Abstract
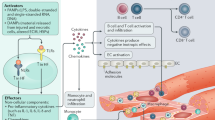
An intense, stereotyped inflammatory response occurs in response to ischaemic and non-ischaemic injury to the myocardium. The NACHT, LRR and PYD domains-containing protein 3 (NLRP3) inflammasome is a finely regulated macromolecular protein complex that senses the injury and triggers and amplifies the inflammatory response by activation of caspase 1; cleavage of pro-inflammatory cytokines, such as pro-IL-1β and pro-IL-18, to their mature forms; and induction of inflammatory cell death (pyroptosis). Inhibitors of the NLRP3 inflammasome and blockers of IL-1β and IL-18 activity have been shown to reduce injury to the myocardium and pericardium, favour resolution of the inflammation and preserve cardiac function. In this Review, we discuss the components of the NLRP3 inflammasome and how it is formed and activated in various ischaemic and non-ischaemic cardiac pathologies (acute myocardial infarction, cardiac dysfunction and remodelling, atherothrombosis, myocarditis and pericarditis, cardiotoxicity and cardiac sarcoidosis). We also summarize current preclinical and clinical evidence from studies of agents that target the NLRP3 inflammasome and related cytokines.
Key points
-
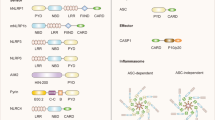
The NACHT, LRR and PYD domains-containing protein 3 (NLRP3) inflammasome is a macromolecular, intracellular structure that functions as a sensor for injury.
-
NLRP3 inflammasome activation amplifies the inflammatory response and tissue injury by regulating the processing and release of IL-1β and IL-18 and causing cell death by pyroptosis.
-
Preclinical studies with genetically modified mouse models and the use of targeted inhibitors have shown that inhibiting activation of the NLRP3 inflammasome reduces inflammatory injury and adverse remodelling.
-
Colchicine, a non-selective NLRP3 inflammasome inhibitor, has been shown to be efficacious in the treatment of pericarditis and in reducing atherothrombotic risk in patients with coronary artery disease.
-
IL-1 blockers have been shown in phase Ib–III trials to reduce cardiovascular risk and morbidity across a wide range of cardiovascular diseases, including myocardial infarction, heart failure, acute myocarditis and recurrent pericarditis.
-
Targeted NLRP3 inflammasome inhibitors and blockers of IL-18 and IL-6, downstream of IL-1, are in clinical testing for various cardiovascular diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Tsao, C. W. et al. Heart disease and stroke statistics — 2022 update: a report from the American Heart Association. Circulation 145, e153–e639 (2022).
Lenz, A., Franklin, G. A. & Cheadle, W. G. Systemic inflammation after trauma. Injury 38, 1336–1345 (2007).
Abbate, A. et al. Interleukin-1 and the inflammasome as therapeutic targets in cardiovascular disease. Circ. Res. 126, 1260–1280 (2020).
Toldo, S. & Abbate, A. The NLRP3 inflammasome in acute myocardial infarction. Nat. Rev. Cardiol. 15, 203–214 (2018).
Westman, P. C. et al. Inflammation as a driver of adverse left ventricular remodeling after acute myocardial infarction. J. Am. Coll. Cardiol. 67, 2050–2060 (2016).
Seropian, I. M., Toldo, S., Van Tassell, B. W. & Abbate, A. Anti-inflammatory strategies for ventricular remodeling following ST-segment elevation acute myocardial infarction. J. Am. Coll. Cardiol. 63, 1593–1603 (2014).
Gao, X.-M., White, D. A., Dart, A. M. & Du, X.-J. Post-infarct cardiac rupture: recent insights on pathogenesis and therapeutic interventions. Pharmacol. Ther. 134, 156–179 (2012).
Abbate, A. et al. Alterations in the interleukin-1/interleukin-1 receptor antagonist balance modulate cardiac remodeling following myocardial infarction in the mouse. PLoS ONE 6, e27923 (2011).
Savvatis, K. et al. Interleukin-23 deficiency leads to impaired wound healing and adverse prognosis after myocardial infarction. Circ. Heart Fail. 7, 161–171 (2014).
Dinarello, C. A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 281, 8–27 (2018).
Swanson, K. V., Deng, M. & Ting, J. P.-Y. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 19, 477–489 (2019).
Zheng, D., Liwinski, T. & Elinav, E. Inflammasome activation and regulation: toward a better understanding of complex mechanisms. Cell Discov. 6, 1–22 (2020).
Viganò, E. et al. Human caspase-4 and caspase-5 regulate the one-step non-canonical inflammasome activation in monocytes. Nat. Commun. 6, 8761 (2015).
Matikainen, S., Nyman, T. A. & Cypryk, W. Function and regulation of noncanonical caspase-4/5/11 inflammasome. J. Immunol. 204, 3063–3069 (2020).
Ma, Q. Pharmacological inhibition of the NLRP3 inflammasome: structure, molecular activation, and inhibitor-NLRP3 interaction. Pharmacol. Rev. 75, 487–520 (2023).
Lu, A. et al. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell 156, 1193–1206 (2014).
Dinarello, C. A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 117, 3720–3732 (2011).
Westermann, D. et al. Cardioprotective and anti-inflammatory effects of interleukin converting enzyme inhibition in experimental diabetic cardiomyopathy. Diabetes 56, 1834–1841 (2007).
Xia, S. et al. Gasdermin D pore structure reveals preferential release of mature interleukin-1. Nature 593, 607–611 (2021).
Santa Cruz Garcia, A. B., Schnur, K. P., Malik, A. B. & Mo, G. C. H. Gasdermin D pores are dynamically regulated by local phosphoinositide circuitry. Nat. Commun. 13, 52 (2022).
Erickson, H. P. Size and shape of protein molecules at the nanometer level determined by sedimentation, gel filtration, and electron microscopy. Biol. Proced. Online 11, 32–51 (2009).
Rider, P., Carmi, Y., Voronov, E. & Apte, R. N. Interleukin-1α. Semin. Immunol. 25, 430–438 (2013).
Gross, O. et al. Inflammasome activators induce interleukin-1α secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity 36, 388–400 (2012).
Merkle, S. et al. A role for caspase-1 in heart failure. Circ. Res. 100, 645–653 (2007).
Liu, X. et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535, 153–158 (2016).
Kayagaki, N. et al. NINJ1 mediates plasma membrane rupture during lytic cell death. Nature 591, 131–136 (2021).
Magnani, L., Colantuoni, M. & Mortellaro, A. Gasdermins: new therapeutic targets in host defense, inflammatory diseases, and cancer. Front. Immunol. 13, 898298 (2022).
Tsuchiya, K. et al. Gasdermin D mediates the maturation and release of IL-1α downstream of inflammasomes. Cell Rep. 34, 108887 (2021).
Shao, W., Yeretssian, G., Doiron, K., Hussain, S. N. & Saleh, M. The caspase-1 digestome identifies the glycolysis pathway as a target during infection and septic shock. J. Biol. Chem. 282, 36321–36329 (2007).
Shen, J. et al. Caspase-1 recognizes extended cleavage sites in its natural substrates. Atherosclerosis 210, 422–429 (2010).
Downs, K. P., Nguyen, H., Dorfleutner, A. & Stehlik, C. An overview of the non-canonical inflammasome. Mol. Asp. Med. 76, 100924 (2020).
Toldo, S. et al. Independent roles of the priming and the triggering of the NLRP3 inflammasome in the heart. Cardiovasc. Res. 105, 203–212 (2015).
Li, X. et al. NOD2 deficiency protects against cardiac remodeling after myocardial infarction in mice. Cell Physiol. Biochem. 32, 1857–1866 (2013).
Chevriaux, A. et al. Cathepsin B is required for NLRP3 inflammasome activation in macrophages, through NLRP3 interaction. Front. Cell Dev. Biol. 8, 167 (2020).
Rajamäki, K. et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS ONE 5, e11765 (2010).
Toldo, S., Mauro, A. G., Cutter, Z. & Abbate, A. Inflammasome, pyroptosis, and cytokines in myocardial ischemia–reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 315, H1553–H1568 (2018).
Takahashi, M. NLRP3 inflammasome as a novel player in myocardial infarction. Int. Heart J. 55, 101–105 (2014).
Mezzaroma, E., Abbate, A. & Toldo, S. The inflammasome in heart failure. Curr. Opin. Physiol. 19, 105–112 (2021).
Netea, M. G. et al. Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood 113, 2324–2335 (2009).
Mezzaroma, E. et al. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc. Natl Acad. Sci. USA 108, 19725–19730 (2011).
Suetomi, T. et al. Inflammation and NLRP3 inflammasome activation initiated in response to pressure overload by Ca2+/calmodulin-dependent protein kinase II δ signaling in cardiomyocytes are essential for adverse cardiac remodeling. Circulation 138, 2530–2544 (2018).
Willeford, A. et al. CaMKIIδ-mediated inflammatory gene expression and inflammasome activation in cardiomyocytes initiate inflammation and induce fibrosis. JCI Insight 3, e97054 (2018).
Marchetti, C. et al. Pharmacologic inhibition of the NLRP3 inflammasome preserves cardiac function after ischemic and nonischemic injury in the mouse. J. Cardiovasc. Pharmacol. 66, 1 (2015).
Wang, H. et al. Inflammasome-independent NLRP3 is required for epithelial-mesenchymal transition in colon cancer cells. Exp. Cell Res. 342, 184–192 (2016).
Wang, W. et al. Inflammasome-independent NLRP3 augments TGF-β signaling in kidney epithelium. J. Immunol. 190, 1239–1249 (2013).
Zuurbier, C. J. et al. Deletion of the innate immune NLRP3 receptor abolishes cardiac ischemic preconditioning and is associated with decreased Il-6/STAT3 signaling. PLoS ONE 7, e40643 (2012).
He, Y., Zeng, M. Y., Yang, D., Motro, B. & Núñez, G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature 530, 354–357 (2016).
Ito, M. et al. Bruton’s tyrosine kinase is essential for NLRP3 inflammasome activation and contributes to ischaemic brain injury. Nat. Commun. 6, 7360 (2015).
O’Riordan, C. E. et al. Bruton’s tyrosine kinase inhibition attenuates the cardiac dysfunction caused by cecal ligation and puncture in mice. Front. Immunol. 10, 2129 (2019).
Liu, Y. et al. TXNIP mediates NLRP3 inflammasome activation in cardiac microvascular endothelial cells as a novel mechanism in myocardial ischemia/reperfusion injury. Basic Res. Cardiol. 109, 415 (2014).
He, K. et al. Inhibition of NLRP3 inflammasome by thioredoxin-interacting protein in mouse Kupffer cells as a regulatory mechanism for non-alcoholic fatty liver disease development. Oncotarget 8, 37657–37672 (2017).
Elshaer, S. L. et al. Deletion of TXNIP mitigates high-fat diet-impaired angiogenesis and prevents inflammation in a mouse model of critical limb ischemia. Antioxid. Basel Switz. 6, 47 (2017).
Masters, S. L. et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat. Immunol. 11, 897–904 (2010).
Muri, J., Thut, H., Feng, Q. & Kopf, M. Thioredoxin-1 distinctly promotes NF-κB target DNA binding and NLRP3 inflammasome activation independently of Txnip. eLife 9, e53627 (2020).
Sun, Q., Fan, J., Billiar, T. R. & Scott, M. J. Inflammasome and autophagy regulation: a two-way street. Mol. Med. 23, 188–195 (2017).
Saitoh, T. et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature 456, 264–268 (2008).
Nakahira, K. et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol. 12, 222–230 (2011).
Shi, C.-S. et al. Activation of autophagy by inflammatory signals limits IL-1β production by targeting ubiquitinated inflammasomes for destruction. Nat. Immunol. 13, 255–263 (2012).
Li, L. et al. ATP6AP2 knockdown in cardiomyocyte deteriorates heart function via compromising autophagic flux and NLRP3 inflammasome activation. Cell Death Discov. 8, 161 (2022).
Mao, S., Chen, P., Pan, W., Gao, L. & Zhang, M. Exacerbated post‐infarct pathological myocardial remodelling in diabetes is associated with impaired autophagy and aggravated NLRP3 inflammasome activation. ESC Heart Fail. 9, 303–317 (2021).
Zhang, D. et al. Activation of autophagy inhibits nucleotide‐binding oligomerization domain‐like receptor protein 3 inflammasome activation and attenuates myocardial ischemia–reperfusion injury in diabetic rats. J. Diabetes Investig. 11, 1126–1136 (2020).
Paik, S., Kim, J. K., Silwal, P., Sasakawa, C. & Jo, E.-K. An update on the regulatory mechanisms of NLRP3 inflammasome activation. Cell. Mol. Immunol. 18, 1141–1160 (2021).
Kim, J. S., He, L., Qian, T. & Lemasters, J. J. Role of the mitochondrial permeability transition in apoptotic and necrotic death after ischemia/reperfusion injury to hepatocytes. Curr. Mol. Med. 3, 527–535 (2003).
Zhou, R., Yazdi, A. S., Menu, P. & Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225 (2011).
Sadatomi, D. et al. Mitochondrial function is required for extracellular ATP-induced NLRP3 inflammasome activation. J. Biochem. 161, 503–512 (2017).
Park, S. et al. Defective mitochondrial fission augments NLRP3 inflammasome activation. Sci. Rep. 5, 15489 (2015).
Mills, E. L., Kelly, B. & O’Neill, L. A. J. Mitochondria are the powerhouses of immunity. Nat. Immunol. 18, 488–498 (2017).
Iyer, S. S. et al. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity 39, 311–323 (2013).
Dagvadorj, J. et al. Recruitment of pro-IL-1α to mitochondrial cardiolipin, via shared LC3 binding domain, inhibits mitophagy and drives maximal NLRP3 activation. Proc. Natl Acad. Sci. USA 118, e2015632118 (2021).
Pereira, A. C. et al. Mitochondria fusion upon SERCA inhibition prevents activation of the NLRP3 inflammasome in human monocytes. Cells 11, 433 (2022).
Kawaguchi, M. et al. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation 123, 594–604 (2011).
Wang, L., Qu, P., Zhao, J. & Chang, Y. NLRP3 and downstream cytokine expression elevated in the monocytes of patients with coronary artery disease. Arch. Med. Sci. 10, 791–800 (2014).
Toldo, S. et al. Inhibition of the NLRP3 inflammasome limits the inflammatory injury following myocardial ischemia–reperfusion in the mouse. Int. J. Cardiol. 209, 215–220 (2016).
Sandanger, Ø. et al. The NLRP3 inflammasome is up-regulated in cardiac fibroblasts and mediates myocardial ischaemia–reperfusion injury. Cardiovasc. Res. 99, 164–174 (2013).
Mastrocola, R. et al. Pharmacological inhibition of NLRP3 inflammasome attenuates myocardial ischemia/reperfusion injury by activation of RISK and mitochondrial pathways. Oxid. Med. Cell Longev. 2016, 5271251 (2016).
Takahashi, M. Cell-specific roles of NLRP3 inflammasome in myocardial infarction. J. Cardiovasc. Pharmacol. 74, 188 (2019).
Toldo, S. et al. The inflammasome in myocardial injury and cardiac remodeling. Antioxid. Redox Signal. 22, 1146–1161 (2015).
Toldo, S. et al. Induction of microRNA-21 with exogenous hydrogen sulfide attenuates myocardial ischemic and inflammatory injury in mice. Circ. Cardiovasc. Genet. 7, 311–320 (2014).
Jong, W. M. C. et al. Nlrp3 plays no role in acute cardiac infarction due to low cardiac expression. Int. J. Cardiol. 177, 41–43 (2014).
Toldo, S. et al. The NLRP3 inflammasome inhibitor, OLT1177 (Dapansutrile), reduces infarct size and preserves contractile function after ischemia–reperfusion injury in the mouse. J. Cardiovasc. Pharmacol. 73, 215–222 (2019).
Abbate, A. et al. Anakinra, a recombinant human interleukin-1 receptor antagonist, inhibits apoptosis in experimental acute myocardial infarction. Circulation 117, 2670–2683 (2008).
Toldo, S. et al. Interleukin-1β blockade improves left ventricular systolic/diastolic function and restores contractility reserve in severe ischemic cardiomyopathy in the mouse. J. Cardiovasc. Pharmacol. 64, 1–6 (2014).
Toldo, S. et al. Interleukin-1β blockade improves cardiac remodelling after myocardial infarction without interrupting the inflammasome in the mouse. Exp. Physiol. 98, 734–745 (2013).
Lugrin, J. et al. Cutting edge: IL-1α is a crucial danger signal triggering acute myocardial inflammation during myocardial infarction. J. Immunol. 194, 499–503 (2015).
Van Tassell, B. W. et al. Interleukin-1 trap attenuates cardiac remodeling after experimental acute myocardial infarction in mice. J. Cardiovasc. Pharmacol. 55, 117–122 (2010).
Mauro, A. G. et al. Reduction of myocardial ischemia–reperfusion injury by inhibiting interleukin-1 alpha. J. Cardiovasc. Pharmacol. 69, 156–160 (2017).
Sager, H. B. et al. Targeting interleukin-1β reduces leukocyte production after acute myocardial infarction. Circulation 132, 1880–1890 (2015).
England, H., Summersgill, H. R., Edye, M. E., Rothwell, N. J. & Brough, D. Release of interleukin-1α or interleukin-1β depends on mechanism of cell death. J. Biol. Chem. 289, 15942–15950 (2014).
Pomerantz, B. J., Reznikov, L. L., Harken, A. H. & Dinarello, C. A. Inhibition of caspase 1 reduces human myocardial ischemic dysfunction via inhibition of IL-18 and IL-1beta. Proc. Natl Acad. Sci. USA 98, 2871–2876 (2001).
Venkatachalam, K. et al. Neutralization of interleukin-18 ameliorates ischemia/reperfusion-induced myocardial injury. J. Biol. Chem. 284, 7853–7865 (2009).
Gu, H. et al. The protective role of interleukin-18 binding protein in a murine model of cardiac ischemia/reperfusion injury. Transpl. Int. 28, 1436–1444 (2015).
Quader, M., Mezzaroma, E., Kenning, K. & Toldo, S. Modulation of interleukin-1 and -18 mediated injury in donation after circulatory death mouse hearts. J. Surg. Res. 257, 468–476 (2021).
Pörksen, G. et al. Periodic fever, mild arthralgias, and reversible moderate and severe organ inflammation associated with the V198M mutation in the CIAS1 gene in three German patients — expanding phenotype of CIAS1 related autoinflammatory syndrome. Eur. J. Haematol. 73, 123–127 (2004).
Kumar, A. et al. Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. J. Exp. Med. 183, 949–958 (1996).
Fujimura, K. et al. NLRP3 inflammasome-driven IL-1β and IL-18 contribute to lipopolysaccharide-induced septic cardiomyopathy. J. Mol. Cell. Cardiol. 180, 58–68 (2023).
Van Tassell, B. W., Toldo, S., Mezzaroma, E. & Abbate, A. Targeting interleukin-1 in heart disease. Circulation 128, 1910–1923 (2013).
Toldo, S. et al. Interleukin-18 mediates interleukin-1-induced cardiac dysfunction. Am. J. Physiol. Heart Circ. Physiol. 306, H1025–H1031 (2014).
Van Tassell, B. W., Seropian, I. M., Toldo, S., Mezzaroma, E. & Abbate, A. Interleukin-1β induces a reversible cardiomyopathy in the mouse. Inflamm. Res. 62, 637–640 (2013).
Takahashi, M. NLRP3 inflammasome as a key driver of vascular disease. Cardiovasc. Res. 118, 372–385 (2022).
Duewell, P. et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361 (2010).
Alexander, M. R. et al. Genetic inactivation of IL-1 signaling enhances atherosclerotic plaque instability and reduces outward vessel remodeling in advanced atherosclerosis in mice. J. Clin. Invest. 122, 70–79 (2012).
Dragoljevic, D. et al. Inhibition of interleukin-1β signalling promotes atherosclerotic lesion remodelling in mice with inflammatory arthritis. Clin. Transl. Immunol. 9, e1206 (2020).
Hettwer, J. et al. Interleukin-1β suppression dampens inflammatory leucocyte production and uptake in atherosclerosis. Cardiovasc. Res. 118, 2778–2791 (2022).
Jiang, M. et al. Caspase-11-gasdermin D-mediated pyroptosis is involved in the pathogenesis of atherosclerosis. Front. Pharmacol. 12, 657486 (2021).
Toldo, S. et al. Formation of the inflammasome in acute myocarditis. Int. J. Cardiol. 171, e119–e121 (2014).
Pappritz, K. et al. Colchicine prevents disease progression in viral myocarditis via modulating the NLRP3 inflammasome in the cardiosplenic axis. ESC Heart Fail. 9, 925–941 (2022).
Yu, Y. et al. Inhibition of calpain alleviates coxsackievirus B3-induced myocarditis through suppressing the canonical NLRP3 inflammasome/caspase-1-mediated and noncanonical caspase-11-mediated pyroptosis pathways. Am. J. Transl. Res. 12, 1954–1964 (2020).
Kron, J. et al. Inflammasome formation in granulomas in cardiac sarcoidosis. Circ. Arrhythm. Electrophysiol. 12, e007582 (2019).
Huppertz, C. et al. The NLRP3 inflammasome pathway is activated in sarcoidosis and involved in granuloma formation. Eur. Respir. J. 55, 1900119 (2020).
Zhang, W. et al. Cardiac fibroblasts contribute to myocardial dysfunction in mice with sepsis: the role of NLRP3 inflammasome activation. PLoS ONE 9, e107639 (2014).
Mauro, A. G. et al. NLRP3-mediated inflammation in cardio-oncology: sterile yet harmful. Transl. Res. J. Lab. Clin. Med. 252, 9–20 (2023).
Zeng, C. et al. NLRP3 inflammasome-mediated pyroptosis contributes to the pathogenesis of non-ischemic dilated cardiomyopathy. Redox Biol. 34, 101523 (2020).
Li, R. et al. Triptolide attenuates pressure overload-induced myocardial remodeling in mice via the inhibition of NLRP3 inflammasome expression. Biochem. Biophys. Res. Commun. 485, 69–75 (2017).
Dang, S. et al. Blockade of β-adrenergic signaling suppresses inflammasome and alleviates cardiac fibrosis. Ann. Transl. Med. 8, 127 (2020).
Zhang, L., Ai, C., Bai, M., Niu, J. & Zhang, Z. NLRP3 inflammasome/pyroptosis: a key driving force in diabetic cardiomyopathy. Int. J. Mol. Sci. 23, 10632 (2022).
Sokolova, M. et al. NLRP3 inflammasome promotes myocardial remodeling during diet-induced obesity. Front. Immunol. 10, 1621 (2019).
Carbone, S. et al. An orally available NLRP3 inflammasome inhibitor prevents western diet-induced cardiac dysfunction in mice. J. Cardiovasc. Pharmacol. 72, 303–307 (2018).
Shen, S. et al. Colchicine alleviates inflammation and improves diastolic dysfunction in heart failure rats with preserved ejection fraction. Eur. J. Pharmacol. 929, 175126 (2022).
Xia, Y.-Y. et al. Involvement of pyroptosis pathway in epicardial adipose tissue — myocardium axis in experimental heart failure with preserved ejection fraction. Biochem. Biophys. Res. Commun. 636, 62–70 (2022).
Yao, C. et al. Enhanced cardiomyocyte NLRP3 inflammasome signaling promotes atrial fibrillation. Circulation 138, 2227–2242 (2018).
Dubuisson, N. et al. Inhibiting the inflammasome with MCC950 counteracts muscle pyroptosis and improves Duchenne muscular dystrophy. Front. Immunol. 13, 1049076 (2022).
Mauro, A. G. et al. The role of NLRP3 inflammasome in pericarditis: potential for therapeutic approaches. JACC Basic Transl. Sci. 6, 137–150 (2021).
Vecchié, A. et al. Interleukin-1 and the NLRP3 inflammasome in pericardial disease. Curr. Cardiol. Rep. 23, 157 (2021).
Lamkanfi, M. et al. Glyburide inhibits the cryopyrin/Nalp3 inflammasome. J. Cell Biol. 187, 61–70 (2009).
Marchetti, C. et al. A novel pharmacologic inhibitor of the NLRP3 inflammasome limits myocardial injury after ischemia–reperfusion in the mouse. J. Cardiovasc. Pharmacol. 63, 316–322 (2014).
Fulp, J. et al. Structural insights of benzenesulfonamide analogues as NLRP3 inflammasome inhibitors: design, synthesis, and biological characterization. J. Med. Chem. 61, 5412–5423 (2018).
Coll, R. C. et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 21, 248–255 (2015).
van Hout, G. P. J. et al. The selective NLRP3-inflammasome inhibitor MCC950 reduces infarct size and preserves cardiac function in a pig model of myocardial infarction. Eur. Heart J. 38, 828–836 (2017).
Gao, R. et al. The selective NLRP3-inflammasome inhibitor MCC950 reduces myocardial fibrosis and improves cardiac remodeling in a mouse model of myocardial infarction. Int. Immunopharmacol. 74, 105575 (2019).
Wang, M. et al. MCC950, a selective NLRP3 inhibitor, attenuates adverse cardiac remodeling following heart failure through improving the cardiometabolic dysfunction in obese mice. Front. Cardiovasc. Med. 9, 727424 (2022).
Zheng, G. et al. The selective NLRP3-inflammasome inhibitor MCC950 mitigates post-resuscitation myocardial dysfunction and improves survival in a rat model of cardiac arrest and resuscitation. Cardiovasc. Drugs Ther. 37, 423–433 (2023).
van der Heijden, T. et al. NLRP3 inflammasome inhibition by MCC950 reduces atherosclerotic lesion development in apolipoprotein E-deficient mice — brief report. Arterioscler. Thromb. Vasc. Biol. 37, 1457–1461 (2017).
Ren, P. et al. Targeting the NLRP3 inflammasome with inhibitor MCC950 prevents aortic aneurysms and dissections in mice. J. Am. Heart Assoc. 9, e014044 (2020).
Marchetti, C. et al. OLT1177, a β-sulfonyl nitrile compound, safe in humans, inhibits the NLRP3 inflammasome and reverses the metabolic cost of inflammation. Proc. Natl Acad. Sci. USA 115, E1530–E1539 (2018).
Marchetti, C. et al. NLRP3 inflammasome inhibitor OLT1177 suppresses joint inflammation in murine models of acute arthritis. Arthritis Res. Ther. 20, 169 (2018).
Aliaga, J. et al. Preservation of contractile reserve and diastolic function by inhibiting the NLRP3 inflammasome with OLT1177® (Dapansutrile) in a mouse model of severe ischemic cardiomyopathy due to non-reperfused anterior wall myocardial infarction. Mol. Basel Switz. 26, 3534 (2021).
Oronsky, B. et al. Discovery of RRx-001, a Myc and CD47 downregulating small molecule with tumor targeted cytotoxicity and healthy tissue cytoprotective properties in clinical development. J. Med. Chem. 64, 7261–7271 (2021).
Chen, Y. et al. RRx-001 ameliorates inflammatory diseases by acting as a potent covalent NLRP3 inhibitor. Cell. Mol. Immunol. 18, 1425–1436 (2021).
Juliana, C. et al. Anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome. J. Biol. Chem. 285, 9792–9802 (2010).
Kim, Y. S. et al. BAY 11-7082, a nuclear factor-κB inhibitor, reduces inflammation and apoptosis in a rat cardiac ischemia–reperfusion injury model. Int. Heart J. 51, 348–353 (2010).
Cocco, M. et al. Design, synthesis, and evaluation of acrylamide derivatives as direct NLRP3 inflammasome inhibitors. ChemMedChem 11, 1790–1803 (2016).
Jiang, H. et al. Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J. Exp. Med. 214, 3219–3238 (2017).
Gao, R.-F. et al. The covalent NLRP3-inflammasome inhibitor Oridonin relieves myocardial infarction induced myocardial fibrosis and cardiac remodeling in mice. Int. Immunopharmacol. 90, 107133 (2021).
Zhang, F. et al. Therapeutic potential of colchicine in cardiovascular medicine: a pharmacological review. Acta Pharmacol. Sin. 43, 2173–2190 (2022).
Fujisue, K. et al. Colchicine improves survival, left ventricular remodeling, and chronic cardiac function after acute myocardial infarction. Circ. J. 81, 1174–1182 (2017).
Imazio, M. & Nidorf, M. Colchicine and the heart. Eur. Heart J. 42, 2745–2760 (2021).
Deftereos, S. et al. Anti-inflammatory treatment with colchicine in acute myocardial infarction: a pilot study. Circulation 132, 1395–1403 (2015).
Cole, J. et al. Colchicine to prevent periprocedural myocardial injury in percutaneous coronary intervention: the COPE-PCI pilot trial. Circ. Cardiovasc. Interv. 14, e009992 (2021).
Hosseini, S. H. et al. Preprocedural colchicine in patients with acute ST-elevation myocardial infarction undergoing percutaneous coronary intervention: a randomized controlled trial (PodCAST-PCI). J. Cardiovasc. Pharmacol. 80, 592–599 (2022).
Deftereos, S. et al. Colchicine treatment for the prevention of bare-metal stent restenosis in diabetic patients. J. Am. Coll. Cardiol. 61, 1679–1685 (2013).
Tardif, J.-C. et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N. Engl. J. Med. 381, 2497–2505 (2019).
Nidorf, S. M., Eikelboom, J. W., Budgeon, C. A. & Thompson, P. L. Low-dose colchicine for secondary prevention of cardiovascular disease. J. Am. Coll. Cardiol. 61, 404–410 (2013).
Nidorf, S. M. et al. Colchicine in patients with chronic coronary disease. N. Engl. J. Med. 383, 1838–1847 (2020).
Deftereos, S. et al. Anti-inflammatory treatment with colchicine in stable chronic heart failure: a prospective, randomized study. JACC Heart Fail. 2, 131–137 (2014).
Imazio, M. et al. Colchicine in addition to conventional therapy for acute pericarditis: results of the COlchicine for acute PEricarditis (COPE) trial. Circulation 112, 2012–2016 (2005).
Imazio, M. et al. Colchicine as first-choice therapy for recurrent pericarditis: results of the CORE (COlchicine for REcurrent pericarditis) trial. Arch. Intern. Med. 165, 1987–1991 (2005).
Imazio, M. et al. Colchicine for recurrent pericarditis (CORP): a randomized trial. Ann. Intern. Med. 155, 409–414 (2011).
Imazio, M. et al. A randomized trial of colchicine for acute pericarditis. N. Engl. J. Med. 369, 1522–1528 (2013).
Imazio, M. et al. Efficacy and safety of colchicine for treatment of multiple recurrences of pericarditis (CORP-2): a multicentre, double-blind, placebo-controlled, randomised trial. Lancet Lond. Engl. 383, 2232–2237 (2014).
Tong, D. C. et al. Colchicine in patients with acute coronary syndrome. Circulation 12, 171–181 (2020).
Fiolet, A. T. L. et al. Efficacy and safety of low-dose colchicine in patients with coronary disease: a systematic review and meta-analysis of randomized trials. Eur. Heart J. 42, 2765–2775 (2021).
Abbate, A. et al. Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU-ART] Pilot Study). Am. J. Cardiol. 105, 1371–1377.e1 (2010).
Abbate, A. et al. Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the Virginia Commonwealth University-Anakinra Remodeling Trial (2) (VCU-ART2) pilot study]. Am. J. Cardiol. 111, 1394–1400 (2013).
Abbate, A. et al. Interleukin-1 blockade inhibits the acute inflammatory response in patients with ST-segment-elevation myocardial infarction. J. Am. Heart Assoc. 9, e014941 (2020).
Abbate, A. et al. Interleukin-1 blockade with anakinra and heart failure following ST-segment elevation myocardial infarction: results from a pooled analysis of the VCUART clinical trials. Eur. Heart J. Cardiovasc. Pharmacother. 8, 503–510 (2022).
Morton, A. C. et al. The effect of interleukin-1 receptor antagonist therapy on markers of inflammation in non-ST elevation acute coronary syndromes: the MRC-ILA Heart Study. Eur. Heart J. 36, 377–384 (2015).
Van Tassell, B. W. et al. Effects of interleukin-1 blockade with anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D-HART pilot study). Am. J. Cardiol. 113, 321–327 (2014).
Van Tassell, B. W. et al. Interleukin-1 blockade in recently decompensated systolic heart failure: results from REDHART (Recently Decompensated Heart Failure Anakinra Response Trial). Circ. Heart Fail. 10, e004373 (2017).
Van Tassell, B. W. et al. Enhanced interleukin-1 activity contributes to exercise intolerance in patients with systolic heart failure. PLoS ONE 7, e33438 (2012).
Van Tassell, B. W. et al. Interleukin-1 blockade in acute decompensated heart failure: a randomized, double-blinded, placebo-controlled pilot study. J. Cardiovasc. Pharmacol. 67, 544–551 (2016).
Van Tassell, B. W. et al. IL-1 blockade in patients with heart failure with preserved ejection fraction. Circ. Heart Fail. 11, e005036 (2018).
Arnold, D. D., Yalamanoglu, A. & Boyman, O. Systematic review of safety and efficacy of IL-1-targeted biologics in treating immune-mediated disorders. Front. Immunol. 13, 888392 (2022).
Cavalli, G. et al. Treating life-threatening myocarditis by blocking interleukin-1. Crit. Care Med. 44, e751–e754 (2016).
Kerneis, M. et al. Rationale and design of the ARAMIS trial: anakinra versus placebo, a double blind randomized controlled trial for the treatment of acute myocarditis. Arch. Cardiovasc. Dis. https://doi.org/10.1016/j.acvd.2023.07.004 (2023).
Wohlford, G. F. et al. Acute effects of interleukin-1 blockade using anakinra in patients with acute pericarditis. J. Cardiovasc. Pharmacol. 76, 50–52 (2020).
Brucato, A. et al. Effect of anakinra on recurrent pericarditis among patients with colchicine resistance and corticosteroid dependence: the AIRTRIP randomized clinical trial. J. Am. Med. Assoc. 316, 1906–1912 (2016).
Ikonomidis, I. et al. Inhibition of interleukin-1 by anakinra improves vascular and left ventricular function in patients with rheumatoid arthritis. Circulation 117, 2662–2669 (2008).
Ikonomidis, I. et al. Lowering interleukin-1 activity with anakinra improves myocardial deformation in rheumatoid arthritis. Heart Br. Card. Soc. 95, 1502–1507 (2009).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Everett, B. M. et al. Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation 139, 1289–1299 (2019).
Everett, B. M. et al. Inhibition of interleukin-1β and reduction in atherothrombotic cardiovascular events in the CANTOS trial. J. Am. Coll. Cardiol. 76, 1660–1670 (2020).
Rothman, A. M. et al. Effects of interleukin-1β inhibition on blood pressure, incident hypertension, and residual inflammatory risk: a secondary analysis of CANTOS. Hypertension 75, 477–482 (2020).
Trankle, C. R. et al. Usefulness of canakinumab to improve exercise capacity in patients with long-term systolic heart failure and elevated C-reactive protein. Am. J. Cardiol. 122, 1366–1370 (2018).
Ks, R. et al. A randomized, placebo-controlled trial of canakinumab in patients with peripheral artery disease. Vasc. Med. Lond. Engl. 24, 414–421 (2019).
Svensson, E. C. et al. TET2-driven clonal hematopoiesis and response to canakinumab: an exploratory analysis of the CANTOS randomized clinical trial. JAMA Cardiol. 7, 521–528 (2022).
Klein, A. L. et al. Phase 3 trial of interleukin-1 trap rilonacept in recurrent pericarditis. N. Engl. J. Med. 384, 31–41 (2021).
Myachikova, V. Y. et al. Treatment of idiopathic recurrent pericarditis with goflikicept: phase II/III study results. J. Am. Coll. Cardiol. 82, 30–40 (2023).
Samsonov, M., Bogin, V., Van Tassell, B. W. & Abbate, A. Interleukin-1 blockade with RPH-104 in patients with acute ST-elevation myocardial infarction: study design and rationale. J. Transl. Med. 19, 169 (2021).
Ridker, P. M., MacFadyen, J. G., Thuren, T. & Libby, P. Residual inflammatory risk associated with interleukin-18 and interleukin-6 after successful interleukin-1β inhibition with canakinumab: further rationale for the development of targeted anti-cytokine therapies for the treatment of atherothrombosis. Eur. Heart J. 41, 2153–2163 (2020).
Mistry, P. et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of single-dose antiinterleukin-18 mAb GSK1070806 in healthy and obese subjects. Int. J. Clin. Pharmacol. Ther. 52, 867–879 (2014).
Gabay, C. et al. Open-label, multicentre, dose-escalating phase II clinical trial on the safety and efficacy of tadekinig alfa (IL-18BP) in adult-onset Still’s disease. Ann. Rheum. Dis. 77, 840–847 (2018).
Ridker, P. M. & Rane, M. Interleukin-6 signaling and anti-interleukin-6 therapeutics in cardiovascular disease. Circ. Res. 128, 1728–1746 (2021).
Broch, K. et al. Randomized trial of interleukin-6 receptor inhibition in patients with acute ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 77, 1845–1855 (2021).
Meyer, M. A. S. et al. Treatment effects of interleukin-6 receptor antibodies for modulating the systemic inflammatory response after out-of-hospital cardiac arrest (The IMICA Trial): a double-blinded, placebo-controlled, single-center, randomized, clinical trial. Circulation 143, 1841–1851 (2021).
Christensen, R. H. et al. Aerobic exercise induces cardiac fat loss and alters cardiac muscle mass through an interleukin-6 receptor-dependent mechanism: cardiac analysis of a double-blind randomized controlled clinical trial in abdominally obese humans. Circulation 140, 1684–1686 (2019).
Ridker, P. M. et al. IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Lond. Engl. 397, 2060–2069 (2021).
NIH National Library of Medicine. ZEUS — a research study to look at how ziltivekimab works compared to placebo in people with cardiovascular disease, chronic kidney disease and inflammation (ZEUS). ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05021835 (2023).
NIH National Library of Medicine. A research study to look at how ziltivekimab works compared to placebo in people with heart failure and inflammation (HERMES). ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05636176 (2023).
Wohlford, G. F. et al. Phase 1B, randomized, double-blinded, dose escalation, single-center, repeat dose safety and pharmacodynamics study of the oral NLRP3 inhibitor dapansutrile in subjects with NYHA II-III systolic heart failure. J. Cardiovasc. Pharmacol. 77, 49–60 (2020).
Klück, V. et al. Dapansutrile, an oral selective NLRP3 inflammasome inhibitor, for treatment of gout flares: an open-label, dose-adaptive, proof-of-concept, phase 2a trial. Lancet Rheumatol. 2, e270–e280 (2020).
Madurka, I. et al. DFV890: a new oral NLRP3 inhibitor-tested in an early phase 2a randomised clinical trial in patients with COVID-19 pneumonia and impaired respiratory function. Infection 51, 641–654 (2023).
NIH National Library of Medicine. Study of efficacy, safety and tolerability of DFV890 in patients with knee osteoarthritis. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04886258 (2023).
Parmar, D. V. et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of the oral NLRP3 inflammasome inhibitor ZYIL1: first-in-human phase 1 studies (single ascending dose and multiple ascending dose). Clin. Pharmacol. Drug Dev. 12, 202–211 (2023).
Zydus Lifesciences Limited. A phase 2a, prospective, open-label study to evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of ZYIL1 in subjects with cryopyrin associated periodic syndromes (CAPS). ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT05186051 (2022).
Klughammer, B. et al. P805 Selnoflast, a potent NLRP3 inhibitor — results from a phase 1b experimental medicine study in patients with ulcerative colitis. J. Crohns Colitis 17, i938 (2023).
Inflazome UK Ltd. A phase 1, randomised, double-blind, placebo-controlled, single and multiple ascending dose study to determine the safety, tolerability, pharmacokinetics, pharmacodynamics and food effect of IZD334 in healthy adult participants as well as an open-label cohort to confirm the safety, pharmacokinetics, and pharmacodynamics in adult patients with cryopyrin-associated periodic syndromes. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04086602 (2020).
Clinical Trials Register. https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-000942-32/NL.
Halia Therapeutics, Inc. A phase 1, single-center, randomized, placebo-controlled, single ascending dose study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of HT-6184 in healthy human subjects. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT05447546 (2022).
Zomagen Biosciences, Ltd. A phase 2A, single-arm study to evaluate the safety and clinical activity of VTX2735 in participants with cryopyrin-associated periodic syndrome (CAPS). ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT05812781 (2023).
Pipeline. Nodthera https://www.nodthera.com/pipeline/.
EpicentRx, Inc. A phase 3, controlled, open-label, randomized study of RRx-001 administered sequentially with a platinum doublet or a platinum doublet in third-line or beyond small cell lung cancer. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03699956 (2022).
Akrami, M. et al. Effects of colchicine on major adverse cardiac events in next 6-month period after acute coronary syndrome occurrence; a randomized placebo-control trial. BMC Cardiovasc. Disord. 21, 583 (2021).
Cole, J. et al. COlchicine to prevent periprocedural myocardial injury in percutaneous coronary intervention (COPE-PCI): coronary microvascular physiology pilot substudy. J. Interv. Cardiol. 2022, 1098429 (2022).
Acknowledgements
S.T. is supported by an NIH grant HL150115. A.A. is supported by NIH grants AG076360, HL139943 and HL150115.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
S.T. has received research grants from Cardiol, Kiniksa and Olatec. A.A. has served as a consultant to Cardiol, Implicit Bioscience, Janssen, Kiniksa, Novo Nordisk, Olatec, R-Pharm, Sanofi and Serpin Pharma.
Peer review
Peer review information
Nature Reviews Cardiology thanks Pal Aukrust, Andrew Murphy, Masafumi Takahashi and Zoltan Varga for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Toldo, S., Abbate, A. The role of the NLRP3 inflammasome and pyroptosis in cardiovascular diseases. Nat Rev Cardiol 21, 219–237 (2024). https://doi.org/10.1038/s41569-023-00946-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-023-00946-3
This article is cited by
-
Aerobic exercise mitigates high-fat diet-induced cardiac dysfunction, pyroptosis, and inflammation by inhibiting STING-NLRP3 signaling pathway
Molecular and Cellular Biochemistry (2024)