Abstract
Increases in calorie consumption and sedentary lifestyles are fuelling a global pandemic of cardiometabolic diseases, including coronary artery disease, diabetes mellitus, cardiomyopathy and heart failure. These lifestyle factors, when combined with genetic predispositions, increase the levels of circulating lipids, which can accumulate in non-adipose tissues, including blood vessel walls and the heart. The metabolism of these lipids produces bioactive intermediates that disrupt cellular function and survival. A compelling body of evidence suggests that sphingolipids, such as ceramides, account for much of the tissue damage in these cardiometabolic diseases. In humans, serum ceramide levels are proving to be accurate biomarkers of adverse cardiovascular disease outcomes. In mice and rats, pharmacological inhibition or depletion of enzymes driving de novo ceramide synthesis prevents the development of diabetes, atherosclerosis, hypertension and heart failure. In cultured cells and isolated tissues, ceramides perturb mitochondrial function, block fuel usage, disrupt vasodilatation and promote apoptosis. In this Review, we discuss the body of literature suggesting that ceramides are drivers — and not merely passengers — on the road to cardiovascular disease. Moreover, we explore the feasibility of therapeutic strategies to lower ceramide levels to improve cardiovascular health.
Key points
-
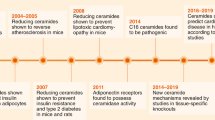
Ceramides have been shown to accumulate in many tissues, including blood vessels and the heart, in individuals with cardiovascular disease (such as hypertension, heart failure and atherosclerosis).
-
Serum ceramide levels are measured clinically as prognostic indicators of major adverse cardiovascular events.
-
Inhibiting ceramide biosynthesis in mice and rats prevents the development of hypertension, atherosclerosis, diabetes mellitus and heart failure.
-
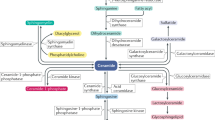
Ceramides have pleiotropic actions that are relevant to metabolic disease, including inhibiting nitric oxide synthase, decreasing insulin sensitivity, altering mitochondrial bioenergetics, and inducing apoptosis and fibrosis.
-
Several enzymes that control ceramide production or metabolism have emerged as attractive therapeutic targets for treating a wide range of cardiometabolic pathologies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hales, C. M., Carroll, M. D., Fryar, C. D. & Ogden, C. L. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief, no. 360 (National Center for Health Statistics, 2020).
Summers, S. A., Chaurasia, B. & Holland, W. L. Metabolic messengers: ceramides. Nat. Metab. 1, 1051–1058 (2019).
Russo, S. B., Ross, J. S. & Cowart, L. A. in Sphingolipids in Disease. Handbook of Experimental Pharmacology vol. 216 (eds Gulbins, E. & Petrache, I.) 373–401 (Springer, 2013).
Poss, A. M. & Summers, S. A. Too much of a good thing? An evolutionary theory to explain the role of ceramides in NAFLD. Front. Endocrinol. 11, 505 (2020).
Hilvo, M., Vasile, V. C., Donato, L. J., Hurme, R. & Laaksonen, R. Ceramides and ceramide scores: clinical applications for cardiometabolic risk stratification. Front. Endocrinol. 11, 570628 (2020).
Hilvo, M. et al. Prediction of residual risk by ceramide-phospholipid score in patients with stable coronary heart disease on optimal medical therapy. J. Am. Heart Assoc. 9, e015258 (2020).
Poss, A. M. et al. Machine learning reveals serum sphingolipids as cholesterol-independent biomarkers of coronary artery disease. J. Clin. Invest. 130, 1363–1376 (2020).
Poss, A. M., Holland, W. L. & Summers, S. A. Risky lipids: refining the ceramide score that measures cardiovascular health. Eur. Heart J. 41, 381–382 (2020).
Mantovani, A. et al. Association between increased plasma ceramides and chronic kidney disease in patients with and without ischemic heart disease. Diabetes Metab. 47, 101152 (2021).
Mantovani, A. & Dugo, C. Ceramides and risk of major adverse cardiovascular events: a meta-analysis of longitudinal studies. J. Clin. Lipidol. 14, 176–185 (2020).
Mantovani, A. et al. Associations between specific plasma ceramides and severity of coronary-artery stenosis assessed by coronary angiography. Diabetes Metab. 46, 150–157 (2020).
Mantovani, A. et al. Association between specific plasma ceramides and high-sensitivity C-reactive protein levels in postmenopausal women with type 2 diabetes. Diabetes Metab. 46, 326–330 (2020).
Anroedh, S. et al. Plasma concentrations of molecular lipid species predict long-term clinical outcome in coronary artery disease patients. J. Lipid Res. 59, 1729–1737 (2018).
Havulinna, A. S. et al. Circulating ceramides predict cardiovascular outcomes in the population-based FINRISK 2002 cohort. Arterioscler. Thromb. Vasc. Biol. 36, 2424–2430 (2016).
Laaksonen, R. et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur. Heart J. 37, 1967–1976 (2016).
Cheng, J. M. et al. Plasma concentrations of molecular lipid species in relation to coronary plaque characteristics and cardiovascular outcome: results of the ATHEROREMO-IVUS study. Atherosclerosis 243, 560–566 (2015).
Tarasov, K. et al. Molecular lipids identify cardiovascular risk and are efficiently lowered by simvastatin and PCSK9 deficiency. J. Clin. Endocrinol. Metab. 99, E45–E52 (2014).
Peterson, L. R. et al. Alterations in plasma triglycerides and ceramides: links with cardiac function in humans with type 2 diabetes. J. Lipid Res. 61, 1065–1074 (2020).
Peterson, L. R. et al. Ceramide remodeling and risk of cardiovascular events and mortality. J. Am. Heart. Assoc. 7, e007931 (2018).
Mikhalkova, D. et al. Bariatric surgery-induced cardiac and lipidomic changes in obesity-related heart failure with preserved ejection fraction. Obesity 26, 284–290 (2018).
Lemaitre, R. N. et al. Plasma ceramides and sphingomyelins in relation to heart failure risk. Circ. Heart Fail. 12, e005708 (2019).
Lemaitre, R. N. et al. Circulating sphingolipids, insulin, HOMA-IR, and HOMA-B: the Strong Heart Family Study. Diabetes 67, 1663–1672 (2018).
Cresci, S. et al. Genetic architecture of circulating very-long-chain (C24:0 and C22:0) ceramide concentrations. J. Lipid Atheroscler. 9, 172–183 (2020).
Javaheri, A., Allegood, J. C., Cowart, L. A. & Chirinos, J. A. Circulating ceramide 16:0 in heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 75, 2273–2275 (2020).
Holland, W. L. et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 5, 167–179 (2007).
Hojjati, M. R. et al. Effect of myriocin on plasma sphingolipid metabolism and atherosclerosis in apoE-deficient mice. J. Biol. Chem. 280, 10284–10289 (2005).
Park, T. S. et al. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J. Lipid Res. 49, 2101–2112 (2008).
Ji, R. et al. Increased de novo ceramide synthesis and accumulation in failing myocardium. JCI Insight 2, e82922 (2017).
Zhang, Q. J. et al. Ceramide mediates vascular dysfunction in diet-induced obesity by PP2A-mediated dephosphorylation of the eNOS-Akt complex. Diabetes 61, 1848–1859 (2012).
Bharath, L. P. et al. Ceramide-initiated protein phosphatase 2A activation contributes to arterial dysfunction in vivo. Diabetes 64, 3914–3926 (2015).
Merrill, A. H. Jr. et al. Sphingolipids–the enigmatic lipid class: biochemistry, physiology, and pathophysiology. Toxicol. Appl. Pharmacol. 142, 208–225 (1997).
Hannun, Y. A. & Obeid, L. M. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 9, 139–150 (2008).
Nikolova-Karakashian, M. N. & Rozenova, K. A. Ceramide in stress response. Adv. Exp. Med. Biol. 688, 86–108 (2010).
Obeid, L. M. & Hannun, Y. A. Ceramide, stress, and a “LAG” in aging. Sci. Aging Knowl. Environ. 2003, PE27 (2003).
Hannun, Y. A. & Obeid, L. M. The ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J. Biol. Chem. 277, 25847–25850 (2002).
Merrill, A. H. Jr. De novo sphingolipid biosynthesis: a necessary, but dangerous, pathway. J. Biol. Chem. 277, 25843–25846 (2002).
Han, G. et al. Identification of small subunits of mammalian serine palmitoyltransferase that confer distinct acyl-CoA substrate specificities. Proc. Natl Acad. Sci. USA 106, 8186–8191 (2009).
Hornemann, T. et al. The SPTLC3 subunit of serine palmitoyltransferase generates short chain sphingoid bases. J. Biol. Chem. 284, 26322–26330 (2009).
Zelnik, I. D., Rozman, B., Rosenfeld-Gur, E., Ben-Dor, S. & Futerman, A. H. A stroll down the CerS lane. Adv. Exp. Med. Biol. 1159, 49–63 (2019).
Laviad, E. L. et al. Characterization of ceramide synthase 2: tissue distribution, substrate specificity, and inhibition by sphingosine 1-phosphate. J. Biol. Chem. 283, 5677–5684 (2008).
Levy, M. & Futerman, A. H. Mammalian ceramide synthases. IUBMB Life 62, 347–356 (2010).
Russo, S. B. et al. Ceramide synthase 5 mediates lipid-induced autophagy and hypertrophy in cardiomyocytes. J. Clin. Invest. 122, 3919–3930 (2012).
Hammerschmidt, P. et al. CerS6-derived sphingolipids interact with Mff and promote mitochondrial fragmentation in obesity. Cell 177, 1536–1552 (2019).
Peters, F. et al. Ceramide synthase 4 regulates stem cell homeostasis and hair follicle cycling. J. Invest. Dermatol. 135, 1501–1509 (2015).
Turpin, S. M. et al. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 20, 678–686 (2014).
Raichur, S. et al. The role of C16:0 ceramide in the development of obesity and type 2 diabetes: CerS6 inhibition as a novel therapeutic approach. Mol. Metab. 21, 36–50 (2019).
Raichur, S. et al. CerS2 haploinsufficiency inhibits β-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab. 20, 687–695 (2014).
Michel, C. & van Echten-Deckert, G. Conversion of dihydroceramide to ceramide occurs at the cytosolic face of the endoplasmic reticulum. FEBS Lett. 416, 153–155 (1997).
Michel, C. et al. Characterization of ceramide synthesis. A dihydroceramide desaturase introduces the 4,5-trans-double bond of sphingosine at the level of dihydroceramide. J. Biol. Chem. 272, 22432–22437 (1997).
Omae, F. et al. DES2 protein is responsible for phytoceramide biosynthesis in the mouse small intestine. Biochem. J. 379, 687–695 (2004).
Hanada, K., Kumagai, K., Tomishige, N. & Yamaji, T. CERT-mediated trafficking of ceramide. Biochim. Biophys. Acta 1791, 684–691 (2009).
Kumagai, K. & Hanada, K. Structure, functions and regulation of CERT, a lipid-transfer protein for the delivery of ceramide at the ER-Golgi membrane contact sites. FEBS Lett. 593, 2366–2377 (2019).
Venkataraman, K. et al. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ. Res. 102, 669–676 (2008).
Hannun, Y. A., Luberto, C. & Argraves, K. M. Enzymes of sphingolipid metabolism: from modular to integrative signaling. Biochemistry 40, 4893–4903 (2001).
Newton, J., Lima, S., Maceyka, M. & Spiegel, S. Revisiting the sphingolipid rheostat: evolving concepts in cancer therapy. Exp. Cell Res. 333, 195–200 (2015).
Cuvillier, O. et al. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature 381, 800–803 (1996).
Wende, A. R., Symons, J. D. & Abel, E. D. Mechanisms of lipotoxicity in the cardiovascular system. Curr. Hypertens. Rep. 14, 517–531 (2012).
Symons, J. D. & Abel, E. D. Lipotoxicity contributes to endothelial dysfunction: a focus on the contribution from ceramide. Rev. Endocr. Metab. Disord. 14, 59–68 (2013).
Dantas, A. P., Igarashi, J. & Michel, T. Sphingosine 1-phosphate and control of vascular tone. Am. J. Physiol. Heart Circ. Physiol. 284, H2045–H2052 (2003).
Igarashi, J. & Michel, T. Sphingosine-1-phosphate and modulation of vascular tone. Cardiovasc. Res. 82, 212–220 (2009).
Kennedy, S., Kane, K. A., Pyne, N. J. & Pyne, S. Targeting sphingosine-1-phosphate signalling for cardioprotection. Curr. Opin. Pharmacol. 9, 194–201 (2009).
Holland, W. L. et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med. 17, 55–63 (2011).
Holland, W. L. et al. Inducible overexpression of adiponectin receptors highlight the roles of adiponectin-induced ceramidase signaling in lipid and glucose homeostasis. Mol. Metab. 6, 267–275 (2017).
Vasiliauskaite-Brooks, I. et al. Structural insights into adiponectin receptors suggest ceramidase activity. Nature 544, 120–123 (2017).
Merrill, A. H., Dennis, E. A., McDonald, J. G. & Fahy, E. Lipidomics technologies at the end of the first decade and the beginning of the next. Adv. Nutr. 4, 565–567 (2013).
Kruger-Genge, A., Blocki, A., Franke, R. P. & Jung, F. Vascular endothelial cell biology: an update. Int. J. Mol. Sci. 20, 4411 (2019).
Vallance, P., Collier, J. & Moncada, S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet 2, 997–1000 (1989).
Triggle, C. R. & Ding, H. A review of endothelial dysfunction in diabetes: a focus on the contribution of a dysfunctional eNOS. J. Am. Soc. Hypertens. 4, 102–115 (2010).
Ross, R. Atherosclerosis–an inflammatory disease. N. Engl. J. Med. 340, 115–126 (1999).
Higashi, Y., Kihara, Y. & Noma, K. Endothelial dysfunction and hypertension in aging. Hypertens. Res. 35, 1039–1047 (2012).
Zhang, D. X., Zou, A. P. & Li, P. L. Ceramide-induced activation of NADPH oxidase and endothelial dysfunction in small coronary arteries. Am. J. Physiol. Heart Circ. Physiol. 284, H605–H612 (2003).
Zheng, T., Li, W., Wang, J., Altura, B. T. & Altura, B. M. Sphingomyelinase and ceramide analogs induce contraction and rises in [Ca2+]i in canine cerebral vascular muscle. Am. J. Physiol. Heart Circ. Physiol. 278, H1421–H1428 (2000).
Li, H. et al. Dual effect of ceramide on human endothelial cells: induction of oxidative stress and transcriptional upregulation of endothelial nitric oxide synthase. Circulation 106, 2250–2256 (2002).
Ogretmen, B. et al. Biochemical mechanisms of the generation of endogenous long chain ceramide in response to exogenous short chain ceramide in the A549 human lung adenocarcinoma cell line. Role for endogenous ceramide in mediating the action of exogenous ceramide. J. Biol. Chem. 277, 12960–12969 (2002).
Chun, L. et al. Inhibition of ceramide synthesis reverses endothelial dysfunction and atherosclerosis in streptozotocin-induced diabetic rats. Diabetes Res. Clin. Pract. 93, 77–85 (2011).
Mount, P. F., Kemp, B. E. & Power, D. A. Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J. Mol. Cell Cardiol. 42, 271–279 (2007).
Smith, A. R., Visioli, F., Frei, B. & Hagen, T. M. Age-related changes in endothelial nitric oxide synthase phosphorylation and nitric oxide dependent vasodilation: evidence for a novel mechanism involving sphingomyelinase and ceramide-activated phosphatase 2A. Aging Cell 5, 391–400 (2006).
Oaks, J. & Ogretmen, B. Regulation of PP2A by sphingolipid metabolism and signaling. Front. Oncol. 4, 388 (2014).
Sukumar, P. et al. Nox2 NADPH oxidase has a critical role in insulin resistance-related endothelial cell dysfunction. Diabetes 62, 2130–2134 (2013).
Rajagopalan, S., Meng, X. P., Ramasamy, S., Harrison, D. G. & Galis, Z. S. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J. Clin. Invest. 98, 2572–2579 (1996).
Hink, U. et al. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ. Res. 88, E14–E22 (2001).
Bryk, D., Olejarz, W. & Zapolska-Downar, D. The role of oxidative stress and NADPH oxidase in the pathogenesis of atherosclerosis. Postepy Hig. Med. Dosw. 71, 57–68 (2017).
Didion, S. P. & Faraci, F. M. Ceramide-induced impairment of endothelial function is prevented by CuZn superoxide dismutase overexpression. Arterioscler. Thromb. Vasc. Biol. 25, 90–95 (2005).
Funai, K., Summers, S. A. & Rutter, J. Reign in the membrane: how common lipids govern mitochondrial function. Curr. Opin. Cell Biol. 63, 162–173 (2020).
Modur, V., Zimmerman, G. A., Prescott, S. M. & McIntyre, T. M. Endothelial cell inflammatory responses to tumor necrosis factor α. Ceramide-dependent and -independent mitogen-activated protein kinase cascades. J. Biol. Chem. 271, 13094–13102 (1996).
Xu, J. et al. Involvement of de novo ceramide biosynthesis in tumor necrosis factor-α/cycloheximide-induced cerebral endothelial cell death. J. Biol. Chem. 273, 16521–16526 (1998).
Camejo, G., Hurt-Camejo, E., Wiklund, O. & Bondjers, G. Association of apo B lipoproteins with arterial proteoglycans: pathological significance and molecular basis. Atherosclerosis 139, 205–222 (1998).
Ross, R. Atherosclerosis is an inflammatory disease. Am. Heart J. 138, S419–S420 (1999).
Hilvo, M. et al. Development and validation of a ceramide- and phospholipid-based cardiovascular risk estimation score for coronary artery disease patients. Eur. Heart J. 41, 371–380 (2020).
Mantovani, A. et al. Association of plasma ceramides with myocardial perfusion in patients with coronary artery disease undergoing stress myocardial perfusion scintigraphy. Arterioscler. Thromb. Vasc. Biol. 38, 2854–2861 (2018).
Meeusen, J. W. et al. Plasma ceramides. Arterioscler. Thromb. Vasc. Biol. 38, 1933–1939 (2018).
Schissel, S. L. et al. Rabbit aorta and human atherosclerotic lesions hydrolyze the sphingomyelin of retained low-density lipoprotein. Proposed role for arterial-wall sphingomyelinase in subendothelial retention and aggregation of atherogenic lipoproteins. J. Clin. Invest. 98, 1455–1464 (1996).
Edsfeldt, A. et al. Sphingolipids contribute to human atherosclerotic plaque inflammation. Arterioscler. Thromb. Vasc. Biol. 36, 1132–1140 (2016).
Park, T. S. et al. Inhibition of sphingomyelin synthesis reduces atherogenesis in apolipoprotein E-knockout mice. Circulation 110, 3465–3471 (2004).
Park, T. S. et al. Modulation of lipoprotein metabolism by inhibition of sphingomyelin synthesis in ApoE knockout mice. Atherosclerosis 189, 264–272 (2006).
Hojjati, M. R., Li, Z. & Jiang, X. C. Serine palmitoyl-CoA transferase (SPT) deficiency and sphingolipid levels in mice. Biochim. Biophys. Acta 1737, 44–51 (2005).
Glaros, E. N. et al. Inhibition of atherosclerosis by the serine palmitoyl transferase inhibitor myriocin is associated with reduced plasma glycosphingolipid concentration. Biochem. Pharmacol. 73, 1340–1346 (2007).
Li, Z. et al. Serine palmitoyltransferase (SPT) deficient mice absorb less cholesterol. Biochim. Biophys. Acta 1791, 297–306 (2009).
Kasumov, T. et al. Ceramide as a mediator of non-alcoholic fatty liver disease and associated atherosclerosis. PLoS ONE 10, e0126910 (2015).
Kurek, K. et al. Inhibition of ceramide de novo synthesis reduces liver lipid accumulation in rats with nonalcoholic fatty liver disease. Liver Int. 34, 1074–1083 (2014).
Chaurasia, B. et al. Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Science 365, 386–392 (2019).
Bikman, B. T. et al. Fenretinide prevents lipid-induced insulin resistance by blocking ceramide biosynthesis. J. Biol. Chem. 287, 17426–17437 (2012).
Mody, N. & McIlroy, G. D. The mechanisms of fenretinide-mediated anti-cancer activity and prevention of obesity and type-2 diabetes. Biochem. Pharmacol. 91, 277–286 (2014).
Koh, I. U. et al. Fenretinide ameliorates insulin resistance and fatty liver in obese mice. Biol. Pharm. Bull. 35, 369–375 (2012).
Busnelli, M. et al. Fenretinide treatment accelerates atherosclerosis development in apoE-deficient mice in spite of beneficial metabolic effects. Br. J. Pharmacol. 177, 328–345 (2020).
Jiang, X. C. et al. Plasma sphingomyelin level as a risk factor for coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 20, 2614–2618 (2000).
Wang, X., Dong, J., Zhao, Y., Li, Y. & Wu, M. Adenovirus-mediated sphingomyelin synthase 2 increases atherosclerotic lesions in ApoE KO mice. Lipids Health Dis. 10, 7 (2011).
Zhao, Y. R., Dong, J. B., Li, Y. & Wu, M. P. Sphingomyelin synthase 2 over-expression induces expression of aortic inflammatory biomarkers and decreases circulating EPCs in ApoE KO mice. Life Sci. 90, 867–873 (2012).
Dong, J. et al. Adenovirus-mediated overexpression of sphingomyelin synthases 1 and 2 increases the atherogenic potential in mice. J. Lipid Res. 47, 1307–1314 (2006).
Liu, J. et al. Sphingomyelin synthase 2 is one of the determinants for plasma and liver sphingomyelin levels in mice. Arterioscler. Thromb. Vasc. Biol. 29, 850–856 (2009).
Liu, J. et al. Macrophage sphingomyelin synthase 2 deficiency decreases atherosclerosis in mice. Circ. Res. 105, 295–303 (2009).
Fan, Y. et al. Selective reduction in the sphingomyelin content of atherogenic lipoproteins inhibits their retention in murine aortas and the subsequent development of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 30, 2114–2120 (2010).
Li, Z. et al. Impact of sphingomyelin synthase 1 deficiency on sphingolipid metabolism and atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 32, 1577–1584 (2012).
Yano, M. et al. Increased oxidative stress impairs adipose tissue function in sphingomyelin synthase 1 null mice. PLoS ONE 8, e61380 (2013).
Kenny, H. C. & Abel, E. D. Heart failure in type 2 diabetes mellitus. Circ. Res. 124, 121–141 (2019).
Tsao, C. W. et al. Temporal trends in the incidence of and mortality associated with heart failure with preserved and reduced ejection fraction. JACC Heart Fail. 6, 678–685 (2018).
van Heerebeek, L. & Paulus, W. J. Understanding heart failure with preserved ejection fraction: where are we today? Neth. Heart J. 24, 227–236 (2016).
Oktay, A. A., Rich, J. D. & Shah, S. J. The emerging epidemic of heart failure with preserved ejection fraction. Curr. Heart Fail. Rep. 10, 401–410 (2013).
Simmonds, S. J., Cuijpers, I., Heymans, S. & Jones, E. A. V. Cellular and molecular differences between HFpEF and HFrEF: a step ahead in an improved pathological understanding. Cells 9, 242 (2020).
Chokshi, A. et al. Ventricular assist device implantation corrects myocardial lipotoxicity, reverses insulin resistance, and normalizes cardiac metabolism in patients with advanced heart failure. Circulation 125, 2844–2853 (2012).
Kato, T. S. et al. Effects of continuous-flow versus pulsatile-flow left ventricular assist devices on myocardial unloading and remodeling. Circ. Heart Fail. 4, 546–553 (2011).
Khan, R. S. et al. Adipose tissue inflammation and adiponectin resistance in patients with advanced heart failure: correction after ventricular assist device implantation. Circ. Heart Fail. 5, 340–348 (2012).
Rogers, J. K. et al. Effect of rosuvastatin on repeat heart failure hospitalizations: the CORONA trial (Controlled Rosuvastatin Multinational Trial in Heart Failure). JACC Heart Fail. 2, 289–297 (2014).
Wang, Z. V. & Scherer, P. E. Adiponectin, the past two decades. J. Mol. Cell Biol. 8, 93–100 (2016).
Hadas, Y. et al. Altering sphingolipid metabolism attenuates cell death and inflammatory response after myocardial infarction. Circulation 141, 916–930 (2020).
Means, C. K. & Brown, J. H. Sphingosine-1-phosphate receptor signalling in the heart. Cardiovasc. Res. 82, 193–200 (2009).
Vessey, D. A., Li, L., Kelley, M. & Karliner, J. S. Combined sphingosine, S1P and ischemic postconditioning rescue the heart after protracted ischemia. Biochem. Biophys. Res. Commun. 375, 425–429 (2008).
Vessey, D. A., Li, L., Kelley, M., Zhang, J. & Karliner, J. S. Sphingosine can pre- and post-condition heart and utilizes a different mechanism from sphingosine 1-phosphate. J. Biochem. Mol. Toxicol. 22, 113–118 (2008).
Vessey, D. A., Kelley, M., Li, L. & Huang, Y. Sphingosine protects aging hearts from ischemia/reperfusion injury: superiority to sphingosine 1-phosphate and ischemic pre- and post-conditioning. Oxid. Med. Cell. Longev. 2, 146–151 (2009).
Hofmann, U. et al. Protective effects of sphingosine-1-phosphate receptor agonist treatment after myocardial ischaemia-reperfusion. Cardiovasc. Res. 83, 285–293 (2009).
Botta, A., Elizbaryan, K., Tashakorinia, P., Lam, N. H. & Sweeney, G. An adiponectin-S1P autocrine axis protects skeletal muscle cells from palmitate-induced cell death. Lipids Health Dis. 19, 156 (2020).
Botta, A. et al. An adiponectin-S1P axis protects against lipid induced insulin resistance and cardiomyocyte cell death via reduction of oxidative stress. Nutr. Metab. 16, 14 (2019).
Gudz, T. I., Tserng, K. Y. & Hoppel, C. L. Direct inhibition of mitochondrial respiratory chain complex III by cell-permeable ceramide. J. Biol. Chem. 272, 24154–24158 (1997).
Di Paola, M., Cocco, T. & Lorusso, M. Ceramide interaction with the respiratory chain of heart mitochondria. Biochemistry 39, 6660–6668 (2000).
Zigdon, H. et al. Ablation of ceramide synthase 2 causes chronic oxidative stress due to disruption of the mitochondrial respiratory chain. J. Biol. Chem. 288, 4947–4956 (2013).
Obeid, L. M., Linardic, C. M., Karolak, L. A. & Hannun, Y. A. Programmed cell death induced by ceramide. Science 259, 1769–1771 (1993).
Tippetts, T. S. et al. Cigarette smoke increases cardiomyocyte ceramide accumulation and inhibits mitochondrial respiration. BMC Cardiovasc. Disord. 14, 165 (2014).
Bielawska, A. E. et al. Ceramide is involved in triggering of cardiomyocyte apoptosis induced by ischemia and reperfusion. Am. J. Pathol. 151, 1257–1263 (1997).
Hickson-Bick, D. L., Buja, L. M. & McMillin, J. B. Palmitate-mediated alterations in the fatty acid metabolism of rat neonatal cardiac myocytes. J. Mol. Cell Cardiol. 32, 511–519 (2000).
Sparagna, G. C., Hickson-Bick, D. L., Buja, L. M. & McMillin, J. B. A metabolic role for mitochondria in palmitate-induced cardiac myocyte apoptosis. Am. J. Physiol. Heart Circ. Physiol. 279, H2124–H2132 (2000).
Sparagna, G. C., Hickson-Bick, D. L., Buja, L. M. & McMillin, J. B. Fatty acid-induced apoptosis in neonatal cardiomyocytes: redox signaling. Antioxid. Redox Signal. 3, 71–79 (2001).
Law, B. A. et al. Lipotoxic very-long-chain ceramides cause mitochondrial dysfunction, oxidative stress, and cell death in cardiomyocytes. FASEB J. 32, 1403–1416 (2018).
Basu, R. et al. Type 1 diabetic cardiomyopathy in the Akita (Ins2WT/C96Y) mouse model is characterized by lipotoxicity and diastolic dysfunction with preserved systolic function. Am. J. Physiol. Heart Circ. Physiol. 297, H2096–H2108 (2009).
Loffredo, F. S., Nikolova, A. P., Pancoast, J. R. & Lee, R. T. Heart failure with preserved ejection fraction: molecular pathways of the aging myocardium. Circ. Res. 115, 97–107 (2014).
Dong, S. et al. microRNA-21 promotes cardiac fibrosis and development of heart failure with preserved left ventricular ejection fraction by up-regulating Bcl-2. Int. J. Clin. Exp. Pathol. 7, 565–574 (2014).
Allouche, M. et al. Influence of Bcl-2 overexpression on the ceramide pathway in daunorubicin-induced apoptosis of leukemic cells. Oncogene 14, 1837–1845 (1997).
Ganesan, V. & Colombini, M. Regulation of ceramide channels by Bcl-2 family proteins. FEBS Lett. 584, 2128–2134 (2010).
Decaudin, D. et al. Bcl-2 and Bcl-XL antagonize the mitochondrial dysfunction preceding nuclear apoptosis induced by chemotherapeutic agents. Cancer Res. 57, 62–67 (1997).
Zhang, J. Autophagy and mitophagy in cellular damage control. Redox Biol. 1, 19–23 (2013).
Schiattarella, G. G. et al. Nitrosative stress drives heart failure with preserved ejection fraction. Nature 568, 351–356 (2019).
Franssen, C. et al. Metabolic comorbidities associated with endothelial inflammation and reduced no-bioavalability as a novel paradigm for heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 63, A970 (2014).
Symons, J. D. et al. Effect of continuous-flow left ventricular assist device support on coronary artery endothelial function in ischemic and nonischemic cardiomyopathy. Circ. Heart Fail. 12, e006085 (2019).
Hulsmans, M. et al. Cardiac macrophages promote diastolic dysfunction. J. Exp. Med. 215, 423–440 (2018).
Albeituni, S. & Stiban, J. Roles of ceramides and other sphingolipids in immune cell function and inflammation. Adv. Exp. Med. Biol. 1161, 169–191 (2019).
Ye, J. Transcription factors activated through RIP (regulated intramembrane proteolysis) and RAT (regulated alternative translocation). J. Biol. Chem. 295, 10271–10280 (2020).
Chen, Q. et al. Inverting the topology of a transmembrane protein by regulating the translocation of the first transmembrane helix. Mol. Cell 63, 567–578 (2016).
Raichur, S. Ceramide synthases are attractive drug targets for treating metabolic diseases. Front. Endocrinol. 11, 483 (2020).
Hla, T. & Kolesnick, R. C16:0-ceramide signals insulin resistance. Cell Metab. 20, 703–705 (2014).
Turpin-Nolan, S. M. & Bruning, J. C. The role of ceramides in metabolic disorders: when size and localization matters. Nat. Rev. Endocrinol. 16, 224–233 (2020).
Lemaitre, R. N. et al. Circulating very long-chain saturated fatty acids and heart failure: the cardiovascular health study. J. Am. Heart Assoc. 7, e010019 (2018).
Russo, S. B., Tidhar, R., Futerman, A. H. & Cowart, L. A. Myristate-derived d16:0 sphingolipids constitute a cardiac sphingolipid pool with distinct synthetic routes and functional properties. J. Biol. Chem. 288, 13397–13409 (2013).
Simons, L. A. An updated review of lipid-modifying therapy. Med. J. Aust. 211, 87–92 (2019).
Wang, N. et al. Intensive LDL cholesterol-lowering treatment beyond current recommendations for the prevention of major vascular events: a systematic review and meta-analysis of randomised trials including 327 037 participants. Lancet Diabetes Endocrinol. 8, 36–49 (2020).
Ng, T. W. et al. Dose-dependent effects of rosuvastatin on the plasma sphingolipidome and phospholipidome in the metabolic syndrome. J. Clin. Endocrinol. Metab. 99, E2335–E2340 (2014).
Ye, Q., Svatikova, A., Meeusen, J. W., Kludtke, E. L. & Kopecky, S. L. Effect of proprotein convertase subtilisin/kexin type 9 inhibitors on plasma ceramide levels. Am. J. Cardiol. 128, 163–167 (2020).
Reforgiato, M. R. et al. Inhibition of ceramide de novo synthesis as a postischemic strategy to reduce myocardial reperfusion injury. Basic Res. Cardiol. 111, 12 (2016).
Ussher, J. R. et al. Inhibition of serine palmitoyl transferase I reduces cardiac ceramide levels and increases glycolysis rates following diet-induced insulin resistance. PLoS ONE 7, e37703 (2012).
Ussher, J. R. et al. Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole-body oxygen consumption. Diabetes 59, 2453–2464 (2010).
Genin, M. J. et al. Imidazopyridine and pyrazolopiperidine derivatives as novel inhibitors of serine palmitoyl transferase. J. Med. Chem. 59, 5904–5910 (2016).
Li, Z. et al. Sphingolipid de novo biosynthesis is essential for intestine cell survival and barrier function. Cell Death Dis. 9, 173 (2018).
Ohta, E. et al. Analysis of development of lesions in mice with serine palmitoyltransferase (SPT) deficiency: Sptlc2 conditional knockout mice. Exp. Anim. 58, 515–524 (2009).
Johansson, H. et al. Effect of fenretinide and low-dose tamoxifen on insulin sensitivity in premenopausal women at high risk for breast cancer. Cancer Res. 68, 9512–9518 (2008).
Yang, G. et al. Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. Am. J. Physiol. Endocrinol. Metab. 297, E211–E224 (2009).
Preitner, F., Mody, N., Graham, T. E., Peroni, O. D. & Kahn, B. B. Long-term fenretinide treatment prevents high-fat diet-induced obesity, insulin resistance, and hepatic steatosis. Am. J. Physiol. Endocrinol. Metab. 297, E1420–E1429 (2009).
Lin, C. H. et al. Fenretinide inhibits macrophage inflammatory mediators and controls hypertension in spontaneously hypertensive rats via the peroxisome proliferator-activated receptor gamma pathway. Drug Des. Devel. Ther. 10, 3591–3597 (2016).
Zhang, Y. Z. et al. AdipoRon alleviates free fatty acid-induced myocardial cell injury via suppressing Nlrp3 inflammasome activation. Diabetes Metab. Syndr. Obes. 12, 2165–2179 (2019).
Fairaq, A., Shawky, N. M., Osman, I., Pichavaram, P. & Segar, L. AdipoRon, an adiponectin receptor agonist, attenuates PDGF-induced VSMC proliferation through inhibition of mTOR signaling independent of AMPK: implications toward suppression of neointimal hyperplasia. Pharmacol. Res. 119, 289–302 (2017).
Kemp, G. J. et al. Abnormalities in exercising skeletal muscle in congestive heart failure can be explained in terms of decreased mitochondrial ATP synthesis, reduced metabolic efficiency, and increased glycogenolysis. Heart 76, 35–41 (1996).
Author information
Authors and Affiliations
Contributions
The authors contributed substantially to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
S.A.S. is a consultant, co-founder and shareholder of Centaurus Therapeutics. The other authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Cardiology thanks X.-C. Jiang, R. Laaksonen and A. Nègre-Salvayre for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Choi, R.H., Tatum, S.M., Symons, J.D. et al. Ceramides and other sphingolipids as drivers of cardiovascular disease. Nat Rev Cardiol 18, 701–711 (2021). https://doi.org/10.1038/s41569-021-00536-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-021-00536-1
This article is cited by
-
Animal models of heart failure with preserved ejection fraction (HFpEF): from metabolic pathobiology to drug discovery
Acta Pharmacologica Sinica (2024)
-
The paradigm change from reactive medical services to 3PM in ischemic stroke: a holistic approach utilising tear fluid multi-omics, mitochondria as a vital biosensor and AI-based multi-professional data interpretation
EPMA Journal (2024)
-
Ceramides are decreased after liraglutide treatment in people with type 2 diabetes: a post hoc analysis of two randomized clinical trials
Lipids in Health and Disease (2023)
-
Metabolic systems approaches update molecular insights of clinical phenotypes and cardiovascular risk in patients with homozygous familial hypercholesterolemia
BMC Medicine (2023)
-
Untargeted metabolomic analysis of ischemic injury in human umbilical vein endothelial cells reveals the involvement of arginine metabolism
Nutrition & Metabolism (2023)