalytical Chemistry (2019)