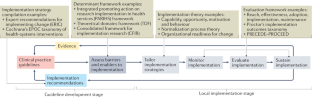
Clinical practice guidelines provide evidence-informed recommendations to improve the delivery of high-quality health care. Despite their ubiquity, the translation of clinical guidelines into routine clinical practice remains suboptimal. We propose the use of implementation science methods in the development of clinical practice guidelines to improve uptake.
This is a preview of subscription content, access via your institution
Relevant articles
Open Access articles citing this article.
-
Assessing the methodological strengths and limitations of the Spanish Society of Medical Oncology (SEOM) guidelines: a critical appraisal using AGREE II and AGREE-REX tool
Clinical and Translational Oncology Open Access 27 June 2023
-
How Can Implementation Science Improve the Care of Familial Hypercholesterolaemia?
Current Atherosclerosis Reports Open Access 20 February 2023
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
References
Grol, R. Successes and failures in the implementation of evidence-based guidelines for clinical practice. Med. Care 39, II46–II54 (2001).
Turner, T., Misso, M., Harris, C. & Green, S. Development of evidence-based clinical practice guidelines (CPGs): comparing approaches. Implement. Sci. 3, 45 (2008).
Brouwers, M. C., Kerkvliet, K. & Spithoff, K. The AGREE Reporting Checklist: a tool to improve reporting of clinical practice guidelines. Br. Med. J. 352, i1152 (2016).
Uchmanowicz, I. et al. Optimising implementation of European guidelines on cardiovascular disease prevention in clinical practice: what is needed? Eur. J. Prev. Cardiol. 28, 426–431 (2020).
Hespe, C. M. et al. Implementing cardiovascular disease preventive care guidelines in general practice: an opportunity missed. Med. J. Aust. 213, 327–328 (2020).
Bonner, C., Fajardo, M. A., Doust, J., McCaffery, K. & Trevena, L. Implementing cardiovascular disease prevention guidelines to translate evidence-based medicine and shared decision making into general practice: theory-based intervention development, qualitative piloting and quantitative feasibility. Implement. Sci. 14, 86 (2019).
Powell, B. J. et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement. Sci. 10, 21 (2015).
Effective Practice and Organisation of Care (EPOC). EPOC Taxonomy 2015 https://epoc.cochrane.org/epoc-taxonomy (2021).
Proctor, E. K., Powell, B. J. & McMillen, J. C. Implementation strategies: recommendations for specifying and reporting. Implement. Sci. 8, 139 (2013).
Nordestgaard, B. G. et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur. Heart J. 34, 3478–3490 (2013).
Acknowledgements
M.N.S. is the recipient of a National Health and Medical Research Council Investigator Grant (Emerging Leader Fellowship) commencing in 2022. L.K.J. and S.S.G. receive funding from the National Heart, Lung, and Blood Institute of the NIH. G.F.W. is a recipient of research grants from the National Health and Medical Research Council and Medical Research Future Funds.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
M.N.S. has received personal fees from Amgen. S.S.G. is a consultant to Esperion for the development of clinical trials for new lipid-lowering agents for children. G.F.W. is a consultant to and recipient of research grants from Amgen, Arrowhead, Regeneron and Sanofi; and a consultant to AstraZeneca, Esperion and Pfizer. L.K.J. declares no competing interests.
Rights and permissions
About this article
Cite this article
Sarkies, M.N., Jones, L.K., Gidding, S.S. et al. Improving clinical practice guidelines with implementation science. Nat Rev Cardiol 19, 3–4 (2022). https://doi.org/10.1038/s41569-021-00645-x
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-021-00645-x
This article is cited by
-
Quality analysis of the clinical practice guidelines for management of impacted maxillary central incisors: a systematic review
Evidence-Based Dentistry (2024)
-
Assessing the methodological strengths and limitations of the Spanish Society of Medical Oncology (SEOM) guidelines: a critical appraisal using AGREE II and AGREE-REX tool
Clinical and Translational Oncology (2023)
-
How Can Implementation Science Improve the Care of Familial Hypercholesterolaemia?
Current Atherosclerosis Reports (2023)
-
International Atherosclerosis Society guidance for implementing best practice in the care of familial hypercholesterolaemia
Nature Reviews Cardiology (2023)
