Abstract
Research conducted in the past 15 years has yielded crucial insights that are reshaping our understanding of the systems physiology of branched-chain amino acid (BCAA) metabolism and the molecular mechanisms underlying the close relationship between BCAA homeostasis and cardiovascular health. The rapidly evolving literature paints a complex picture, in which numerous tissue-specific and disease-specific modes of BCAA regulation initiate a diverse set of molecular mechanisms that connect changes in BCAA homeostasis to the pathogenesis of cardiovascular diseases, including myocardial infarction, ischaemia–reperfusion injury, atherosclerosis, hypertension and heart failure. In this Review, we outline the current understanding of the major factors regulating BCAA abundance and metabolic fate, highlight molecular mechanisms connecting impaired BCAA homeostasis to cardiovascular disease, discuss the epidemiological evidence connecting BCAAs with various cardiovascular disease states and identify current knowledge gaps requiring further investigation.
Key points
-
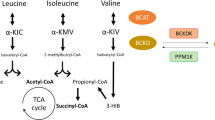
Branched-chain amino acids (BCAAs) — isoleucine, leucine and valine — are essential amino acids that have a crucial role in metabolic homeostasis through nutrient signalling.
-
High circulating concentrations of BCAAs are a hallmark of metabolic disorders, including obesity, insulin resistance and type 2 diabetes mellitus.
-
Mechanisms underlying the association between BCAAs and cardiovascular disease (CVD) are still being defined, but include activation of the serine/threonine protein kinase mTOR, mitochondrial dysfunction, shifts in cardiac substrate utilization and platelet activation.
-
Epidemiological studies have also shown that high plasma BCAA concentrations identify individuals with heart failure, coronary artery disease or hypertension and predict adverse events in these populations.
-
In some other populations (such as African American individuals without CVD and frail elderly individuals), high plasma BCAA concentrations predict improved cardiovascular outcomes.
-
Areas for future research include the roles of tissue-specific BCAA metabolism and inter-organ BCAA metabolic crosstalk in various CVD states and the roles of impaired BCAA metabolism in vascular function and atherosclerosis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ridaura, V. K. et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341, 1241214 (2013).
Pedersen, H. K. et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 535, 376–381 (2016).
Wolfson, R. L. et al. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 351, 43–48 (2016).
Felig, P., Marliss, E. & Cahill, G. F. Plasma amino acid levels and insulin secretion in obesity. N. Engl. J. Med. 281, 811–816 (1969).
White, P. J. & Newgard, C. B. Branched-chain amino acids in disease. Science 363, 582–583 (2019).
White, P. J. et al. Insulin action, type 2 diabetes, and branched-chain amino acids: a two-way street. Mol. Metab. 52, 101261 (2021).
Hunter, W. G. et al. Metabolomic profiling identifies novel circulating biomarkers of mitochondrial dysfunction differentially elevated in heart failure with preserved versus reduced ejection fraction: evidence for shared metabolic impairments in clinical heart failure. J. Am. Heart Assoc. 5, e003190 (2016).
Lanfear, D. E. et al. Targeted metabolomic profiling of plasma and survival in heart failure patients. JACC Heart Fail. 5, 823–832 (2017).
Sun, H. et al. Catabolic defect of branched-chain amino acids promotes heart failure. Circulation 133, 2038–2049 (2016).
Shah, S. H. et al. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circ. Cardiovasc. Genet. 3, 207–214 (2010).
Bhattacharya, S. et al. Validation of the association between a branched chain amino acid metabolite profile and extremes of coronary artery disease in patients referred for cardiac catheterization. Atherosclerosis 232, 191–196 (2014).
Flores-Guerrero, J. L. et al. Concentration of branched-chain amino acids is a strong risk marker for incident hypertension. Hypertension 74, 1428–1435 (2019).
Portero, V. et al. Chronically elevated branched chain amino acid levels are pro-arrhythmic. Cardiovasc. Res. 118, 1742–1757 (2022).
Jang, C. et al. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat. Med. 22, 421–426 (2016).
Green, C. R. et al. Branched-chain amino acid catabolism fuels adipocyte differentiation and lipogenesis. Nat. Chem. Biol. 12, 15–21 (2016).
Yoneshiro, T. et al. BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature 572, 614–619 (2019).
Walejko, J. M. et al. Branched-chain α-ketoacids are preferentially reaminated and activate protein synthesis in the heart. Nat. Commun. 12, 1680 (2021).
Neinast, M. D. et al. Quantitative analysis of the whole-body metabolic fate of branched-chain amino acids. Cell Metab. 29, 417–429.e4 (2019).
Fillmore, N., Wagg, C. S., Zhang, L., Fukushima, A. & Lopaschuk, G. D. Cardiac branched-chain amino acid oxidation is reduced during insulin resistance in the heart. Am. J. Physiol. Endocrinol. Metab. 315, E1046–E1052 (2018).
Nishi, K. et al. Branched-chain keto acid inhibits mitochondrial pyruvate carrier and suppresses gluconeogenesis. SSRN Electron. J. https://doi.org/10.2139/ssrn.4022706 (2022).
Li, R. et al. Time series characteristics of serum branched-chain amino acids for early diagnosis of chronic heart failure. J. Proteome Res. 18, 2121–2128 (2019).
Wang, W. et al. Defective branched chain amino acid catabolism contributes to cardiac dysfunction and remodeling following myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 311, H1160–H1169 (2016).
Uddin, G. M. et al. Impaired branched chain amino acid oxidation contributes to cardiac insulin resistance in heart failure. Cardiovasc. Diabetol. 18, 86 (2019).
Sansbury, B. E. et al. Metabolomic analysis of pressure-overloaded and infarcted mouse hearts. Circ. Heart Fail. 7, 634–642 (2014).
Spyropoulos, F. et al. Metabolomic and transcriptomic signatures of chemogenetic heart failure. Am. J. Physiol. Heart Circ. Physiol. 322, H451–H465 (2022).
Lai, L. et al. Energy metabolic reprogramming in the hypertrophied and early stage failing heart: a multisystems approach. Circ. Heart Fail. 7, 1022–1031 (2014).
Kwon, H. K., Jeong, H., Hwang, D. & Park, Z. Y. Comparative proteomic analysis of mouse models of pathological and physiological cardiac hypertrophy, with selection of biomarkers of pathological hypertrophy by integrative proteogenomics. Biochim. Biophys. acta Proteins Proteom. 1866, 1043–1054 (2018).
Lu, G. et al. Protein phosphatase 2Cm is a critical regulator of branched-chain amino acid catabolism in mice and cultured cells. J. Clin. Invest. 119, 1678–1687 (2009).
Tso, S.-C. et al. Benzothiophene carboxylate derivatives as novel allosteric inhibitors of branched-chain α-ketoacid dehydrogenase kinase. J. Biol. Chem. 289, 20583–20593 (2014).
White, P. J. et al. The BCKDH kinase and phosphatase integrate BCAA and lipid metabolism via regulation of ATP-citrate lyase. Cell Metab. 27, 1281–1293.e7 (2018).
Chen, M. et al. Therapeutic effect of targeting branched-chain amino acid catabolic flux in pressure-overload induced heart failure. J. Am. Heart Assoc. 8, e011625 (2019).
Sciarretta, S., Forte, M., Frati, G. & Sadoshima, J. New insights into the role of mTOR signaling in the cardiovascular system. Circ. Res. 122, 489–505 (2018).
Bueno, O. F. et al. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J. 19, 6341–6350 (2000).
Zhang, L. et al. KLF15 establishes the landscape of diurnal expression in the heart. Cell Rep. 13, 2368–2375 (2015).
Crnko, S., Du Pré, B. C., Sluijter, J. P. G. & Van Laake, L. W. Circadian rhythms and the molecular clock in cardiovascular biology and disease. Nat. Rev. Cardiol. 16, 437–447 (2019).
McGinnis, G. R. et al. Genetic disruption of the cardiomyocyte circadian clock differentially influences insulin-mediated processes in the heart. J. Mol. Cell. Cardiol. 110, 80–95 (2017).
Latimer, M. N. et al. Branched chain amino acids selectively promote cardiac growth at the end of the awake period. J. Mol. Cell. Cardiol. 157, 31–44 (2021).
Fan, L., Hsieh, P. N., Sweet, D. R. & Jain, M. K. Krüppel-like factor 15: regulator of BCAA metabolism and circadian protein rhythmicity. Pharmacol. Res. 130, 123–126 (2018).
Jeyaraj, D. et al. Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature 483, 96–99 (2012).
Haldar, S. M. et al. Klf15 deficiency is a molecular link between heart failure and aortic aneurysm formation. Sci. Transl. Med. 2, 26ra26 (2010).
Prosdocimo, D. A. et al. Kruppel-like factor 15 is a critical regulator of cardiac lipid metabolism. J. Biol. Chem. 289, 5914–5924 (2014).
Lu, G. et al. A novel mitochondrial matrix serine/threonine protein phosphatase regulates the mitochondria permeability transition pore and is essential for cellular survival and development. Genes Dev. 21, 784–796 (2007).
Doehner, W., Frenneaux, M. & Anker, S. D. Metabolic impairment in heart failure: the myocardial and systemic perspective. J. Am. Coll. Cardiol. 64, 1388–1400 (2014).
Murashige, D. et al. Comprehensive quantification of fuel use by the failing and nonfailing human heart. Science 370, 364–368 (2020).
White, P. J. et al. Branched-chain amino acid restriction in Zucker-fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl-glycine export. Mol. Metab. 5, 538–551 (2016).
McGarrah, R. W. et al. Dietary branched-chain amino acid restriction alters fuel selection and reduces triglyceride stores in hearts of Zucker fatty rats. Am. J. Physiol. Endocrinol. Metab. 318, E216–E223 (2020).
Shao, D. et al. Glucose promotes cell growth by suppressing branched-chain amino acid degradation. Nat. Commun. 9, 2935 (2018).
Zhou, Y., Jetton, T. L., Goshorn, S., Lynch, C. J. & She, P. Transamination is required for α-ketoisocaproate but not leucine to stimulate insulin secretion. J. Biol. Chem. 285, 33718–33726 (2010).
Uddin, G. M. et al. Deletion of BCATm increases insulin-stimulated glucose oxidation in the heart. Metabolism 124, 154871 (2021).
Raffel, S. et al. BCAT1 restricts αKG levels in AML stem cells leading to IDHmut-like DNA hypermethylation. Nature 551, 384–388 (2017).
Leenders, J. J. et al. Regulation of cardiac gene expression by KLF15, a repressor of myocardin activity. J. Biol. Chem. 285, 27449–27456 (2010).
Grajeda-Iglesias, C., Rom, O. & Aviram, M. Branched-chain amino acids and atherosclerosis: friends or foes? Curr. Opin. Lipidol. 29, 166–169 (2018).
Zhao, Y. et al. Leucine supplementation via drinking water reduces atherosclerotic lesions in apoE null mice. Acta Pharmacol. Sin. 37, 196–203 (2016).
Rom, O. et al. Atherogenicity of amino acids in the lipid-laden macrophage model system in vitro and in atherosclerotic mice: a key role for triglyceride metabolism. J. Nutr. Biochem. 45, 24–38 (2017).
Wu, G. et al. Dietary supplementation with watermelon pomace juice enhances arginine availability and ameliorates the metabolic syndrome in Zucker diabetic fatty rats. J. Nutr. 137, 2680–2685 (2007).
Kakoki, M. et al. Amino acids as modulators of endothelium-derived nitric oxide. Am. J. Physiol. Ren. Physiol. 291, F297–F304 (2006).
Schachter, D. & Sang, J. C. Aortic leucine-to-glutamate pathway: metabolic route and regulation of contractile responses. Am. J. Physiol. Heart Circ. Physiol. 282, H1135–H1148 (2002).
Reho, J. J., Guo, D. F. & Rahmouni, K. Mechanistic target of rapamycin complex 1 signaling modulates vascular endothelial function through reactive oxygen species. J. Am. Heart Assoc. 8, e010662 (2019).
Zhenyukh, O. et al. Branched-chain amino acids promote endothelial dysfunction through increased reactive oxygen species generation and inflammation. J. Cell. Mol. Med. 22, 4948–4962 (2018).
Xu, Y. et al. Branched-chain amino acid catabolism promotes thrombosis risk by enhancing tropomodulin-3 propionylation in platelets. Circulation 142, 49–64 (2020).
Li, T. et al. Defective branched-chain amino acid catabolism disrupts glucose metabolism and sensitizes the heart to ischemia-reperfusion injury. Cell Metab. 25, 374–385 (2017).
Lian, K. et al. PP2Cm overexpression alleviates MI/R injury mediated by a BCAA catabolism defect and oxidative stress in diabetic mice. Eur. J. Pharmacol. 866, 172796 (2020).
Adabag, A. S., Luepker, R. V., Roger, V. L. & Gersh, B. J. Sudden cardiac death: epidemiology and risk factors. Nat. Rev. Cardiol. 7, 216–225 (2010).
Fontana, L., Partridge, L. & Longo, V. D. Extending healthy life span–from yeast to humans. Science 328, 321–326 (2010).
Le Couteur, D. G. et al. Branched chain amino acids, aging and age-related health. Ageing Res. Rev. 64, 101198 (2020).
Green, C. L. et al. The effects of graded levels of calorie restriction: XIII. Global metabolomics screen reveals graded changes in circulating amino acids, vitamins, and bile acids in the plasma of C57BL/6 mice. J. Gerontol. A Biol. Sci. Med. Sci. 74, 16–26 (2019).
Solon-Biet, S. M. et al. Branched-chain amino acids impact health and lifespan indirectly via amino acid balance and appetite control. Nat. Metab. 1, 532–545 (2019).
Richardson, N. E. et al. Lifelong restriction of dietary branched-chain amino acids has sex-specific benefits for frailty and lifespan in mice. Nat. Aging 1, 73–86 (2021).
Du, X. et al. Increased branched-chain amino acid levels are associated with long-term adverse cardiovascular events in patients with STEMI and acute heart failure. Life Sci. 209, 167–172 (2018).
Ahmad, T. et al. Prognostic implications of long-chain acylcarnitines in heart failure and reversibility with mechanical circulatory support. J. Am. Coll. Cardiol. 67, 291–299 (2016).
Lim, L. L. et al. Circulating branched-chain amino acids and incident heart failure in type 2 diabetes: the Hong Kong diabetes register. Diabetes Metab. Res. Rev. 36, e3253 (2020).
Nemutlu, E. et al. Cardiac resynchronization therapy induces adaptive metabolic transitions in the metabolomic profile of heart failure. J. Card. Fail. 21, 460–469 (2015).
Zhang, Z. Y. et al. Diastolic left ventricular function in relation to circulating metabolic biomarkers in a population study. Eur. J. Prev. Cardiol. 26, 22–32 (2019).
Shah, S. H. et al. Baseline metabolomic profiles predict cardiovascular events in patients at risk for coronary artery disease. Am. Heart J. 163, 844–850.e1 (2012).
Du, X. et al. Relationships between circulating branched chain amino acid concentrations and risk of adverse cardiovascular events in patients with STEMI treated with PCI. Sci. Rep. 8, 15809 (2018).
Ruiz-Canela, M. et al. Plasma branched-chain amino acids and incident cardiovascular disease in the PREDIMED trial. Clin. Chem. 62, 582–592 (2016).
Magnusson, M. et al. A diabetes-predictive amino acid score and future cardiovascular disease. Eur. Heart J. 34, 1982–1989 (2013).
Tobias, D. K. et al. Circulating branched-chain amino acids and incident cardiovascular disease in a prospective cohort of US women. Circ. Genom. Precis. Med. 11, e002157 (2018).
Chevli, P. A. et al. Plasma metabolomic profiling in subclinical atherosclerosis: the Diabetes Heart Study. Cardiovasc. Diabetol. 20, 231 (2021).
Cruz, D. E. et al. Metabolomic analysis of coronary heart disease in an African American cohort from the Jackson Heart Study. JAMA Cardiol. 7, 184–194 (2022).
Yamaguchi, N. et al. Plasma free amino acid profiles evaluate risk of metabolic syndrome, diabetes, dyslipidemia, and hypertension in a large Asian population. Environ. Health Prev. Med. 22, 35 (2017).
Yang, R. et al. Association of branched-chain amino acids with carotid intima-media thickness and coronary artery disease risk factors. PLoS ONE 9, e99598 (2014).
Teymoori, F., Asghari, G., Mirmiran, P. & Azizi, F. Dietary amino acids and incidence of hypertension: a principle component analysis approach. Sci. Rep. 7, 16838 (2017).
Jennings, A. et al. Amino acid intakes are inversely associated with arterial stiffness and central blood pressure in women. J. Nutr. 145, 2130–2138 (2015).
Kimberly, W. T., Wang, Y., Pham, L., Furie, K. L. & Gerszten, R. E. Metabolite profiling identifies a branched chain amino acid signature in acute cardioembolic stroke. Stroke 44, 1389–1395 (2013).
Le Couteur, D. G. et al. Branched chain amino acids, cardiometabolic risk factors and outcomes in older men: the Concord Health and Ageing in Men Project. J. Gerontol. A Biol. Sci. Med. Sci. 75, 1805–1810 (2020).
Lerman, J. B. et al. Plasma metabolites associated with functional and clinical outcomes in heart failure with reduced ejection fraction with and without type 2 diabetes. Sci. Rep. 12, 9183 (2022).
Tsuji, S. et al. Nutritional status of outpatients with chronic stable heart failure based on serum amino acid concentration. J. Cardiol. 72, 458–465 (2018).
Blackburn, P. R. et al. Maple syrup urine disease: mechanisms and management. Appl. Clin. Genet. 10, 57–66 (2017).
Fontana, L. et al. Decreased consumption of branched-chain amino acids improves metabolic health. Cell Rep. 16, 520–530 (2016).
Tuchman, M. et al. Cross-sectional multicenter study of patients with urea cycle disorders in the United States. Mol. Genet. Metab. 94, 397–402 (2008).
Brunetti-Pierri, N. et al. Phenylbutyrate therapy for maple syrup urine disease. Hum. Mol. Genet. 20, 631–640 (2011).
Acknowledgements
The authors are supported by NIH grants K08HL135275 (R.W.M.) and R01HL160689 (R.W.M.), American Diabetes Association Pathway to Stop Diabetes Award 1-16-INI-17 (P.J.W.), the Edna and Fred L. Mandel Jr. Foundation (R.W.M.) and the Duke School of Medicine Strong Start Award (R.W.M.).
Author information
Authors and Affiliations
Contributions
Both authors contributed substantially to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cardiology thanks Gary Lopaschuk, Yibin Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
McGarrah, R.W., White, P.J. Branched-chain amino acids in cardiovascular disease. Nat Rev Cardiol 20, 77–89 (2023). https://doi.org/10.1038/s41569-022-00760-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-022-00760-3
This article is cited by
-
Dysregulated cellular metabolism in atherosclerosis: mediators and therapeutic opportunities
Nature Metabolism (2024)
-
Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases
Signal Transduction and Targeted Therapy (2024)
-
From Energy Metabolic Change to Precision Therapy: a Holistic View of Energy Metabolism in Heart Failure
Journal of Cardiovascular Translational Research (2024)
-
Identification of a leucine-mediated threshold effect governing macrophage mTOR signalling and cardiovascular risk
Nature Metabolism (2024)
-
Circulating metabolites may illustrate relationship of alcohol consumption with cardiovascular disease
BMC Medicine (2023)