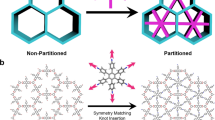
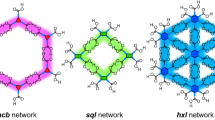
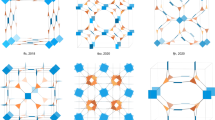
Abstract
In the past decade, covalent organic frameworks (COFs) have emerged as a new class of highly ordered crystalline organic porous polymers. They have attracted tremendous research interest because of their unique structures and potential applications in gas storage and separation, energy storage, catalysis and optoelectronic materials development. Although the skeletons and pore structures of COFs are customizable through judicious selection of chemical building blocks, COF materials have been mainly limited to uniform pore structures with homogeneous pore environments. Two-dimensional COFs with complex multipore structures are largely unexplored, perhaps owing to the challenges that are inherent in designing selective syntheses. Simple tessellation has been remarkably successful in the preparation of regular 2D COFs, but building multiporous systems requires the aid of mathematical design. In this Perspective, we discuss four different approaches to tessellated 2D COFS with a focus on the mathematical rules for their application. A comparison of these strategies should provide guidance to those designing new applications of COF materials.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Doonan, C. J., Tranchemontagne, D. J., Glover, T. G., Hunt, J. R. & Yaghi, O. M. Exceptional ammonia uptake by a covalent organic framework. Nat. Chem. 2, 235–238 (2010).
Furukawa, H. & Yaghi, O. M. Storage of hydrogen, methane, and carbon dioxide in highly porous covalent organic frameworks for clean energy applications. J. Am. Chem. Soc. 131, 8875–8883 (2009).
Han, S. S., Furukawa, H., Yaghi, O. M. & Goddard, W. A. Covalent organic frameworks as exceptional hydrogen storage materials. J. Am. Chem. Soc. 130, 11580–11581 (2008).
Lin, S. et al. Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water. Science 349, 1208–1213 (2015).
Xu, H., Gao, J. & Jiang, D. L. Stable, crystalline, porous, covalent organic frameworks as a platform for chiral organocatalysts. Nat. Chem. 7, 905–912 (2015).
Ding, S. Y. et al. Construction of covalent organic framework for catalysis: Pd/COF-LZU1 in Suzuki–Miyaura coupling reaction. J. Am. Chem. Soc. 133, 19816–19822 (2011).
Dogru, M. et al. A photoconductive thienothiophene-based covalent organic framework showing charge transfer towards included fullerene. Angew. Chem. Int. Ed. 52, 2920–2924 (2013).
Colson, J. C. et al. Oriented 2D covalent organic framework thin films on single-layer graphene. Science 332, 228–231 (2011).
Wan, S., Guo, J., Kim, J., Ihee, H. & Jiang, D. L. A belt-shaped, blue luminescent, and semiconducting covalent organic framework. Angew. Chem. Int. Ed. 47, 8826–8830 (2008).
Ma, H. P. et al. Cationic covalent organic frameworks: a simple platform of anionic exchange for porosity tuning and proton conduction. J. Am. Chem. Soc. 138, 5897–5903 (2016).
Xu, H. & Jiang, D. L. Covalent organic frameworks: crossing the channel. Nat. Chem. 6, 564–566 (2014).
Chandra, S. et al. Phosphoric acid loaded azo (-N=N-) based covalent organic framework for proton conduction. J. Am. Chem. Soc. 136, 6570–6573 (2014).
Côté, A. P. et al. Porous, crystalline, covalent organic frameworks. Science 310, 1166–1170 (2005).
Huang, N., Wang, P. & Jiang, D. L. Covalent organic frameworks: a materials platform for structural and functional designs. Nat. Rev. Mater. 1, 16068 (2016).
Ding, S. Y. & Wang, W. Covalent organic frameworks (COFs): from design to applications. Chem. Soc. Rev. 42, 548–568 (2013).
Colson, J. W. & Dichtel, W. R. Rationally synthesized two-dimensional polymers. Nat. Chem. 5, 453–465 (2013).
Feng, X., Ding, X. & Jiang, D. Covalent organic frameworks. Chem. Soc. Rev. 41, 6010–6022 (2012).
Uribe-Romo, F. J. & Dichtel, W. R. Two-dimensional materials polymers stripped down. Nat. Chem. 4, 244–245 (2012).
Ohmori, O., Kawano, M. & Fujita, M. A two-in-one crystal: uptake of two different guests into two distinct channels of a biporous coordination network. Angew. Chem. Int. Ed. 44, 1962–1964 (2005).
Seo, M., Kim, S., Oh, J., Kim, S. J. & Hillmyer, M. A. Hierarchically porous polymers from hyper-cross-linked block polymer precursors. J. Am. Chem. Soc. 137, 600–603 (2015).
Sun, Q., Dai, Z. F., Meng, X. J. & Xiao, F. S. Porous polymer catalysts with hierarchical structures. Chem. Soc. Rev. 44, 6018–6034 (2015).
Sai, H. et al. Hierarchical porous polymer scaffolds from block copolymers. Science 341, 530–534 (2013).
Furukawa, H., Muller, U. & Yaghi, O. M. “Heterogeneity within order” in metal–organic frameworks. Angew. Chem. Int. Ed. 54, 3417–3430 (2015).
Fang, Z. L., Bueken, B., De Vos, D. E. & Fischer, R. A. Defect-engineered metal–organic frameworks. Angew. Chem. Int. Ed. 54, 7234–7254 (2015).
Wong-Foy, A. G., Lebel, O. & Matzger, A. J. Porous crystal derived from a tricarboxylate linker with two distinct binding motifs. J. Am. Chem. Soc. 129, 15740–15741 (2007).
Schnobrich, J. K. et al. Linker-directed vertex desymmetrization for the production of coordination polymers with high porosity. J. Am. Chem. Soc. 132, 13941–13948 (2010).
Choi, K. M., Jeon, H. J., Kang, J. K. & Yaghi, O. M. Heterogeneity within order in crystals of a porous metal–organic framework. J. Am. Chem. Soc. 133, 11920–11923 (2011).
Cliffe, M. J. et al. Correlated defect nanoregions in a metal–organic framework. Nat. Commun. 5, 4176 (2014).
Xie, Y. B. et al. Unusual preservation of polyhedral molecular building units in a metal–organic framework with evident desymmetrization in ligand design. Chem. Commun. 50, 563–565 (2014).
Zhu, Y., Wan, S., Jin, Y. & Zhang, W. Desymmetrized vertex design for the synthesis of covalent organic frameworks with periodically heterogeneous pore structures. J. Am. Chem. Soc. 137, 13772–13775 (2015).
Wilson, A., Gasparini, G. & Matile, S. Functional systems with orthogonal dynamic covalent bonds. Chem. Soc. Rev. 43, 1948–1962 (2014).
Jin, Y., Wang, Q., Taynton, P. & Zhang, W. Dynamic covalent chemistry approaches toward macrocycles, molecular cages, and polymers. Acc. Chem. Res. 47, 1575–1586 (2014).
Chen, X. et al. Designed synthesis of double-stage two-dimensional covalent organic frameworks. Sci. Rep. 5, 14650 (2015).
Zeng, Y. F. et al. Covalent organic frameworks formed with two types of covalent bonds based on orthogonal reactions. J. Am. Chem. Soc. 137, 1020–1023 (2015).
Nguyen, H. L. et al. A titanium–organic framework as an exemplar of combining the chemistry of metal– and covalent–organic frameworks. J. Am. Chem. Soc. 138, 4330–4333 (2016).
Zhou, T.-Y., Xu, S.-Q., Wen, Q., Pang, Z.-F. & Zhao, X. One-step construction of two different kinds of pores in a 2D covalent organic framework. J. Am. Chem. Soc. 136, 15885–15888 (2014).
Dalapati, S., Jin, E., Addicoat, M., Heine, T. & Jiang, D. Highly emissive covalent organic frameworks. J. Am. Chem. Soc. 138, 5797–5800 (2016).
Ascherl, L. et al. Molecular docking sites designed for the generation of highly crystalline covalent organic frameworks. Nat. Chem. 8, 310–316 (2016).
Pang, Z.-F., Zhou, T.-Y., Liang, R.-R., Qi, Q.-Y. & Zhao, X. Regulating the topology of 2D covalent organic frameworks by the rational introduction of substituents. Chem. Sci. 8, 3866–3870 (2017).
Smith, B. J. & Dichtel, W. R. Mechanistic studies of two-dimensional covalent organic frameworks rapidly polymerized from initially homogenous conditions. J. Am. Chem. Soc. 136, 8783–8789 (2014).
Smith, B. J., Hwang, N., Chavez, A. D., Novotney, J. L. & Dichtel, W. R. Growth rates and water stability of 2D boronate ester covalent organic frameworks. Chem. Commun. 51, 7532–7535 (2015).
Smith, B. J., Overholts, A. C., Hwang, N. & Dichtel, W. R. Insight into the crystallization of amorphous imine-linked polymer networks to 2D covalent organic frameworks. Chem. Commun. 52, 3690–3693 (2016).
Feng, X., Dong, Y. & Jiang, D. Star-shaped two-dimensional covalent organic frameworks. CrystEngComm 15, 1508–1511 (2013).
Baldwin, L. A., Crowe, J. W., Shannon, M. D., Jaroniec, C. P. & McGrier, P. L. 2D covalent organic frameworks with alternating triangular and hexagonal pores. Chem. Mater. 27, 6169–6172 (2015).
Yang, H. S. et al. Mesoporous 2D covalent organic frameworks based on shape-persistent arylene–ethynylene macrocycles. Chem. Sci. 6, 4049–4053 (2015).
Du, Y. et al. Ionic covalent organic frameworks with spiroborate linkage. Angew. Chem. Int. Ed. 55, 1737–1741 (2016).
Hisaki, I., Nakagawa, S., Tohnai, N. & Miyata, M. A. C3-symmetric macrocycle-based, hydrogen-bonded, multiporous hexagonal network as a motif of porous molecular crystals. Angew. Chem. Int. Ed. 54, 3008–3012 (2015).
Xu, S. Q., Zhan, T. G., Wen, Q., Pang, Z. F. & Zhao, X. Diversity of covalent organic frameworks (COFs): a 2D COF containing two kinds of triangular micropores of different sizes. ACS Macro Lett. 5, 99–102 (2016).
Xu, S. Q., Liang, R. R., Zhan, T. G., Qi, Q. Y. & Zhao, X. Construction of 2D covalent organic frameworks by taking advantage of the variable orientation of imine bonds. Chem. Commun. 53, 2431–2434 (2017).
Zhang, W. & Moore, J. S. Shape-persistent macrocycles: structures and synthetic approaches from arylene and ethynylene building blocks. Angew. Chem. Int. Ed. 45, 4416–4439 (2006).
Iyoda, M., Yamakawa, J. & Rahman, M. J. Conjugated macrocycles: concepts and applications. Angew. Chem. Int. Ed. 50, 10522–10553 (2011).
Grave, C. & Schlüter, A. D. Shape-persistent, nano-sized macrocycles. Eur. J. Org. Chem. 2002, 3075–3098 (2002).
Yang, H., Liu, Z. & Zhang, W. Multidentate triphenolsilane-based alkyne metathesis catalysts. Adv. Synth. Catal. 355, 885–890 (2013).
Zhang, W. & Moore, J. S. Arylene ethynylene macrocycles prepared by precipitation-driven alkyne metathesis. J. Am. Chem. Soc. 126, 12796–12796 (2004).
Deng, H. X. et al. Multiple functional groups of varying ratios in metal–organic frameworks. Science 327, 846–850 (2010).
Kong, X. Q. et al. Mapping of functional groups in metal–organic frameworks. Science 341, 882–885 (2013).
Bunck, D. N. & Dichtel, W. R. Mixed linker strategies for organic framework functionalization. Chem. Eur. J. 19, 818–827 (2013).
Crowe, J. W., Baldwin, L. A. & McGrier, P. L. Luminescent covalent organic frameworks containing a homogeneous and heterogeneous distribution of dehydrobenzoannulene vertex units. J. Am. Chem. Soc. 138, 10120–10123 (2016).
Pang, Z.-F. et al. Construction of covalent organic frameworks bearing three different kinds of pores through the heterostructural mixed linker strategy. J. Am. Chem. Soc. 138, 4710–4713 (2016).
Huang, N. et al. Multiple-component covalent organic frameworks. Nat. Commun. 7, 12325 (2016).
Tian, Y. et al. Two-dimensional dual-pore covalent organic frameworks obtained from the combination of two D2h symmetrical building blocks. Chem. Commun. 52, 11704–11707 (2016).
Yin, Z.-J. et al. Ultrahigh volatile iodine uptake by hollow microspheres formed from a heteropore covalent organic framework. Chem. Commun.http://dx.doi.org/10.1039/C7CC01045A (2017).
Qian, C., Xu, S. Q., Jiang, G. F., Zhan, T. G. & Zhao, X. Precision construction of 2D heteropore covalent organic frameworks by a multiple-linking-site strategy. Chem. Eur. J. 22, 17784–17789 (2016).
Keith, C. T., Borisy, A. A. & Stockwell, B. R. Multicomponent therapeutics for networked systems. Nat. Rev. Drug Discov. 4, 71–78 (2005).
Elliott, E. L., Hartley, C. S. & Moore, J. S. Covalent ladder formation becomes kinetically trapped beyond four rungs. Chem. Commun. 47, 5028–5030 (2011).
Acknowledgements
The authors thank the University of Colorado Boulder and the K. C. Wong Education Foundation for funding support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Jin, Y., Hu, Y. & Zhang, W. Tessellated multiporous two-dimensional covalent organic frameworks. Nat Rev Chem 1, 0056 (2017). https://doi.org/10.1038/s41570-017-0056
Published:
DOI: https://doi.org/10.1038/s41570-017-0056
This article is cited by
-
Creating amphiphilic porosity in two-dimensional covalent organic frameworks via steric-hindrance-mediated precision hydrophilic-hydrophobic microphase separation
Nature Communications (2024)
-
Linkage conversions in single-crystalline covalent organic frameworks
Nature Chemistry (2024)
-
Metal-free 2-isocyanobiaryl-based cyclization reactions: phenanthridine framework synthesis
Molecular Diversity (2024)
-
Advances of Electrochemical and Electrochemiluminescent Sensors Based on Covalent Organic Frameworks
Nano-Micro Letters (2024)
-
Hydrogen-bonding and π-π interaction promoted solution-processable covalent organic frameworks
Nature Communications (2023)