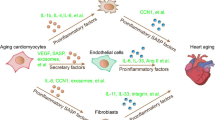
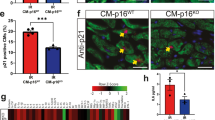
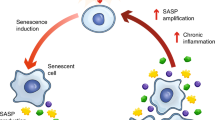
Abstract
Cellular senescence, classically defined as stable cell cycle arrest, is implicated in biological processes such as embryogenesis, wound healing and ageing. Senescent cells have a complex senescence-associated secretory phenotype (SASP), involving a range of pro-inflammatory factors with important paracrine and autocrine effects on cell and tissue biology. Clinical evidence and experimental studies link cellular senescence, senescent cell accumulation, and the production and release of SASP components with age-related cardiac pathologies such as heart failure, myocardial ischaemia and infarction, and cancer chemotherapy-related cardiotoxicity. However, the precise role of senescent cells in these conditions is unclear and, in some instances, both detrimental and beneficial effects have been reported. The involvement of cellular senescence in other important entities, such as cardiac arrhythmias and remodelling, is poorly understood. In this Review, we summarize the basic biology of cellular senescence and discuss what is known about the role of cellular senescence and the SASP in heart disease. We then consider the various approaches that are being developed to prevent the accumulation of senescent cells and their consequences. Many of these strategies are applicable in vivo and some are being investigated for non-cardiac indications in clinical trials. We end by considering important knowledge gaps, directions for future research and the potential implications for improving the management of patients with heart disease.
Key points
-
Cellular senescence refers to the set of changes noted in cells damaged by various stress factors such as dysfunctional telomeres, DNA damage and expression of certain oncogenes, which also occur in cells of aged individuals.
-
Senescent cells have the potential to influence neighbouring cells through secreted cytokines, chemokines, matrix remodelling proteases, growth factors and lipids, collectively referred to as the senescence-associated secretory phenotype.
-
Senescent cells that are transiently present in the heart in response to temporary stress can be beneficial, whereas the long-term accumulation of senescent cells can impair heart function and promote cardiac disease.
-
Acute cellular senescence has important physiological roles in heart development and regeneration; however, senescent cells accumulate progressively in the heart during ageing and cause an age-related decline in heart function.
-
Therapeutic interventions that target senescent cells have the potential to attenuate cardiac dysfunction and improve disease outcomes.
-
Despite a growing number of studies investigating the role of senescence in cardiac ageing and disease, important knowledge gaps remain, especially related to the potential therapeutic benefits of targeting cellular senescence.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Kuilman, T., Michaloglou, C., Mooi, W. J. & Peeper, D. S. The essence of senescence. Genes Dev. 24, 2463–2479 (2010).
Campisi, J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 75, 685–705 (2013).
Khosla, S., Farr, J. N., Tchkonia, T. & Kirkland, J. L. The role of cellular senescence in ageing and endocrine disease. Nat. Rev. Endocrinol. 16, 263–275 (2020).
Frey, N., Venturelli, S., Zender, L. & Bitzer, M. Cellular senescence in gastrointestinal diseases: from pathogenesis to therapeutics. Nat. Rev. Gastroenterol. Hepatol. 15, 81–95 (2018).
Sturmlechner, I., Durik, M., Sieben, C. J., Baker, D. J. & van Deursen, J. M. Cellular senescence in renal ageing and disease. Nat. Rev. Nephrol. 13, 77–89 (2017).
Shimizu, I. & Minamino, T. Cellular senescence in cardiac diseases. J. Cardiol. 74, 313–319 (2019).
Demaria, M. et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 31, 722–733 (2014).
Storer, M. et al. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 155, 1119–1130 (2013).
Kennedy, B. K. et al. Geroscience: linking aging to chronic disease. Cell 159, 709–713 (2014).
Paez-Ribes, M., Gonzalez-Gualda, E., Doherty, G. J. & Munoz-Espin, D. Targeting senescent cells in translational medicine. EMBO Mol. Med. 11, e10234 (2019).
Farr, J. N. et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat. Med. 23, 1072–1079 (2017).
Musi, N. et al. Tau protein aggregation is associated with cellular senescence in the brain. Aging Cell 17, e12840 (2018).
Roos, C. M. et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell 15, 973–977 (2016).
Xu, M. et al. Targeting senescent cells enhances adipogenesis and metabolic function in old age. eLife 4, e12997 (2015).
Xu, E. & Wen, H. X. Risk factors of cerebrovascular diseases and their intervention and management. Chin. J. Contemp. Neurol. Neurosurg. 15, 20–26 (2015).
Xu, M. et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 24, 1246–1256 (2018).
Spallarossa, P. et al. Doxorubicin induces senescence or apoptosis in rat neonatal cardiomyocytes by regulating the expression levels of the telomere binding factors 1 and 2. Am. J. Physiol. Heart Circ. Physiol. 297, H2169–H2181 (2009).
Januzzi, J. L. et al. IGFBP7 (insulin-like growth factor-binding protein-7) and neprilysin inhibition in patients with heart failure. Circ. Heart Fail. 11, e005133 (2018).
Weissman, A. Essays upon Heredity and Kindred Biological Problems (Clarendon, 1891).
Levine, H. J. Rest heart rate and life expectancy. J. Am. Coll. Cardiol. 30, 1104–1106 (1997).
O’Rourke, M. F., Safar, M. E. & Dzau, V. The cardiovascular continuum extended: aging effects on the aorta and microvasculature. Vasc. Med. 15, 461–468 (2010).
Hacker, T. A., McKiernan, S. H., Douglas, P. S., Wanagat, J. & Aiken, J. M. Age-related changes in cardiac structure and function in Fischer 344 x Brown Norway hybrid rats. Am. J. Physiol. Heart Circ. Physiol. 290, H304–H311 (2006).
Hayflick, L. & Moorhead, P. S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 25, 585–621 (1961).
Greider, C. W. & Blackburn, E. H. The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell 51, 887–898 (1987).
Harley, C. B., Futcher, A. B. & Greider, C. W. Telomeres shorten during ageing of human fibroblasts. Nature 345, 458–460 (1990).
Chang, E. & Harley, C. B. Telomere length and replicative aging in human vascular tissues. Proc. Natl Acad. Sci. USA 92, 11190–11194 (1995).
Voghel, G. et al. Cellular senescence in endothelial cells from atherosclerotic patients is accelerated by oxidative stress associated with cardiovascular risk factors. Mech. Ageing Dev. 128, 662–671 (2007).
Razgonova, M. P. et al. Telomerase and telomeres in aging theory and chronographic aging theory (Review). Mol. Med. Rep. 22, 1679–1694 (2020).
Serrano, M., Lin, A. W., McCurrach, M. E., Beach, D. & Lowe, S. W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593–602 (1997).
Mallette, F. A. & Ferbeyre, G. The DNA damage signaling pathway connects oncogenic stress to cellular senescence. Cell Cycle 6, 1831–1836 (2007).
Borghesan, M., Hoogaars, W. M. H., Varela-Eirin, M., Talma, N. & Demaria, M. A senescence-centric view of aging: implications for longevity and disease. Trends Cell Biol. 30, 777–791 (2020).
Gorgoulis, V. et al. Cellular senescence: defining a path forward. Cell 179, 813–827 (2019).
Anderson, R. et al. Length-independent telomere damage drives post-mitotic cardiomyocyte senescence. EMBO J. 38, e100492 (2019).
Zhu, Y. et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14, 644–658 (2015).
Chang, J. et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 22, 78–83 (2016).
Childs, B. G. et al. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 354, 472–477 (2016).
Bussian, T. J. et al. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature 562, 578–582 (2018).
Hickson, L. J. et al. Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine 47, 446–456 (2019).
Justice, J. N. et al. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine 40, 554–563 (2019).
Munoz-Espin, D. et al. Programmed cell senescence during mammalian embryonic development. Cell 155, 1104–1118 (2013).
Jun, J. I. & Lau, L. F. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat. Cell Biol. 12, 676–685 (2010).
Feng, T. et al. CCN1-induced cellular senescence promotes heart regeneration. Circulation 139, 2495–2498 (2019).
Grosse, L. et al. Defined p16(High) senescent cell types are indispensable for mouse healthspan. Cell Metab. 32, 87–99.e6 (2020).
Deschênes-Simard, X. et al. Tumor suppressor activity of the ERK/MAPK pathway by promoting selective protein degradation. Genes Dev. 27, 900–915 (2013).
Tran, D. et al. Insulin-like growth factor-1 regulates the SIRT1-p53 pathway in cellular senescence. Aging Cell 13, 669–678 (2014).
Bueno, M. et al. Mitochondria, aging, and cellular senescence: implications for scleroderma. Curr. Rheumatol. Rep. 22, 37 (2020).
Nacarelli, T., Azar, A. & Sell, C. Mitochondrial stress induces cellular senescence in an mTORC1-dependent manner. Free Radic. Biol. Med. 95, 133–154 (2016).
Chapman, J., Fielder, E. & Passos, J. F. Mitochondrial dysfunction and cell senescence: deciphering a complex relationship. FEBS Lett. 593, 1566–1579 (2019).
Sahin, E. et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature 470, 359–365 (2011).
Du, W. W. et al. The microRNA miR-17-3p inhibits mouse cardiac fibroblast senescence by targeting Par4. J. Cell Sci. 128, 293–304 (2015).
Ito, T., Yagi, S. & Yamakuchi, M. MicroRNA-34a regulation of endothelial senescence. Biochem. Biophys. Res. Commun. 398, 735–740 (2010).
Jazbutyte, V. et al. MicroRNA-22 increases senescence and activates cardiac fibroblasts in the aging heart. Age 35, 747–762 (2013).
Bilsland, A. E., Revie, J. & Keith, W. MicroRNA and senescence: the senectome, integration and distributed control. Crit. Rev. Oncog. 18, 373–390 (2013).
Yosef, R. et al. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat. Commun. 7, 11190 (2016).
Ahn, J. S. et al. Aging-associated increase of gelsolin for apoptosis resistance. Biochem. Biophys. Res. Commun. 312, 1335–1341 (2003).
d’Adda di Fagagna, F. Living on a break: cellular senescence as a DNA-damage response. Nat. Rev. Cancer 8, 512–522 (2008).
Baar, M. P. et al. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell 169, 132–147.e16 (2017).
Coppe, J. P. et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 6, 2853–2868 (2008).
Acosta, J. C. et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133, 1006–1018 (2008).
Kuilman, T. et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 133, 1019–1031 (2008).
Childs, B. G., Durik, M., Baker, D. J. & van Deursen, J. M. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat. Med. 21, 1424–1435 (2015).
Martini, H. et al. Aging induces cardiac mesenchymal stromal cell senescence and promotes endothelial cell fate of the CD90+ subset. Aging Cell 18, e13015 (2019).
Sokolova, M. et al. Palmitate promotes inflammatory responses and cellular senescence in cardiac fibroblasts. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1862, 234–245 (2017).
Matsushita, H. et al. eNOS activity is reduced in senescent human endothelial cells: preservation by hTERT immortalization. Circ. Res. 89, 793–798 (2001).
Bochenek, M. L., Schütz, E. & Schäfer, K. Endothelial cell senescence and thrombosis: ageing clots. Thromb. Res. 147, 36–45 (2016).
Tang, X., Li, P. H. & Chen, H. Z. Cardiomyocyte senescence and cellular communications within myocardial microenvironments. Front. Endocrinol. 11, 280 (2020).
Li, H. et al. Targeting age-related pathways in heart failure. Circ. Res. 126, 533–551 (2020).
Sun, T., Ghosh, A. K., Eren, M., Miyata, T. & Vaughan, D. E. PAI-1 contributes to homocysteine-induced cellular senescence. Cell. Signal. 64, 109394 (2019).
Franceschi, C. et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. NY Acad. Sci. 908, 244–254 (2000).
Liberale, L., Montecucco, F., Tardif, J. C., Libby, P. & Camici, G. G. Inflamm-ageing: the role of inflammation in age-dependent cardiovascular disease. Eur. Heart J. 41, 2974–2982 (2020).
Straub, R. H. & Schradin, C. Chronic inflammatory systemic diseases: an evolutionary trade-off between acutely beneficial but chronically harmful programs. Evol. Med. Public Health 2016, 37–51 (2016).
van Deursen, J. M. The role of senescent cells in ageing. Nature 509, 439–446 (2014).
He, S. & Sharpless, N. E. Senescence in health and disease. Cell 169, 1000–1011 (2017).
Baker, D. J. et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236 (2011).
Jeon, O. H. et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med. 23, 775–781 (2017).
Palmer, A. K. et al. Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell 18, e12950 (2019).
Robbins, P. D. et al. Senolytic drugs: reducing senescent cell viability to extend health span. Annu. Rev. Pharmacol. Toxicol. 61, 779–803 (2021).
Ellison-Hughes, G. M. Senescent cells: targeting and therapeutic potential of senolytics in age-related diseases with a particular focus on the heart. Expert Opin. Ther. Targets 24, 819–823 (2020).
Cianflone, E. et al. Targeting cardiac stem cell senescence to treat cardiac aging and disease. Cells 9, 1558 (2020).
Moiseeva, O., Deschênes-Simard, X., Pollak, M. & Ferbeyre, G. Metformin, aging and cancer. Aging 5, 330–331 (2013).
Di Micco, R., Krizhanovsky, V., Baker, D. & d’Adda di Fagagna, F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 22, 75–95 (2021).
Park, J. H. et al. Pharmacological inhibition of mTOR attenuates replicative cell senescence and improves cellular function via regulating the STAT3-PIM1 axis in human cardiac progenitor cells. Exp. Mol. Med. 52, 615–628 (2020).
Noren Hooten, N. et al. Metformin-mediated increase in DICER1 regulates microRNA expression and cellular senescence. Aging Cell 15, 572–581 (2016).
Macip, S. et al. Inhibition of p21-mediated ROS accumulation can rescue p21-induced senescence. EMBO J. 21, 2180–2188 (2002).
Kosugi, R. et al. Angiotensin II receptor antagonist attenuates expression of aging markers in diabetic mouse heart. Circ. J. 70, 482–488 (2006).
Katsuumi, G. et al. Catecholamine-induced senescence of endothelial cells and bone marrow cells promotes cardiac dysfunction in mice. Int. Heart J. 59, 837–844 (2018).
Baker, D. J. et al. Naturally occurring p16 Ink4a-positive cells shorten healthy lifespan. Nature 530, 184–189 (2016).
Li, J. et al. Long-term repopulation of aged bone marrow stem cells using young Sca-1 cells promotes aged heart rejuvenation. Aging Cell 18, e13026 (2019).
Cui, S. et al. Postinfarction hearts are protected by premature senescent cardiomyocytes via GATA4-dependent CCN1 secretion. J. Am. Heart Assoc. 7, e009111 (2018).
Alam, P. et al. Inhibition of senescence-associated genes Rb1 and Meis2 in adult cardiomyocytes results in cell cycle reentry and cardiac repair post-myocardial infarction. J. Am. Heart Assoc. 8, e012089 (2019).
Manzella, N. et al. Monoamine oxidase-A is a novel driver of stress-induced premature senescence through inhibition of parkin-mediated mitophagy. Aging Cell 17, e12811 (2018).
von Zglinicki, T., Wan, T. & Miwa, S. Senescence in post-mitotic cells: a driver of aging? Antioxid. Redox Signal. 34, 308–323 (2021).
Sapieha, P. & Mallette, F. A. Cellular senescence in postmitotic cells: beyond growth arrest. Trends Cell Biol. 28, 595–607 (2018).
Lorda-Diez, C. I. et al. Cell senescence, apoptosis and DNA damage cooperate in the remodeling processes accounting for heart morphogenesis. J. Anat. 234, 815–829 (2019).
Kirkland, J. L. & Tchkonia, T. Cellular senescence: a translational perspective. EBioMedicine 21, 21–28 (2017).
Cheng, H. L. et al. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc. Natl Acad. Sci. USA 100, 10794–10799 (2003).
Wu, Y. et al. Pax8 plays a pivotal role in regulation of cardiomyocyte growth and senescence. J. Cell. Mol. Med. 20, 644–654 (2016).
North, B. J. & Sinclair, D. A. The intersection between aging and cardiovascular disease. Circ. Res. 110, 1097–1108 (2012).
Laredo, M., Waldmann, V., Khairy, P. & Nattel, S. Age as a critical determinant of atrial fibrillation: a two-sided relationship. Can. J. Cardiol. 34, 1396–1406 (2018).
Kuo, C. L., Pilling, L. C., Kuchel, G. A., Ferrucci, L. & Melzer, D. Telomere length and aging-related outcomes in humans: a Mendelian randomization study in 261,000 older participants. Aging Cell 18, e13017 (2019).
Noly, P. E. et al. Reduction of plasma angiopoietin-like 2 after cardiac surgery is related to tissue inflammation and senescence status of patients. J. Thorac. Cardiovasc. Surg. 158, 792–802.e5 (2019).
Bujak, M. et al. Aging-related defects are associated with adverse cardiac remodeling in a mouse model of reperfused myocardial infarction. J. Am. Coll. Cardiol. 51, 1384–1392 (2008).
Gude, N. A., Broughton, K. M., Firouzi, F. & Sussman, M. A. Cardiac ageing: extrinsic and intrinsic factors in cellular renewal and senescence. Nat. Rev. Cardiol. 15, 523–542 (2018).
Nattel, S. Molecular and cellular mechanisms of atrial fibrosis in atrial fibrillation. JACC Clin. Electrophysiol. 3, 425–435 (2017).
Sawaki, D. et al. Visceral adipose tissue drives cardiac aging through modulation of fibroblast senescence by osteopontin production. Circulation 138, 809–822 (2018).
Meyer, K., Hodwin, B., Ramanujam, D., Engelhardt, S. & Sarikas, A. Essential role for premature senescence of myofibroblasts in myocardial fibrosis. J. Am. Coll. Cardiol. 67, 2018–2028 (2016).
Zhu, F. et al. Senescent cardiac fibroblast is critical for cardiac fibrosis after myocardial infarction. PLoS ONE 8, e74535 (2013).
Azar, A., Lawrence, I., Jofre, S., Mell, J. & Sell, C. Distinct patterns of gene expression in human cardiac fibroblasts exposed to rapamycin treatment or methionine restriction. Ann. NY Acad. Sci. 1418, 95–105 (2018).
Li, W. Q. et al. Calcitonin gene-related peptide inhibits the cardiac fibroblasts senescence in cardiac fibrosis via up-regulating klotho expression. Eur. J. Pharmacol. 843, 96–103 (2019).
Bonda, T. A. et al. Interleukin-6 affects aging-related changes of the PPARα-PGC-1α axis in the myocardium. J. Interferon Cytokine Res. 37, 513–521 (2017).
Lyu, G. et al. TGF-β signaling alters H4K20me3 status via miR-29 and contributes to cellular senescence and cardiac aging. Nat. Commun. 9, 2560 (2018).
Rawal, S. et al. Down-regulation of miR-15a/b accelerates fibrotic remodelling in the type 2 diabetic human and mouse heart. Clin. Sci. 131, 847–863 (2017).
Lin, R. et al. miR-1468-3p promotes aging-related cardiac fibrosis. Mol. Ther. Nucleic Acids 20, 589–605 (2020).
Huang, Z. P. & Wang, D. Z. miR-22 in smooth muscle cells: a potential therapy for cardiovascular disease. Circulation 137, 1842–1845 (2018).
Tijsen, A. J. et al. The microRNA-15 family inhibits the TGFβ-pathway in the heart. Cardiovasc. Res. 104, 61–71 (2014).
Gutiérrez-Fernández, A. et al. Loss of MT1-MMP causes cell senescence and nuclear defects which can be reversed by retinoic acid. EMBO J. 34, 1875–1888 (2015).
Abdelfatah, N. et al. Characterization of a unique form of arrhythmic cardiomyopathy caused by recessive mutation in LEMD2. JACC Basic Transl. Sci. 4, 204–221 (2019).
Jia, L. et al. Haplodeficiency of ataxia telangiectasia mutated accelerates heart failure after myocardial infarction. J. Am. Heart Assoc. 6, e006349 (2017).
Xie, J. et al. Premature senescence of cardiac fibroblasts and atrial fibrosis in patients with atrial fibrillation. Oncotarget 8, 57981–57990 (2017).
Nakamura, M. & Sadoshima, J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 15, 387–407 (2018).
Oldfield, C. J., Duhamel, T. A. & Dhalla, N. S. Mechanisms for the transition from physiological to pathological cardiac hypertrophy. Can. J. Physiol. Pharmacol. 98, 74–84 (2020).
Dai, D. F. et al. Age-dependent cardiomyopathy in mitochondrial mutator mice is attenuated by overexpression of catalase targeted to mitochondria. Aging Cell 9, 536–544 (2010).
Morin, D. et al. Hsp22 overexpression induces myocardial hypertrophy, senescence and reduced life span through enhanced oxidative stress. Free Radic. Biol. Med. 137, 194–200 (2019).
Cataldi, A. et al. p53 and telomerase control rat myocardial tissue response to hypoxia and ageing. Eur. J. Histochem. 53, 209–216 (2009).
Li, Y. et al. SIRT3 deficiency exacerbates p53/Parkin-mediated mitophagy inhibition and promotes mitochondrial dysfunction: implication for aged hearts. Int. J. Mol. Med. 41, 3517–3526 (2018).
Zha, Z., Wang, J., Wang, X., Lu, M. & Guo, Y. Involvement of PINK1/Parkin-mediated mitophagy in AGE-induced cardiomyocyte aging. Int. J. Cardiol. 227, 201–208 (2017).
Dong, R. et al. Bradykinin inhibits oxidative stress-induced cardiomyocytes senescence via regulating redox state. PLoS ONE 8, e77034 (2013).
Takahashi, K. et al. Premature cardiac senescence in DahlS.Z-Lepr(fa)/Lepr(fa) rats as a new animal model of metabolic syndrome. Nagoya J. Med. Sci. 76, 35–49 (2014).
Ock, S. et al. Deletion of IGF-1 receptors in cardiomyocytes attenuates cardiac aging in male mice. Endocrinology 157, 336–345 (2016).
Hua, Y. et al. Cathepsin K knockout alleviates aging-induced cardiac dysfunction. Aging Cell 14, 345–351 (2015).
Sun, R. et al. Senescence as a novel mechanism involved in β-adrenergic receptor mediated cardiac hypertrophy. PLoS ONE 12, e0182668 (2017).
Sheng, Y. et al. Opposing effects on cardiac function by calorie restriction in different-aged mice. Aging Cell 16, 1155–1167 (2017).
Louhelainen, M. et al. Effects of calcium sensitizer OR-1986 on a cardiovascular mortality and myocardial remodelling in hypertensive Dahl/Rapp rats. J. Physiol. Pharmacol. 60, 41–47 (2009).
Ewer, M. S. & Ewer, S. M. Cardiotoxicity of anticancer treatments. Nat. Rev. Cardiol. 12, 547–558 (2015).
Herrmann, J. Adverse cardiac effects of cancer therapies: cardiotoxicity and arrhythmia. Nat. Rev. Cardiol. 17, 474–502 (2020).
Piegari, E. et al. Doxorubicin induces senescence and impairs function of human cardiac progenitor cells. Basic Res. Cardiol. 108, 334 (2013).
Lazzarini, E. et al. The human amniotic fluid stem cell secretome effectively counteracts doxorubicin-induced cardiotoxicity. Sci. Rep. 6, 29994 (2016).
Werner, C. et al. Effects of physical exercise on myocardial telomere-regulating proteins, survival pathways, and apoptosis. J. Am. Coll. Cardiol. 52, 470–482 (2008).
Ghosh, A. K. et al. A small molecule inhibitor of PAI-1 protects against doxorubicin-induced cellular senescence. Oncotarget 7, 72443–72457 (2016).
Altieri, P. et al. Testosterone antagonizes doxorubicin-induced senescence of cardiomyocytes. J. Am. Heart Assoc. 5, e002383 (2016).
Altieri, P. et al. Inhibition of doxorubicin-induced senescence by PPARδ activation agonists in cardiac muscle cells: cooperation between PPARδ and Bcl6. PLoS ONE 7, e46126 (2012).
Xia, W. & Hou, M. Mesenchymal stem cells confer resistance to doxorubicin-induced cardiac senescence by inhibiting microRNA-34a. Oncol. Lett. 15, 10037–10046 (2018).
Piegari, E. et al. MicroRNA-34a regulates doxorubicin-induced cardiotoxicity in rat. Oncotarget 7, 62312–62326 (2016).
Du, W. W. et al. Foxo3 circular RNA promotes cardiac senescence by modulating multiple factors associated with stress and senescence responses. Eur. Heart J. 38, 1402–1412 (2017).
Xie, Z., Xia, W. & Hou, M. Long intergenic noncoding RNAp21 mediates cardiac senescence via the Wnt/betacatenin signaling pathway in doxorubicin-induced cardiotoxicity. Mol. Med. Rep. 17, 2695–2704 (2018).
Kim, E. J. et al. Involvement of corin downregulation in ionizing radiation-induced senescence of myocardial cells. Int. J. Mol. Med. 35, 731–738 (2015).
Alessio, N. et al. Increase of circulating IGFBP-4 following genotoxic stress and its implication for senescence. eLife 9, e54523 (2020).
Saleh, Y., Abdelkarim, O., Herzallah, K. & Abela, G. S. Anthracycline-induced cardiotoxicity: mechanisms of action, incidence, risk factors, prevention, and treatment. Heart Fail Rev. 26, 1159–1173 (2021).
Braunwald, E. Biomarkers in heart failure. N. Engl. J. Med. 358, 2148–2159 (2008).
Din, S. et al. Metabolic dysfunction consistent with premature aging results from deletion of pim kinases. Circ. Res. 115, 376–387 (2014).
Inuzuka, Y. et al. Suppression of phosphoinositide 3-kinase prevents cardiac aging in mice. Circulation 120, 1695–1703 (2009).
Bertagnolli, M. et al. Transient neonatal high oxygen exposure leads to early adult cardiac dysfunction, remodeling, and activation of the Renin-Angiotensin system. Hypertension 63, 143–150 (2014).
Rota, M. et al. Diabetes promotes cardiac stem cell aging and heart failure, which are prevented by deletion of the p66shc gene. Circ. Res. 99, 42–52 (2006).
Caragnano, A. et al. Autophagy and inflammasome activation in dilated cardiomyopathy. J. Clin. Med. 8, 1519 (2019).
Neves, M. F., Cunha, A. R., Cunha, M. R., Gismondi, R. A. & Oigman, W. The role of renin-angiotensin-aldosterone system and its new components in arterial stiffness and vascular aging. High Blood Press. Cardiovasc. Prev. 25, 137–145 (2018).
Minamino, T. & Komuro, I. Vascular cell senescence: contribution to atherosclerosis. Circ. Res. 100, 15–26 (2007).
Jia, K. et al. Senolytic agent navitoclax inhibits angiotensin II-induced heart failure in mice. J. Cardiovasc. Pharmacol. 76, 452–460 (2020).
Sciarretta, S., Maejima, Y., Zablocki, D. & Sadoshima, J. The role of autophagy in the heart. Annu. Rev. Physiol. 80, 1–26 (2018).
Russomanno, G. et al. The anti-ageing molecule sirt1 mediates beneficial effects of cardiac rehabilitation. Immun. Ageing 14, 7 (2017).
Youn, J. C. et al. Increased frequency of CD4+CD57+ senescent T cells in patients with newly diagnosed acute heart failure: exploring new pathogenic mechanisms with clinical relevance. Sci. Rep. 9, 12887 (2019).
Toldo, S. & Abbate, A. The NLRP3 inflammasome in acute myocardial infarction. Nat. Rev. Cardiol. 15, 203–214 (2018).
Frangogiannis, N. G. Pathophysiology of myocardial infarction. Compr. Physiol. 5, 1841–1875 (2015).
Boon, R. A. & Dimmeler, S. MicroRNAs in myocardial infarction. Nat. Rev. Cardiol. 12, 135–142 (2015).
Rodriguez, J. A. et al. Selective increase of cardiomyocyte derived extracellular vesicles after experimental myocardial infarction and functional effects on the endothelium. Thrombosis Res. 170, 1–9 (2018).
Walaszczyk, A. et al. Pharmacological clearance of senescent cells improves survival and recovery in aged mice following acute myocardial infarction. Aging Cell 18, e12945 (2019).
Nishimura, A. et al. Hypoxia-induced interaction of filamin with Drp1 causes mitochondrial hyperfission-associated myocardial senescence. Sci. Signal. 11, eaat5185 (2018).
Lin, B., Feng, D. & Xu, J. Cardioprotective effects of microRNA-18a on acute myocardial infarction by promoting cardiomyocyte autophagy and suppressing cellular senescence via brain derived neurotrophic factor. Cell Biosci. 9, 38 (2019).
Louhelainen, M. et al. Oral levosimendan prevents postinfarct heart failure and cardiac remodeling in diabetic Goto-Kakizaki rats. J. Hypertens. 27, 2094–2107 (2009).
Wen, Z., Mai, Z., Chen, Y., Wang, J. F. & Geng, D. F. Angiotensin II receptor blocker reverses heart failure by attenuating local oxidative stress and preserving resident stem cells in rats with myocardial infarction. Am. J. Transl. Res. 10, 2387–2401 (2018).
Shibamoto, M. et al. Activation of dna damage response and cellular senescence in cardiac fibroblasts limit cardiac fibrosis after myocardial infarction. Int. Heart J. 60, 944–957 (2019).
Du, G. Q. et al. Targeted myocardial delivery of GDF11 gene rejuvenates the aged mouse heart and enhances myocardial regeneration after ischemia-reperfusion injury. Basic Res. Cardiol. 112, 7 (2017).
Dookun, E. et al. Clearance of senescent cells during cardiac ischemia-reperfusion injury improves recovery. Aging Cell 19, e13249 (2020).
Andrade, J., Khairy, P., Dobrev, D. & Nattel, S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ. Res. 114, 1453–1468 (2014).
Jesel, L. et al. Atrial fibrillation progression is associated with cell senescence burden as determined by p53 and p16 expression. J. Clin. Med. 9, 36 (2019).
Aharonov, A. et al. ERBB2 drives YAP activation and EMT-like processes during cardiac regeneration. Nat. Cell Biol. 22, 1346–1356 (2020).
Yutzey, K. E. Cardiomyocyte proliferation: teaching an old dogma new tricks. Circ. Res. 120, 627–629 (2017).
Rubin, N., Harrison, M. R., Krainock, M., Kim, R. & Lien, C. L. Recent advancements in understanding endogenous heart regeneration-insights from adult zebrafish and neonatal mice. Semin. Cell Dev. Biol. 58, 34–40 (2016).
Eschenhagen, T. et al. Cardiomyocyte regeneration: a consensus statement. Circulation 136, 680–686 (2017).
He, L., Nguyen, N. B., Ardehali, R. & Zhou, B. Heart regeneration by endogenous stem cells and cardiomyocyte proliferation: controversy, fallacy, and progress. Circulation 142, 275–291 (2020).
Menasché, P. Cell therapy trials for heart regeneration - lessons learned and future directions. Nat. Rev. Cardiol. 15, 659–671 (2018).
Zhang, M. et al. Bone marrow mesenchymal stem cell transplantation retards the natural senescence of rat hearts. Stem Cell Transl. Med. 4, 494–502 (2015).
Song, H. F. et al. Aged human multipotent mesenchymal stromal cells can be rejuvenated by neuron-derived neurotrophic factor and improve heart function after injury. JACC Basic Transl. Sci. 2, 702–716 (2017).
Khan, M., Mohsin, S., Khan, S. N. & Riazuddin, S. Repair of senescent myocardium by mesenchymal stem cells is dependent on the age of donor mice. J. Cell. Mol. Med. 15, 1515–1527 (2011).
Zhang, Y. et al. Macrophage migration inhibitory factor rejuvenates aged human mesenchymal stem cells and improves myocardial repair. Aging 11, 12641–12660 (2019).
Lewis-McDougall, F. C. et al. Aged-senescent cells contribute to impaired heart regeneration. Aging Cell 18, e12931 (2019).
Khatiwala, R. V. et al. Inhibition of p16INK4A to rejuvenate aging human cardiac progenitor cells via the upregulation of anti-oxidant and NFκB signal pathways. Stem Cell Rev. Rep. 14, 612–625 (2018).
Hariharan, N. et al. Nucleostemin rejuvenates cardiac progenitor cells and antagonizes myocardial aging. J. Am. Coll. Cardiol. 65, 133–147 (2015).
Matsumoto, C. et al. Short telomeres induce p53 and autophagy and modulate age-associated changes in cardiac progenitor cell fate. Stem Cell 36, 868–880 (2018).
Castaldi, A. et al. Decline in cellular function of aged mouse c-kit+ cardiac progenitor cells. J. Physiol. 595, 6249–6262 (2017).
Fomison-Nurse, I. et al. Diabetes induces the activation of pro-ageing MIR-34a in the heart, but has differential effects on cardiomyocytes and cardiac progenitor cells. Cell Death Differ. 25, 1336–1349 (2018).
Toko, H. et al. Differential regulation of cellular senescence and differentiation by prolyl isomerase Pin1 in cardiac progenitor cells. J. Biol. Chem. 289, 5348–5356 (2014).
Zhao, L. et al. TERT assists GDF11 to rejuvenate senescent VEGFR2+/CD133+ cells in elderly patients with myocardial infarction. Lab. Invest. 99, 1661–1688 (2019).
Hong, Y. et al. miR-155-5p inhibition rejuvenates aged mesenchymal stem cells and enhances cardioprotection following infarction. Aging Cell 19, e13128 (2020).
Choudhery, M. S. et al. Mesenchymal stem cells conditioned with glucose depletion augments their ability to repair-infarcted myocardium. J. Cell. Mol. Med. 16, 2518–2529 (2012).
Samse, K. et al. Functional effect of Pim1 depends upon intracellular localization in human cardiac progenitor cells. J. Biol. Chem. 290, 13935–13947 (2015).
Rafatian, G. et al. Mybl2 rejuvenates heart explant-derived cells from aged donors after myocardial infarction. Aging Cell 19, e13174 (2020).
Dong, J. et al. MiR-10a rejuvenates aged human mesenchymal stem cells and improves heart function after myocardial infarction through KLF4. Stem Cell Res. Ther. 9, 151 (2018).
Mohsin, S. et al. Rejuvenation of human cardiac progenitor cells with pim-1 kinase. Circ. Res. 113, 1169–1179 (2013).
Fu, C. et al. Bradykinin protects cardiac c-kit positive cells from high-glucose-induced senescence through B2 receptor signaling pathway. J. Cell. Biochem. 120, 17731–17743 (2019).
Nakamura, T. et al. Age-related increase in Wnt inhibitor causes a senescence-like phenotype in human cardiac stem cells. Biochem. Biophys. Res. Commun. 487, 653–659 (2017).
Leone, M. & Engel, F. B. Advances in heart regeneration based on cardiomyocyte proliferation and regenerative potential of binucleated cardiomyocytes and polyploidization. Clin. Sci. 133, 1229–1253 (2019).
Sarig, R. et al. Transient p53-mediated regenerative senescence in the injured heart. Circulation 139, 2491–2494 (2019).
Dimri, G. P. et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl Acad. Sci. USA 92, 9363–9367 (1995).
Hoenicke, L. & Zender, L. Immune surveillance of senescent cells — biological significance in cancer- and non-cancer pathologies. Carcinogenesis 33, 1123–1126 (2012).
Ritschka, B. et al. The senescence-associated secretory phenotype induces cellular plasticity and tissue regeneration. Genes Dev. 31, 172–183 (2017).
Muñoz-Espín, D. et al. Programmed cell senescence during mammalian embryonic development. Cell 155, 1104–1118 (2013).
Zhu, Y. et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 15, 428–435 (2016).
Acknowledgements
The authors thank L. Lefebvre (Montreal Heart Institute, Canada) for valuable secretarial help with manuscript preparation and submission. The authors received funding from the Canadian Institutes of Health Research (grant 148401 to S.N.; grants 166110 and 162446 to E.T.), Heart and Stroke Foundation of Canada (18-0022032 to S.N.), the Montreal Heart Institute Foundation (S.N. and E.T.) and a doctoral training scholarship from the Fonds de Recherche en Santé du Québec (M.M.).
Review criteria
This Review includes an introductory narrative section, followed by a systematic review of the role of cellular senescence in heart disease. Electronic databases, including Medline, Scopus and the Cochrane library, were searched with the following search formula: (“senescence” OR “senescent” OR “SASP”) [title/abstract] AND (“heart” OR “cardiac”) [title/abstract]. Only papers with English full texts were reviewed. The primary search results were screened based on their abstracts to find potentially relevant studies; only studies in which more than one marker of senescence (Box 1) were used to identify senescent cells were included. In many excluded papers, the term ‘senescence’ was used synonymously with the ageing process, without defining senescence per se. Articles were selected based on the exploration of a role for cell senescence in cardiac development, physiology and/or disease. The full texts of the selected articles were then checked by two independent investigators, and final articles were chosen by consensus to form the primary literature base for this Review. A limited number of additional citations were included if needed to support or detail a key concept related to the selected literature. A consort diagram of the systematic search approach and results is available as Supplementary Figure 1.
Author information
Authors and Affiliations
Contributions
M.M. researched data for the article. M.M., M.A., E.T., G.F. and S.N. wrote the manuscript. All the authors contributed to discussion of the content and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Cardiology thanks G. Ellison-Hughes and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Senolytics
-
Therapeutic compounds that kill senescent cells.
- Telomerase
-
Enzyme responsible for the maintenance of telomere length by the addition of telomere repeat sequences to the end of telomeres.
- Telomere
-
A specific complex at the end of linear eukaryotic chromosomes consisting of repeat DNA sequences and associated proteins; critically short telomeres cause cellular senescence.
- Immunosenescence
-
Senescence of the immune cells that are responsible for recognizing and eliminating senescent cells.
- microRNAs
-
(miRNAs). Endogenous small RNAs that regulate gene expression.
- PML nuclear bodies
-
Promyelocytic leukaemia protein (PML) nuclear bodies are membraneless structures located in the nucleus of eukaryotic cells and are involved in DNA damage, DNA repair, telomere homeostasis and p53-associated apoptosis.
- INK-ATTAC
-
Transgenic mouse model in which senescent cells express a ‘suicide’ gene (Casp8, encoding caspase 8) and undergo apoptosis following the treatment of mice with a polymerizing agent (AP20187) that activates caspase.
- p16-3MR
-
Transgenic mouse model in which senescent cells express the herpes simplex virus thymidine kinase and can therefore be eliminated following the treatment of mice with ganciclovir.
Rights and permissions
About this article
Cite this article
Mehdizadeh, M., Aguilar, M., Thorin, E. et al. The role of cellular senescence in cardiac disease: basic biology and clinical relevance. Nat Rev Cardiol 19, 250–264 (2022). https://doi.org/10.1038/s41569-021-00624-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-021-00624-2
This article is cited by
-
Cardiovascular disease and cancer: shared risk factors and mechanisms
Nature Reviews Cardiology (2024)
-
The key cellular senescence related molecule RRM2 regulates prostate cancer progression and resistance to docetaxel treatment
Cell & Bioscience (2023)
-
ERBB2-PTGS2 axis promotes intervertebral disc degeneration by regulating senescence of nucleus pulposus cells
BMC Musculoskeletal Disorders (2023)
-
Metabolic landscape in cardiac aging: insights into molecular biology and therapeutic implications
Signal Transduction and Targeted Therapy (2023)
-
Hallmarks of cardiovascular ageing
Nature Reviews Cardiology (2023)