Abstract
Myocardial infarction (MI), as a result of thrombosis or vascular occlusion, is the most prevalent cause of morbidity and mortality among all cardiovascular diseases. The devastating consequences of MI are compounded by the complexities of cellular functions involved in the initiation and resolution of early-onset inflammation and the longer-term effects related to scar formation. The resultant tissue damage can occur as early as 1 h after MI and activates inflammatory signalling pathways to elicit an immune response. Macrophages are one of the most active cell types during all stages after MI, including the cardioprotective, inflammatory and tissue repair phases. In this Review, we describe the phenotypes of cardiac macrophage involved in MI and their cardioprotective functions. A specific subset of macrophages called resident cardiac macrophages (RCMs) are derived from yolk sac progenitor cells and are maintained as a self-renewing population, although their numbers decrease with age. We explore sophisticated sequencing techniques that demonstrate the cardioprotective properties of this cardiac macrophage phenotype. Furthermore, we discuss the interactions between cardiac macrophages and other important cell types involved in the pathology and resolution of inflammation after MI. We summarize new and promising therapeutic approaches that target macrophage-mediated inflammation and the cardioprotective properties of RCMs after MI. Finally, we discuss future directions for the study of RCMs in MI and cardiovascular health in general.
Key points
-
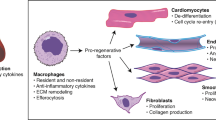
Macrophages are one of the most active cell types participating in the cardiac remodelling events that occur during the inflammatory, proliferative and reparative phases after a myocardial infarction (MI).
-
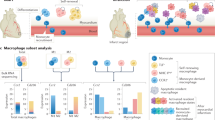
Immediately after an MI, macrophages that accumulate in the infarcted heart tissue are derived from either the bone marrow or splenic reservoirs and produce cytokines that mediate inflammation.
-
Resident cardiac macrophages have an essential role in the development and functioning of the normal heart, whereas after an MI, the surviving resident cardiac macrophages act to offset the inflammatory response mediated by recruited macrophages.
-
In the infarcted heart, macrophages interact with several cell types, including cardiomyocytes, endothelial cells, fibroblasts and lymphocytes, that contribute to the cardiac remodelling that occurs after an MI.
-
A deeper understanding of the role of macrophages in the infarcted heart might lead to macrophage-specific therapeutic approaches to mitigate tissue damage after an MI.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Benjamin, E. J. et al. Heart disease and stroke statistics — 2019 update: a report from the American Heart Association. Circulation 139, e56–e528 (2019).
Nowbar, A. N., Gitto, M., Howard, J. P., Francis, D. P. & Al-Lamee, R. Mortality from ischemic heart disease. Circ. Cardiovasc. Qual. Outcomes 12, e005375 (2019).
Ambrose, J. A. & Dangas, G. Unstable angina: current concepts of pathogenesis and treatment. Arch. Intern. Med. 160, 25–37 (2000).
Newby, D. E. & Fox, K. A. A. Unstable angina: the first 48 hours and later in-hospital management. Br. Med. Bull. 59, 69–87 (2001).
Braunwald, E. Unstable angina and non-ST elevation myocardial infarction. Am. J. Respir. Crit. Care Med. 185, 924–932 (2012).
Swap, C. J. & Nagurney, J. T. Value and limitations of chest pain history in the evaluation of patients with suspected acute coronary syndromes. JAMA 294, 2623–2629 (2005).
Amsterdam, E. A. et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 130, 2354–2394 (2014).
Bagai, A., Dangas, G. D., Stone, G. W. & Granger, C. B. Reperfusion strategies in acute coronary syndromes. Circ. Res. 114, 1918–1928 (2014).
Crea, F. & Libby, P. Acute coronary syndromes: the way forward from mechanisms to precision treatment. Circulation 136, 1155–1166 (2017).
Gerber, Y. et al. Contemporary trends in heart failure with reduced and preserved ejection fraction after myocardial infarction: a community study. Am. J. Epidemiol. 178, 1272–1280 (2013).
Ezekowitz, J. A. et al. Declining in-hospital mortality and increasing heart failure incidence in elderly patients with first myocardial infarction. J. Am. Coll. Cardiol. 53, 13–20 (2009).
Chen, J., Hsieh, A. F., Dharmarajan, K., Masoudi, F. A. & Krumholz, H. M. National trends in heart failure hospitalization after acute myocardial infarction for Medicare beneficiaries: 1998–2010. Circulation 128, 2577–2584 (2013).
Velagaleti, R. S. et al. Long-term trends in the incidence of heart failure after myocardial infarction. Circulation 118, 2057–2062 (2008).
Milanlouei, S. et al. A systematic comprehensive longitudinal evaluation of dietary factors associated with acute myocardial infarction and fatal coronary heart disease. Nat. Commun. 11, 6074 (2020).
Yu, E., Malik, V. S. & Hu, F. B. Cardiovascular disease prevention by diet modification: JACC health promotion series. J. Am. Coll. Cardiol. 72, 914–926 (2018).
Khera, A. V. et al. Genetic risk, adherence to a healthy lifestyle, and coronary disease. N. Engl. J. Med. 375, 2349–2358 (2016).
Yu, X. H., Fu, Y. C., Zhang, D. W., Yin, K. & Tang, C. K. Foam cells in atherosclerosis. Clin. Chim. Acta 424, 245–252 (2013).
Seidman, M. A., Mitchell, R. N. & Stone, J. R. Cellular and Molecular Pathobiology of Cardiovascular Disease Ch. 12 (eds Willis, M. S., Homeister, J. W. & Stone, J. R.) 221–237 (Elsevier, 2014).
Seimon, T. & Tabas, I. Mechanisms and consequences of macrophage apoptosis in atherosclerosis. J. Lipid Res. 50, S382–S387 (2009).
Martinet, W., Schrijvers, D. M. & De Meyer, G. R. Necrotic cell death in atherosclerosis. Basic Res. Cardiol. 106, 749–760 (2011).
de Villiers, W. J. & Smart, E. J. Macrophage scavenger receptors and foam cell formation. J. Leukoc. Biol. 66, 740–746 (1999).
Chistiakov, D. A., Bobryshev, Y. V. & Orekhov, A. N. Macrophage‐mediated cholesterol handling in atherosclerosis. J. Cell. Mol. Med. 20, 17–28 (2016).
Dohi, T. et al. Non-fibroatheroma lesion phenotype and long-term clinical outcomes: a substudy analysis from the PROSPECT study. JACC Cardiovasc. Imaging 6, 908–916 (2013).
Hansson, G. K., Libby, P. & Tabas, I. Inflammation and plaque vulnerability. J. Intern. Med. 278, 483–493 (2015).
Stone, G. W. et al. A prospective natural-history study of coronary atherosclerosis. N. Engl. J. Med. 364, 226–235 (2011).
Brown, A. J. et al. Role of biomechanical forces in the natural history of coronary atherosclerosis. Nat. Rev. Cardiol. 13, 210–220 (2016).
Huang, X. et al. 3D MRI-based multicomponent thin layer structure only plaque models for atherosclerotic plaques. J. Biomech. 49, 2726–2733 (2016).
Virmani, R., Kolodgie Frank, D., Burke Allen, P., Farb, A. & Schwartz Stephen, M. Lessons from sudden coronary death. Arterioscler. Thromb. Vasc. Biol. 20, 1262–1275 (2000).
Bentzon, J. F., Otsuka, F., Virmani, R. & Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 114, 1852–1866 (2014).
Verma, S. et al. Fundamentals of reperfusion injury for the clinical cardiologist. Circulation 105, 2332–2336 (2002).
Naito, H. et al. Therapeutic strategies for ischemia reperfusion injury in emergency medicine. Acute Med. Surg. 7, e501 (2020).
Hausenloy, D. J. & Yellon, D. M. Myocardial ischemia–reperfusion injury: a neglected therapeutic target. J. Clin. Invest. 123, 92–100 (2013).
Heusch, G. et al. Coronary microembolization. Circulation 120, 1822–1836 (2009).
Ito, H. No-reflow phenomenon and prognosis in patients with acute myocardial infarction. Nat. Clin. Pract. Cardiovasc. Med. 3, 499–506 (2006).
Visan, I. Myocardial infarct inflammation. Nat. Immunol. 19, 99 (2018).
Nahrendorf, M. et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 204, 3037–3047 (2007).
Van der Borght, K. & Lambrecht, B. N. Heart macrophages and dendritic cells in sickness and in health: a tale of a complicated marriage. Cell. Immunol. 330, 105–113 (2018).
Swirski, F. K. et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325, 612–616 (2009).
Halade, G. V., Norris, P. C., Kain, V., Serhan, C. N. & Ingle, K. A. Splenic leukocytes define the resolution of inflammation in heart failure. Sci. Signal. 11, eaao1818 (2018).
Lee, W. W. et al. PET/MRI of inflammation in myocardial infarction. J. Am. Coll. Cardiol. 59, 153–163 (2012).
Jung, K. et al. Endoscopic time-lapse imaging of immune cells in infarcted mouse hearts. Circ. Res. 112, 891–899 (2013).
Heidt, T. et al. Differential contribution of monocytes to heart macrophages in steady-state and after myocardial infarction. Circ. Res. 115, 284–295 (2014).
Bajpai, G. et al. Tissue resident CCR2– and CCR2+ cardiac macrophages differentially orchestrate monocyte recruitment and fate specification following myocardial injury. Circ. Res. 124, 263–278 (2019).
Sager, H. B. et al. Proliferation and recruitment contribute to myocardial macrophage expansion in chronic heart failure. Circ. Res. 119, 853–864 (2016).
Dick, S. A. et al. Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction. Nat. Immunol. 20, 29–39 (2019).
van der Laan, A. M. et al. Monocyte subset accumulation in the human heart following acute myocardial infarction and the role of the spleen as monocyte reservoir. Eur. Heart J. 35, 376–385 (2014).
Hilgendorf, I. et al. Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ. Res. 114, 1611–1622 (2014).
Sutton, M. G. S. J. & Sharpe, N. Left ventricular remodeling after myocardial infarction. Circulation 101, 2981–2988 (2000).
Pinto, A. R., Godwin, J. W. & Rosenthal, N. A. Macrophages in cardiac homeostasis, injury responses and progenitor cell mobilisation. Stem Cell Res. 13, 705–714 (2014).
Yamasaki, S. et al. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat. Immunol. 9, 1179–1188 (2008).
van Furth, R. et al. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull. World Health Organ. 46, 845–852 (1972).
Schulz, C. et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336, 86–90 (2012).
Hashimoto, D. et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 38, 792–804 (2013).
Yona, S. et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38, 79–91 (2013).
Chakarov, S. et al. Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science 363, eaau0964 (2019).
Lavine, K. J. et al. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc. Natl Acad. Sci. USA 111, 16029–16034 (2014).
Epelman, S. et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 40, 91–104 (2014).
Thornley, T. B. et al. Fragile TIM-4-expressing tissue resident macrophages are migratory and immunoregulatory. J. Clin. Invest. 124, 3443–3454 (2014).
Honold, L. & Nahrendorf, M. Resident and monocyte-derived macrophages in cardiovascular disease. Circ. Res. 122, 113–127 (2018).
Marsh, S. A. et al. Rapid fall in circulating non-classical monocytes in ST elevation myocardial infarction patients correlates with cardiac injury. FASEB J. 35, e21604 (2021).
Tsujioka, H. et al. Impact of heterogeneity of human peripheral blood monocyte subsets on myocardial salvage in patients with primary acute myocardial infarction. J. Am. Coll. Cardiol. 54, 130–138 (2009).
Bajpai, G. et al. The human heart contains distinct macrophage subsets with divergent origins and functions. Nat. Med. 24, 1234–1245 (2018).
Leid, J. et al. Primitive embryonic macrophages are required for coronary development and maturation. Circ. Res. 118, 1498–1511 (2016).
Aurora, A. B. et al. Macrophages are required for neonatal heart regeneration. J. Clin. Invest. 124, 1382–1392 (2014).
Hulsmans, M. et al. Macrophages facilitate electrical conduction in the heart. Cell 169, 510–522.e20 (2017).
Grune, J. et al. Neutrophils incite and macrophages avert electrical storm after myocardial infarction. Nat. Cardiovasc. Res. 1, 649–664 (2022).
Lim, G. B. Macrophages and neutrophils modulate arrhythmia risk after myocardial infarction. Nat. Rev. Cardiol. 19, 573 (2022).
DeBerge, M. et al. MerTK cleavage on resident cardiac macrophages compromises repair after myocardial ischemia–reperfusion injury. Circ. Res. 121, 930–940 (2017).
Penberthy, K. K. & Ravichandran, K. S. Apoptotic cell recognition receptors and scavenger receptors. Immunol. Rev. 269, 44–59 (2016).
Wan, E. et al. Enhanced efferocytosis of apoptotic cardiomyocytes through myeloid-epithelial-reproductive tyrosine kinase links acute inflammation resolution to cardiac repair after infarction. Circ. Res. 113, 1004–1012 (2013).
Howangyin, K.-Y. et al. Myeloid-epithelial-reproductive receptor tyrosine kinase and milk fat globule epidermal growth factor 8 coordinately improve remodeling after myocardial infarction via local delivery of vascular endothelial growth factor. Circulation 133, 826–839 (2016).
Saljoughian, N. et al. Role of cardiac macrophages in controlling the infection and homeostasis of steady state heart function in aging [abstract]. J. Immunol. 204, 75.1 (2020).
Nicolás-Ávila, J. A. et al. A network of macrophages supports mitochondrial homeostasis in the heart. Cell 183, 94–109.e23 (2020).
Pepine, C. J. New concepts in the pathophysiology of acute myocardial infarction. Am. J. Cardiol. 64, 2B–8B (1989).
Frangogiannis, N. G. The inflammatory response in myocardial injury, repair, and remodelling. Nat. Rev. Cardiol. 11, 255–265 (2014).
Prabhu, S. D. & Frangogiannis, N. G. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ. Res. 119, 91–112 (2016).
Salminen, A., Kaarniranta, K. & Kauppinen, A. Immunosenescence: the potential role of myeloid-derived suppressor cells (MDSC) in age-related immune deficiency. Cell. Mol. Life Sci. 76, 1901–1918 (2019).
Kain, V. et al. Resolution agonist 15-epi-lipoxin A4 programs early activation of resolving phase in post-myocardial infarction healing. Sci. Rep. 7, 9999 (2017).
Kanisicak, O. et al. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat. Commun. 7, 12260 (2016).
Dewald, O. et al. Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. Am. J. Pathol. 164, 665–677 (2004).
Celle, T. D. et al. Long-term structural and functional consequences of cardiac ischaemia–reperfusion injury in vivo in mice. Exp. Physiol. 89, 605–615 (2004).
Cabrera-Fuentes, H. A. et al. Regulation of monocyte/macrophage polarisation by extracellular RNA. Thromb. Haemost. 113, 473–481 (2015).
Simsekyilmaz, S. et al. Role of extracellular RNA in atherosclerotic plaque formation in mice. Circulation 129, 598–606 (2014).
Arslan, F., de Kleijn, D. P. & Pasterkamp, G. Innate immune signaling in cardiac ischemia. Nat. Rev. Cardiol. 8, 292–300 (2011).
Oka, T. et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature 485, 251–255 (2012).
Raggi, F. et al. Regulation of human macrophage M1–M2 polarization balance by hypoxia and the triggering receptor expressed on myeloid cells-1. Front. Immunol. 8, 1097 (2017).
Saxena, A. et al. IL-1 induces proinflammatory leukocyte infiltration and regulates fibroblast phenotype in the infarcted myocardium. J. Immunol. 191, 4838–4848 (2013).
Kaikita, K. et al. Targeted deletion of CC chemokine receptor 2 attenuates left ventricular remodeling after experimental myocardial infarction. Am. J. Pathol. 165, 439–447 (2004).
Hayasaki, T. et al. CC chemokine receptor-2 deficiency attenuates oxidative stress and infarct size caused by myocardial ischemia–reperfusion in mice. Circ. J. 70, 342–351 (2006).
Dewald, O. et al. CCL2/monocyte chemoattractant protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ. Res. 96, 881–889 (2005).
Liehn, E. A. et al. A new monocyte chemotactic protein-1/chemokine CC motif ligand-2 competitor limiting neointima formation and myocardial ischemia/reperfusion injury in mice. J. Am. Coll. Cardiol. 56, 1847–1857 (2010).
Majmudar, M. D. et al. Monocyte-directed RNAi targeting CCR2 improves infarct healing in atherosclerosis-prone mice. Circulation 127, 2038–2046 (2013).
Leuschner, F. et al. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J. Exp. Med. 209, 123–137 (2012).
Davies, L. C. et al. A quantifiable proliferative burst of tissue macrophages restores homeostatic macrophage populations after acute inflammation. Eur. J. Immunol. 41, 2155–2164 (2011).
Calderone, A. p38 MAPK and the compromised regenerative response of the infarcted adult heart. Cardiovasc. Regen. Med. 3, e1508 (2018).
Leblond, A. L. et al. Systemic and cardiac depletion of M2 macrophage through CSF-1R signaling inhibition alters cardiac function post myocardial infarction. PLoS ONE 10, e0137515 (2015).
Kain, V., Prabhu, S. D. & Halade, G. V. Inflammation revisited: inflammation versus resolution of inflammation following myocardial infarction. Basic Res. Cardiol. 109, 444 (2014).
Huang, S. & Frangogiannis, N. G. Anti-inflammatory therapies in myocardial infarction: failures, hopes and challenges. Br. J. Pharmacol. 175, 1377–1400 (2018).
Yan, X. et al. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J. Mol. Cell. Cardiol. 62, 24–35 (2013).
Huynh, M.-L. N., Fadok, V. A. & Henson, P. M. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-β1 secretion and the resolution of inflammation. J. Clin. Invest. 109, 41–50 (2002).
Dobaczewski, M., Chen, W. & Frangogiannis, N. G. Transforming growth factor (TGF)-beta signaling in cardiac remodeling. J. Mol. Cell. Cardiol. 51, 600–606 (2011).
Vajen, T. et al. Blocking CCL5-CXCL4 heteromerization preserves heart function after myocardial infarction by attenuating leukocyte recruitment and NETosis. Sci. Rep. 8, 10647 (2018).
Molawi, K. et al. Progressive replacement of embryo-derived cardiac macrophages with age. J. Exp. Med. 211, 2151–2158 (2014).
Jadapalli, J. K. & Halade, G. V. Unified nexus of macrophages and maresins in cardiac reparative mechanisms. FASEB J. 32, 5227–5237 (2018).
Bournazou, I. et al. Apoptotic human cells inhibit migration of granulocytes via release of lactoferrin. J. Clin. Invest. 119, 20–32 (2009).
Eghbalzadeh, K. et al. Compromised anti-inflammatory action of neutrophil extracellular traps in PAD4-deficient mice contributes to aggravated acute inflammation after myocardial infarction. Front. Immunol. 10, 2313 (2019).
Hashimoto, S., Suzuki, T., Dong, H. Y., Yamazaki, N. & Matsushima, K. Serial analysis of gene expression in human monocytes and macrophages. Blood 94, 837–844 (1999).
McCurdy, S. M. et al. SPARC mediates early extracellular matrix remodeling following myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 301, H497–H505 (2011).
Schellings, M. W. et al. Absence of SPARC results in increased cardiac rupture and dysfunction after acute myocardial infarction. J. Exp. Med. 206, 113–123 (2009).
Huang, W. et al. Molecular strategy to reduce in vivo collagen barrier promotes entry of NCX1 positive inducible pluripotent stem cells (iPSC(NCX¹+)) into ischemic (or injured) myocardium. PLoS ONE 8, e70023 (2013).
Dai, B. et al. Reduced collagen deposition in infarcted myocardium facilitates induced pluripotent stem cell engraftment and angiomyogenesis for improvement of left ventricular function. J. Am. Coll. Cardiol. 58, 2118–2127 (2011).
Knutson, A. K., Williams, A. L., Boisvert, W. A. & Shohet, R. V. HIF in the heart: development, metabolism, ischemia, and atherosclerosis. J. Clin. Invest. 131, e137557 (2021).
Du, Y. et al. Hypoxia-inducible factor 1 alpha (HIF-1alpha)/vascular endothelial growth factor (VEGF) pathway participates in angiogenesis of myocardial infarction in muscone-treated mice: preliminary study. Med. Sci. Monit. 24, 8870–8877 (2018).
Neri, M., Riezzo, I., Pascale, N., Pomara, C. & Turillazzi, E. Ischemia/reperfusion injury following acute myocardial infarction: a critical issue for clinicians and forensic pathologists. Mediators Inflamm. 2017, 7018393 (2017).
Bergmann, O. et al. Dynamics of cell generation and turnover in the human heart. Cell 161, 1566–1575 (2015).
Nag, A. C. Study of non-muscle cells of the adult mammalian heart: a fine structural analysis and distribution. Cytobios 28, 41–61 (1980).
Pinto, A. R. et al. Revisiting cardiac cellular composition. Circ. Res. 118, 400–409 (2016).
Tang, Y., Nyengaard, J. R., Andersen, J. B., Baandrup, U. & Gundersen, H. J. The application of stereological methods for estimating structural parameters in the human heart. Anat. Rec. 292, 1630–1647 (2009).
Frangogiannis, N. G. et al. Critical role of monocyte chemoattractant protein-1/CC chemokine ligand 2 in the pathogenesis of ischemic cardiomyopathy. Circulation 115, 584–592 (2007).
Gwechenberger, M. et al. Cardiac myocytes produce interleukin-6 in culture and in viable border zone of reperfused infarctions. Circulation 99, 546–551 (1999).
Zhang, S. et al. Cardiomyocytes induce macrophage receptor shedding to suppress phagocytosis. J. Mol. Cell. Cardiol. 87, 171–179 (2015).
Monnerat, G. et al. Macrophage-dependent IL-1beta production induces cardiac arrhythmias in diabetic mice. Nat. Commun. 7, 13344 (2016).
Wang, B. et al. Macrophage-derived exosomal Mir-155 regulating cardiomyocyte pyroptosis and hypertrophy in uremic cardiomyopathy. JACC Basic Transl. Sci. 5, 148–166 (2020).
Hu, J. et al. MicroRNA-155 inhibition attenuates endoplasmic reticulum stress-induced cardiomyocyte apoptosis following myocardial infarction via reducing macrophage inflammation. Eur. J. Pharmacol. 857, 172449 (2019).
Almeida Paiva, R. et al. Ischaemia alters the effects of cardiomyocyte-derived extracellular vesicles on macrophage activation. J. Cell. Mol. Med. 23, 1137–1151 (2019).
Zlatanova, I. et al. Iron regulator hepcidin impairs macrophage-dependent cardiac repair after injury. Circulation 139, 1530–1547 (2019).
Wong, N. R. et al. Resident cardiac macrophages mediate adaptive myocardial remodeling. Immunity 54, 2072–2088.e7 (2021).
Song, E. et al. Influence of alternatively and classically activated macrophages on fibrogenic activities of human fibroblasts. Cell. Immunol. 204, 19–28 (2000).
Cao, B., Guo, Z., Zhu, Y. & Xu, W. The potential role of PDGF, IGF-1, TGF-beta expression in idiopathic pulmonary fibrosis. Chin. Med. J. 113, 776–782 (2000).
Spiller, K. L. et al. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials 35, 4477–4488 (2014).
Ferraro, B. et al. Pro-angiogenic macrophage phenotype to promote myocardial repair. J. Am. Coll. Cardiol. 73, 2990–3002 (2019).
Behzadian, M. A., Wang, X. L., Jiang, B. & Caldwell, R. B. Angiostatic role of astrocytes: suppression of vascular endothelial cell growth by TGF-beta and other inhibitory factor(s). Glia 15, 480–490 (1995).
Chen, M. et al. Persistent inflammation subverts thrombospondin-1-induced regulation of retinal angiogenesis and is driven by CCR2 ligation. Am. J. Pathol. 180, 235–245 (2012).
Sato, N. et al. Actions of TNF and IFN-gamma on angiogenesis in vitro. J. Invest. Dermatol. 95, 85S–89S (1990).
Patterson, B. C. & Sang, Q. A. Angiostatin-converting enzyme activities of human matrilysin (MMP-7) and gelatinase B/type IV collagenase (MMP-9). J. Biol. Chem. 272, 28823–28825 (1997).
Falcone, D. J., Khan, K. M., Layne, T. & Fernandes, L. Macrophage formation of angiostatin during inflammation. A byproduct of the activation of plasminogen. J. Biol. Chem. 273, 31480–31485 (1998).
Lepidi, S. et al. MMP9 production by human monocyte-derived macrophages is decreased on polymerized type I collagen. J. Vasc. Surg. 34, 1111–1118 (2001).
Zouggari, Y. et al. Regulatory T cells modulate postischemic neovascularization. Circulation 120, 1415–1425 (2009).
DeRuiter, M. C. et al. Embryonic endothelial cells transdifferentiate into mesenchymal cells expressing smooth muscle actins in vivo and in vitro. Circ. Res. 80, 444–451 (1997).
Kenswil, K. J. G. et al. Endothelium-derived stromal cells contribute to hematopoietic bone marrow niche formation. Cell Stem Cell 28, 653–670.e11 (2021).
Zeisberg, E. M. et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 13, 952–961 (2007).
Alonso-Herranz, L. et al. Macrophages promote endothelial-to-mesenchymal transition via MT1-MMP/TGFβ1 after myocardial infarction. eLife 9, e57920 (2020).
Huleihel, M., Douvdevani, A., Segal, S. & Apte, R. N. Regulation of interleukin 1 generation in immune-activated fibroblasts. Eur. J. Immunol. 20, 731–738 (1990).
Akbar, M. et al. Fibroblast activation and inflammation in frozen shoulder. PLoS ONE 14, e0215301 (2019).
Blythe, N. M. et al. Mechanically activated Piezo1 channels of cardiac fibroblasts stimulate p38 mitogen-activated protein kinase activity and interleukin-6 secretion. J. Biol. Chem. 294, 17395–17408 (2019).
Sandanger, O. et al. The NLRP3 inflammasome is up-regulated in cardiac fibroblasts and mediates myocardial ischaemia–reperfusion injury. Cardiovasc. Res. 99, 164–174 (2013).
Ma, F. et al. Macrophage-stimulated cardiac fibroblast production of IL-6 is essential for TGF beta/Smad activation and cardiac fibrosis induced by angiotensin II. PLoS ONE 7, e35144 (2012).
Wang, C. et al. Macrophage-derived mir-155-containing exosomes suppress fibroblast proliferation and promote fibroblast inflammation during cardiac injury. Mol. Ther. 25, 192–204 (2017).
Wu, X. et al. miR-155 inhibits the formation of hypertrophic scar fibroblasts by targeting HIF-1alpha via PI3K/AKT pathway. J. Mol. Histol. 49, 377–387 (2018).
Yue, Y. et al. M2b macrophages regulate cardiac fibroblast activation and alleviate cardiac fibrosis after reperfusion injury. Circ. J. 84, 626–635 (2020).
Palmen, M. et al. Fibroblast growth factor-1 improves cardiac functional recovery and enhances cell survival after ischemia and reperfusion: a fibroblast growth factor receptor, protein kinase C, and tyrosine kinase-dependent mechanism. J. Am. Coll. Cardiol. 44, 1113–1123 (2004).
Dhingra, S., Sharma, A. K., Arora, R. C., Slezak, J. & Singal, P. K. IL-10 attenuates TNF-alpha-induced NFκB pathway activation and cardiomyocyte apoptosis. Cardiovasc. Res. 82, 59–66 (2009).
Korf-Klingebiel, M. et al. Myeloid-derived growth factor (C19orf10) mediates cardiac repair following myocardial infarction. Nat. Med. 21, 140–149 (2015).
Cambier, L. et al. Y RNA fragment in extracellular vesicles confers cardioprotection via modulation of IL-10 expression and secretion. EMBO Mol. Med. 9, 337–352 (2017).
Jung, M. et al. IL-10 improves cardiac remodeling after myocardial infarction by stimulating M2 macrophage polarization and fibroblast activation. Basic Res. Cardiol. 112, 33 (2017).
Simoes, F. C. et al. Macrophages directly contribute collagen to scar formation during zebrafish heart regeneration and mouse heart repair. Nat. Commun. 11, 600 (2020).
Haider, N. et al. Transition of macrophages to fibroblast-like cells in healing myocardial infarction. J. Am. Coll. Cardiol. 74, 3124–3135 (2019).
Bansal, S. S. et al. Activated T lymphocytes are essential drivers of pathological remodeling in ischemic heart failure. Circ. Heart Fail. 10, e003688 (2017).
Ilatovskaya, D. V. et al. CD8+ T-cells negatively regulate inflammation post-myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 317, H581–H596 (2019).
Patel, B. et al. CCR2+ monocyte-derived infiltrating macrophages are required for adverse cardiac remodeling during pressure overload. JACC Basic Transl. Sci. 3, 230–244 (2018).
Weirather, J. et al. Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ. Res. 115, 55–67 (2014).
Tang, T. T. et al. Regulatory T cells ameliorate cardiac remodeling after myocardial infarction. Basic Res. Cardiol. 107, 232 (2012).
Saxena, A. et al. Regulatory T cells are recruited in the infarcted mouse myocardium and may modulate fibroblast phenotype and function. Am. J. Physiol. Heart Circ. Physiol. 307, H1233–H1242 (2014).
Rieckmann, M. et al. Myocardial infarction triggers cardioprotective antigen-specific T helper cell responses. J. Clin. Invest 129, 4922–4936 (2019).
Bansal, S. S. et al. Dysfunctional and proinflammatory regulatory T-lymphocytes are essential for adverse cardiac remodeling in ischemic cardiomyopathy. Circulation 139, 206–221 (2019).
Frantz, S., Bauersachs, J. & Ertl, G. Post-infarct remodelling: contribution of wound healing and inflammation. Cardiovasc. Res. 81, 474–481 (2009).
Fujiwara, M. et al. Nanoparticle incorporating Toll-like receptor 4 inhibitor attenuates myocardial ischaemia–reperfusion injury by inhibiting monocyte-mediated inflammation in mice. Cardiovasc. Res 115, 1244–1255 (2019).
Gilbert, J. et al. Effect of CC chemokine receptor 2 CCR2 blockade on serum C-reactive protein in individuals at atherosclerotic risk and with a single nucleotide polymorphism of the monocyte chemoattractant protein-1 promoter region. Am. J. Cardiol. 107, 906–911 (2011).
Schroer, A. K. et al. Cadherin-11 blockade reduces inflammation-driven fibrotic remodeling and improves outcomes after myocardial infarction. JCI Insight 4, e131545 (2019).
Wang, J. et al. Effect of CCR2 inhibitor-loaded lipid micelles on inflammatory cell migration and cardiac function after myocardial infarction. Int. J. Nanomed. 13, 6441–6451 (2018).
Wu, Z. et al. EGFP-EGF1-conjugated poly (lactic-co-glycolic acid) nanoparticles as a carrier for the delivery of CCR2–shRNA to atherosclerotic macrophage in vitro. Sci. Rep. 10, 19636 (2020).
Kubota, A., Suto, A., Suzuki, K., Kobayashi, Y. & Nakajima, H. Matrix metalloproteinase-12 produced by Ly6Clow macrophages prolongs the survival after myocardial infarction by preventing neutrophil influx. J. Mol. Cell. Cardiol. 131, 41–52 (2019).
Ogawa, H. et al. Adipolin/CTRP12 protects against pathological vascular remodelling through suppression of smooth muscle cell growth and macrophage inflammatory response. Cardiovasc. Res. 116, 237–249 (2020).
Takikawa, T. et al. Adipolin/C1q/Tnf-related protein 12 prevents adverse cardiac remodeling after myocardial infarction. PLoS ONE 15, e0243483 (2020).
Liu, W. et al. Calhex231 ameliorates myocardial fibrosis post myocardial infarction in rats through the autophagy–NLRP3 inflammasome pathway in macrophages. J. Cell. Mol. Med. 24, 13440–13453 (2020).
Cheng, Y. & Rong, J. Macrophage polarization as a therapeutic target in myocardial infarction. Curr. Drug. Targets 19, 651–662 (2018).
ter Horst, E. N. et al. Modulators of macrophage polarization influence healing of the infarcted myocardium. Int. J. Mol. Sci. 16, 29583–29591 (2015).
Courties, G. et al. In vivo silencing of the transcription factor IRF5 reprograms the macrophage phenotype and improves infarct healing. J. Am. Coll. Cardiol. 63, 1556–1566 (2014).
Jia, D. et al. Interleukin-35 promotes macrophage survival and improves wound healing after myocardial infarction in mice. Circ. Res. 124, 1323–1336 (2019).
Li, J. et al. CD226 deletion improves post-infarction healing via modulating macrophage polarization in mice. Theranostics 10, 2422–2435 (2020).
Ma, Y. et al. Matrix metalloproteinase-28 deletion exacerbates cardiac dysfunction and rupture after myocardial infarction in mice by inhibiting M2 macrophage activation. Circ. Res. 112, 675–688 (2013).
Shintani, Y. et al. IL-4 as a repurposed biological drug for myocardial infarction through augmentation of reparative cardiac macrophages: proof-of-concept data in mice. Sci. Rep. 7, 6877 (2017).
Zhou, L. S. et al. Silencing collapsin response mediator protein-2 reprograms macrophage phenotype and improves infarct healing in experimental myocardial infarction model. J. Inflamm. 12, 11 (2015).
Peet, C., Ivetic, A., Bromage, D. I. & Shah, A. M. Cardiac monocytes and macrophages after myocardial infarction. Cardiovasc. Res. 116, 1101–1112 (2020).
Yang, M. et al. Deficiency of GATA3-positive macrophages improves cardiac function following myocardial infarction or pressure overload hypertrophy. J. Am. Coll. Cardiol. 72, 885–904 (2018).
Zhang, S., Chen, R., Chakrabarti, S. & Su, Z. Resident macrophages as potential therapeutic targets for cardiac ageing and injury. Clin. Transl. Immunol. 9, e1167 (2020).
Rao, D. D., Vorhies, J. S., Senzer, N. & Nemunaitis, J. siRNA vs. shRNA: similarities and differences. Adv. Drug Deliv. Rev. 61, 746–759 (2009).
van Rooijen, N. & Hendrikx, E. Liposomes for specific depletion of macrophages from organs and tissues. Methods Mol. Biol. 605, 189–203 (2010).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Everett, B. M. et al. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am. Heart J. 166, 199–207.e15 (2013).
Ridker, P. M. et al. Low-dose methotrexate for the prevention of atherosclerotic events. N. Engl. J. Med. 380, 752–762 (2019).
Tardif, J. C. et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N. Engl. J. Med. 381, 2497–2505 (2019).
Nidorf, S. M. et al. Colchicine in patients with chronic coronary disease. N. Engl. J. Med. 383, 1838–1847 (2020).
Tong, D. C. et al. Colchicine in patients with acute coronary syndrome: The Australian COPS randomized clinical trial. Circulation 142, 1890–1900 (2020).
Broch, K. et al. Randomized trial of interleukin-6 receptor inhibition in patients with acute ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 77, 1845–1855 (2021).
Dalbeth, N., Lauterio, T. J. & Wolfe, H. R. Mechanism of action of colchicine in the treatment of gout. Clin. Ther. 36, 1465–1479 (2014).
Riebeling, T. et al. Primidone blocks RIPK1-driven cell death and inflammation. Cell Death Differ. 28, 1610–1626 (2021).
Luo, P. et al. Bazedoxifene exhibits anti-inflammation and anti-atherosclerotic effects via inhibition of IL-6/IL-6R/STAT3 signaling. Eur. J. Pharmacol. 893, 173822 (2021).
Ramos, G. C. et al. Myocardial aging as a T-cell-mediated phenomenon. Proc. Natl Acad. Sci. USA 114, E2420–E2429 (2017).
Ma, Y. et al. Deriving a cardiac ageing signature to reveal MMP-9-dependent inflammatory signalling in senescence. Cardiovasc. Res. 106, 421–431 (2015).
Esfahani, N. S. et al. Aging influences the cardiac macrophage phenotype and function during steady state and during inflammation. Aging Cell 20, e13438 (2021).
Cochain, C. et al. Single-cell RNA-seq reveals the transcriptional landscape and heterogeneity of aortic macrophages in murine atherosclerosis. Circ. Res. 122, 1661–1674 (2018).
Dick, S. A., Zaman, R. & Epelman, S. Using high-dimensional approaches to probe monocytes and macrophages in cardiovascular disease. Front. Immunol. 10, 2146 (2019).
Willemsen, L. & de Winther, M. P. J. Macrophage subsets in atherosclerosis as defined by single-cell technologies. J. Pathol. 250, 705–714 (2020).
Acknowledgements
The authors are grateful to L. N. Boisvert (John A. Burns School of Medicine, University of Hawaii, USA) for her expert editing of the manuscript before submission.
Author information
Authors and Affiliations
Contributions
The authors contributed substantially to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cardiology thanks Kristine Deleon-Pennell, Nikolaos Frangogiannis and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yap, J., Irei, J., Lozano-Gerona, J. et al. Macrophages in cardiac remodelling after myocardial infarction. Nat Rev Cardiol 20, 373–385 (2023). https://doi.org/10.1038/s41569-022-00823-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-022-00823-5
This article is cited by
-
The neutrophil-to-apolipoprotein A1 ratio is associated with adverse outcomes in patients with acute decompensated heart failure at different glucose metabolic states: a retrospective cohort study
Lipids in Health and Disease (2024)
-
Association of relative hand grip strength with myocardial infarction and angina pectoris in the Korean population: a large-scale cross-sectional study
BMC Public Health (2024)
-
Cuproptosis and copper deficiency in ischemic vascular injury and repair
Apoptosis (2024)
-
Excretory/secretory products from Trichinella spiralis adult worms ameliorate myocardial infarction by inducing M2 macrophage polarization in a mouse model
Parasites & Vectors (2023)
-
Unlocking the potential of microRNAs: machine learning identifies key biomarkers for myocardial infarction diagnosis
Cardiovascular Diabetology (2023)