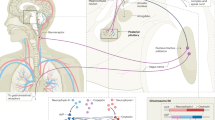
Abstract
Investigations into the mixed muscle–secretory phenotype of cardiomyocytes from the atrial appendages of the heart led to the discovery that these cells produce, in a regulated manner, two polypeptide hormones — the natriuretic peptides — referred to as atrial natriuretic factor or atrial natriuretic peptide (ANP) and brain or B-type natriuretic peptide (BNP), thereby demonstrating an endocrine function for the heart. Studies on the gene encoding ANP (NPPA) initiated the field of modern research into gene regulation in the cardiovascular system. Additionally, ANP and BNP were found to be the natural ligands for cell membrane-bound guanylyl cyclase receptors that mediate the effects of natriuretic peptides through the generation of intracellular cGMP, which interacts with specific enzymes and ion channels. Natriuretic peptides have many physiological actions and participate in numerous pathophysiological processes. Important clinical entities associated with natriuretic peptide research include heart failure, obesity and systemic hypertension. Plasma levels of natriuretic peptides have proven to be powerful diagnostic and prognostic biomarkers of heart disease. Development of pharmacological agents that are based on natriuretic peptides is an area of active research, with vast potential benefits for the treatment of cardiovascular disease.
Key points
-
The dual contractile–secretory phenotype of atrial (appendage) cardiomyocytes underlies the regulated secretion of two natriuretic peptide (NP) hormones: atrial NP and B-type NP.
-
Investigation into the genes encoding NPs has provided valuable insights into gene regulation in the cardiovascular system.
-
NPs are ligands for receptors that are membrane-bound guanylyl cyclases, thereby signalling through increases in intracellular levels of cGMP, which in turn interact with cGMP-dependent enzymes and ion channels in many cell types.
-
In addition to their roles in water and electrolyte balance, NPs participate in seemingly unrelated processes, such as immune response and lipid metabolism.
-
Plasma levels of NPs are useful clinical indicators in the diagnosis and prognosis of cardiovascular diseases.
-
NPs, their chemical derivatives and pharmacological agents that slow their catabolic rate are areas of active research that have produced useful new approaches in the treatment of systemic hypertension and heart failure.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
McGrath, M. F., de Bold, M. L. & de Bold, A. J. The endocrine function of the heart. Trends Endocrinol. Metab. 16, 469–477 (2005).
Jamieson, J. D. & Palade, G. E. Specific granules in atrial muscle cells. J. Cell Biol. 23, 151–172 (1964).
de Bold, A. J., Borenstein, H. B., Veress, A. T. & Sonnenberg, H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extracts in rats. Life Sci. 28, 89–94 (1981).
Flynn, T. G., de Bold, M. L. & de Bold, A. J. The amino acid sequence of an atrial peptide with potent diuretic and natriuretic properties. Biochem. Biophys. Res. Commun. 117, 859–865 (1983).
Dzau, V. J. et al. Nomenclature for atrial peptides. N. Engl. J. Med. 316, 1278–1279 (1987).
Sudoh, T., Kangawa, K., Minamino, N. & Matsuo, H. A new natriuretic peptide in porcine brain. Nature 332, 78–81 (1988).
Sudoh, T., Minamino, N., Kangawa, K. & Matsuo, H. C-type natriuretic peptide (CNP): a new member of natriuretic peptide family identified in porcine brain. Biochem. Biophys. Res. Commun. 168, 863–870 (1990).
Komatsu, Y. et al. C-type natriuretic peptide (CNP) in rats and humans. Endocrinology 129, 1104–1106 (1991).
Nishimura, M. et al. Roles of brain angiotensin II and C-type natriuretic peptide in deoxycorticosterone acetate-salt hypertension in rats. J. Hypertens. 16, 1175–1185 (1998).
Ueda, S. et al. Distribution and characterization of immunoreactive porcine C-type natriuretic peptide. Biochem. Biophys. Res. Commun. 175, 759–767 (1991).
Furuya, M. et al. C-type natriuretic peptide is a growth inhibitor of rat vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 177, 927–931 (1991).
Prickett, T. C. et al. C-type natriuretic peptides in coronary disease. Clin. Chem. 63, 316–324 (2017).
Wright, S. P. et al. Amino-terminal pro-C-type natriuretic peptide in heart failure. Hypertension 43, 94–100 (2004).
Moyes, A. J. & Hobbs, A. J. C-type natriuretic peptide: a multifaceted paracrine regulator in the heart and vasculature. Int. J. Mol. Sci. 20, 2281 (2019).
Zeller, R., Bloch, K. D., Williams, B. S., Arceci, R. J. & Seidman, C. E. Localized expression of the atrial natriuretic factor gene during cardiac embryogenesis. Genes Dev. 1, 693–698 (1987).
Bruneau, B. G. et al. A murine model of Holt-Oram syndrome defines roles of the T-box transcription factor Tbx5 in cardiogenesis and disease. Cell 106, 709–721 (2001).
Durocher, D., Chen, C. Y., Ardati, A., Schwartz, R. J. & Nemer, M. The atrial natriuretic factor promoter is a downstream target for Nkx-2.5 in the myocardium. Mol. Cell. Biol. 16, 4648–4655 (1996).
Durocher, D., Charron, F., Warren, R., Schwartz, R. J. & Nemer, M. The cardiac transcription factors Nkx2-5 and GATA-4 are mutual cofactors. EMBO J. 16, 5687–5696 (1997).
Välimaki, M. J. & Ruskoaho, H. J. Targeting GATA4 for cardiac repair. IUBMB Life 72, 68–79 (2020).
Tanaka, M., Chen, Z., Bartunkova, S., Yamasaki, N. & Izumo, S. The cardiac homeobox gene Csx/Nkx2.5 lies genetically upstream of multiple genes essential for heart development. Development 126, 1269–1280 (1999).
Lickert, H. et al. Baf60c is essential for function of BAF chromatin remodelling complexes in heart development. Nature 432, 107–112 (2004).
Habets, P. E. et al. Cooperative action of Tbx2 and Nkx2.5 inhibits ANF expression in the atrioventricular canal: implications for cardiac chamber formation. Genes Dev. 16, 1234–1246 (2002).
Horsthuis, T. et al. Distinct regulation of developmental and heart disease-induced atrial natriuretic factor expression by two separate distal sequences. Circ. Res. 102, 849–859 (2008).
Sergeeva, I. A. et al. Identification of a regulatory domain controlling the Nppa-Nppb gene cluster during heart development and stress. Development 143, 2135–2146 (2016).
Zaidi, S. & Brueckner, M. Genetics and genomics of congenital heart disease. Circ. Res. 120, 923–940 (2017).
Mori, T. et al. Volume overload results in exaggerated cardiac hypertrophy in the atrial natriuretic peptide knockout mouse. Cardiovasc. Res. 61, 771–779 (2004).
Takeuchi, J. K. et al. Chromatin remodelling complex dosage modulates transcription factor function in heart development. Nat. Commun. 2, 187 (2011).
Koshiba-Takeuchi, K. et al. Reptilian heart development and the molecular basis of cardiac chamber evolution. Nature 461, 95–98 (2009).
Luna-Zurita, L. et al. Complex interdependence regulates heterotypic transcription factor distribution and coordinates cardiogenesis. Cell 164, 999–1014 (2016).
Ogawa, T. & de Bold, A. J. Uncoordinated regulation of atrial natriuretic factor and brain natriuretic peptide in lipopolysaccharide-treated rats. Biomarkers 17, 140–149 (2012).
Ogawa, T., Veinot, J. P., Kuroski de Bold, M. L., Georgalis, T. & de Bold, A. J. Angiotensin II receptor antagonism reverts the selective cardiac BNP upregulation and secretion observed in myocarditis. Am. J. Physiol. Heart Circ. Physiol. 294, H2596–H2603 (2008).
Ma, K. K., Ogawa, T. & de Bold, A. J. Selective upregulation of cardiac brain natriuretic peptide at the transcriptional and translational levels by pro-inflammatory cytokines and by conditioned medium derived from mixed lymphocyte reactions via p38 MAP kinase. J. Mol. Cell. Cardiol. 36, 505–513 (2004).
Ma, K. K., Banas, K. & de Bold, A. J. Determinants of inducible brain natriuretic peptide promoter activity. Regul. Pept. 128, 169–176 (2005).
Yasuda, S. & Lew, W. Y. Lipopolysaccharide depresses cardiac contractility and β-adrenergic contractile response by decreasing myofilament response to Ca2+ in cardiac myocytes. Circ. Res. 81, 1011–1020 (1997).
Tomaru, K. K. et al. Transcriptional activation of the BNP gene by lipopolysaccharide is mediated through GATA elements in neonatal rat cardiac myocytes. J. Mol. Cell. Cardiol. 34, 649–659 (2002).
Pemberton, C. J. et al. First identification of circulating prepro-A-type natriuretic peptide (preproANP) signal peptide fragments in humans: initial assessment as cardiovascular biomarkers. Clin. Chem. 58, 757–767 (2012).
Siriwardena, M. et al. B-type natriuretic peptide signal peptide circulates in human blood: evaluation as a potential biomarker of cardiac ischemia. Circulation 122, 255–264 (2010).
Schellenberger, U. et al. The precursor to B-type natriuretic peptide is an O-linked glycoprotein. Arch. Biochem. Biophys. 451, 160–166 (2006).
Seferian, K. R. et al. Immunodetection of glycosylated NT-proBNP circulating in human blood. Clin. Chem. 54, 866–873 (2008).
Crimmins, D. L. & Kao, J. L. A glycosylated form of the human cardiac hormone pro B-type natriuretic peptide is an intrinsically unstructured monomeric protein. Arch. Biochem. Biophys. 475, 36–41 (2008).
Semenov, A. G. et al. Processing of pro-brain natriuretic peptide is suppressed by O-glycosylation in the region close to the cleavage site. Clin. Chem. 55, 489–498 (2009).
Goetze, J. P. B-type natriuretic peptide: from posttranslational processing to clinical measurement. Clin. Chem. 58, 83–91 (2012).
Hansen, L. H. et al. Discovery of O-glycans on atrial natriuretic peptide (ANP) that affect both its proteolytic degradation and potency at its cognate receptor. J. Biol. Chem. 294, 12567–12578 (2019).
Dries, D. L. et al. Corin gene minor allele defined by 2 missense mutations is common in blacks and associated with high blood pressure and hypertension. Circulation 112, 2403–2410 (2005).
Semenov, A. G. et al. Processing of pro-B-type natriuretic peptide: furin and corin as candidate convertases. Clin. Chem. 56, 1166–1176 (2010).
Yan, W., Wu, F., Morser, J. & Wu, Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc. Natl Acad. Sci. USA 97, 8525–8529 (2000).
Ogawa, T., Vatta, M., Bruneau, B. G. & de Bold, A. J. Characterization of natriuretic peptide production by adult heart atria. Am. J. Physiol. Heart Circ. Physiol. 276, H1977–H1986 (1999).
Mangat, H. & de Bold, A. J. Stretch-induced atrial natriuretic factor release utilizes a rapidly depleting pool of newly synthesized hormone. Endocrinology 133, 1398–1403 (1993).
Arvan, P. & Halban, P. A. Sorting ourselves out: seeking consensus on trafficking in the beta-cell. Traffic 5, 53–61 (2004).
de Bold, A. J. Heart atria granularity effects of changes in water-electrolyte balance. Proc. Soc. Exp. Biol. Med. 161, 508–511 (1979).
Bensimon, M. A. & de Bold, A. J. Role of Gi/o protein signalling in ANF stretch-secretion coupling in heart atria [abstract]. J. Mol. Cell. Cardiol. 33, A11 (2001).
Bensimon, M. et al. Participation of G proteins in natriuretic peptide hormone secretion from heart atria. Endocrinology 145, 5313–5321 (2004).
McGrath, M. F. & de Bold, A. J. Transcriptional analysis of the mammalian heart with special reference to its endocrine function. BMC Genomics 10, 254 (2009).
Chang, A. I., McGrath, M. F. & de Bold, A. J. Phospholipase C signaling tonically represses basal atrial natriuretic factor secretion from the atria of the heart. Am. J. Physiol. Heart Circ. Physiol. 304, H1328–H1336 (2013).
Roeske, C. et al. Go protein subunit Goα and the secretory process of the natriuretic peptide hormones ANF and BNP. J. Mol. Endocrinol. 54, 277–288 (2015).
Ogawa, T. et al. Neuroendocrine profiling of humans receiving cardiac allografts. J. Heart Lung Transplant. 24, 1046–1054 (2005).
Ramos, H. & de Bold, A. J. Gene expression, processing and secretion of natriuretic peptides: physiologic and diagnostic implications. Heart Fail. Clin. 2, 255–268 (2006).
Ogawa, T. et al. Evidence for load-dependent and load-independent determinants of cardiac natriuretic peptide production. Circulation 93, 2059–2067 (1996).
Yokota, N. et al. Dissociation of cardiac hypertrophy, myosin heavy chain isoform expression, and natriuretic peptide production in DOCA-salt rats. Am. J. Hypertens. 8, 301–310 (1995).
Murakami, Y. et al. New insights into the mechanism of the elevation of plasma brain natriuretic polypeptide levels in patients with left ventricular hypertrophy. Can. J. Cardiol. 18, 1294–1300 (2002).
Masters, R. G. et al. Discoordinate modulation of natriuretic peptides during acute cardiac allograft rejection in humans. Circulation 100, 287–291 (1999).
McGrath, M. F. & de Bold, A. J. Determinants of natriuretic peptide gene expression. Peptides 26, 933–943 (2005).
de Bold, M. L., Etchepare, A., Martinuk, A. & de Bold, A. J. Cardiac hormones ANF and BNP modulate proliferation in the unidirectional mixed lymphocyte reaction. J. Heart Lung Transplant. 29, 323–326 (2010).
Liu, S., Chirkov, Y. Y. & Horowitz, J. D. Neutrophil-initiated myocardial inflammation and its modulation by B-type natriuretic peptide: a potential therapeutic target. Int. J. Mol. Sci. 20, E129 (2018).
Mohapatra, S. S. Role of natriuretic peptide signaling in modulating asthma and inflammation. Can. J. Physiol. Pharmacol. 85, 754–759 (2007).
Suganami, T. et al. Overexpression of brain natriuretic peptide in mice ameliorates immune-mediated renal injury. J. Am. Soc. Nephrol. 12, 2652–2663 (2001).
Vollmar, A. M. The role of atrial natriuretic peptide in the immune system. Peptides 26, 1086–1094 (2005).
Murad, F., Leitman, D. C., Bennett, B. M., Molina, C. & Waldman, S. A. Regulation of guanylate cyclase by atrial natriuretic factor and the role of cyclic GMP in vasodilation. Am. J. Med. Sci. 294, 139–143 (1987).
Chinkers, M. & Garbers, D. L. The protein kinase domain of the ANP receptor is required for signaling. Science 245, 1392–1394 (1989).
Kumar, R., Grammatikakis, N. & Chinkers, M. Regulation of the atrial natriuretic peptide receptor by heat shock protein 90 complexes. J. Biol. Chem. 276, 11371–11375 (2001).
Potter, L. R. & Garbers, D. L. Dephosphorylation of the guanylyl cyclase-A receptor causes desensitization. J. Biol. Chem. 267, 14531–14534 (1992).
Leitman, D. C. et al. Atrial natriuretic peptide, oxytocin, and vasopressin increase guanosine 3′,5′-monophosphate in LLC-PK1 kidney epithelial cells. Endocrinology 122, 1478–1485 (1988).
McCoy, D. E., Guggino, S. E. & Stanton, B. A. The renal cGMP-gated cation channel: its molecular structure and physiological role. Kidney Int. 48, 1125–1133 (1995).
Kuhn, M. Molecular physiology of natriuretic peptide signalling. Basic Res. Cardiol. 99, 76–82 (2004).
Miyagi, M. & Misono, K. S. Disulfide bond structure of the atrial natriuretic peptide receptor extracellular domain: conserved disulfide bonds among guanylate cyclase-coupled receptors. Biochim. Biophys. Acta 1478, 30–38 (2000).
Miyagi, M., Zhang, X. & Misono, K. S. Glycosylation sites in the atrial natriuretic peptide receptor oligosaccharide structures are not required for hormone binding. Eur. J. Biochem. 267, 5758–5768 (2000).
Potter, L. R. & Hunter, T. Phosphorylation of the kinase homology domain is essential for activation of the A-type natriuretic peptide receptor. Mol. Cell. Biol. 18, 2164–2172 (1998).
Potter, L. R., Yoder, A. R., Flora, D. R., Antos, L. K. & Dickey, D. M. in cGMP: Generators, Effectors and Therapeutic Implications (eds Schmidt, H. H. H. W., Hofmann, F. & Stasch, J. P.) 341–366 (Springer, 2009).
Del Ry, S., Passino, C., Emdin, M. & Giannessi, D. C-type natriuretic peptide and heart failure. Pharmacol. Res. 54, 326–333 (2006).
Garg, R. & Pandey, K. N. Regulation of guanylyl cyclase/natriuretic peptide receptor-A gene expression. Peptides 26, 1009–1023 (2005).
John, S. W. et al. Genetic decreases in atrial natriuretic peptide and salt-sensitive hypertension. Science 267, 679–681 (1995).
Tamura, N. et al. Critical roles of the guanylyl cyclase B receptor in endochondral ossification and development of female reproductive organs. Proc. Natl Acad. Sci. USA 101, 17300–17305 (2004).
Rose, R. A. & Giles, W. R. Natriuretic peptide C receptor signalling in the heart and vasculature. J. Physiol. 586, 353–366 (2008).
Moffatt, P. et al. Osteocrin is a specific ligand of the natriuretic peptide clearance receptor that modulates bone growth. J. Biol. Chem. 282, 36454–36462 (2007).
Matsukawa, N. et al. The natriuretic peptide clearance receptor locally modulates the physiological effects of the natriuretic peptide system. Proc. Natl Acad. Sci. USA 96, 7403–7408 (1999).
Ibrahim, N. & Januzzi, J. L. The potential role of natriuretic peptides and other biomarkers in heart failure diagnosis, prognosis and management. Expert Rev. Cardiovasc. Ther. 13, 1017–1030 (2015).
Vodovar, N. et al. Evolution of natriuretic peptide biomarkers in heart failure: implications for clinical care and clinical trials. Int. J. Cardiol. 254, 215–221 (2018).
Thibault, G. et al. NH2-terminal fragment of rat pro-atrial natriuretic factor in the circulation: identification, radioimmunoassay and half-life. Peptides 9, 47–53 (1988).
Holmes, S. J., Espiner, E. A., Richards, A. M., Yandle, T. G. & Frampton, C. Renal, endocrine, and hemodynamic effects of human brain natriuretic peptide in normal man. J. Clin. Endocrinol. Metab. 76, 91–96 (1993).
Cui, K., Huang, W., Fan, J. & Lei, H. Midregional pro-atrial natriuretic peptide is a superior biomarker to N-terminal pro-B-type natriuretic peptide in the diagnosis of heart failure patients with preserved ejection fraction. Medicine 97, e12277 (2018).
Gangnus, T. & Burckhardt, B. B. Potential and limitations of atrial natriuretic peptide as biomarker in pediatric heart failure — a comparative review. Front. Pediatr. 6, 420 (2018).
Idzikowska, K. & Zielinska, M. Midregional pro-atrial natriuretic peptide, an important member of the natriuretic peptide family: potential role in diagnosis and prognosis of cardiovascular disease. J. Int. Med. Res. 46, 3017–3029 (2018).
Roberts, E. et al. The diagnostic accuracy of the natriuretic peptides in heart failure: systematic review and diagnostic meta-analysis in the acute care setting. BMJ 350, h910 (2015).
Troughton, R. W. et al. Effect of B-type natriuretic peptide-guided treatment of chronic heart failure on total mortality and hospitalization: an individual patient meta-analysis. Eur. Heart J. 35, 1559–1567 (2014).
Volpe, M., Battistoni, A. & Rubattu, S. Natriuretic peptides in heart failure: current achievements and future perspectives. Int. J. Cardiol. 281, 186–189 (2019).
Yancy, C. W. et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 128, e240–e327 (2013).
Saenger, A. K. et al. Specificity of B-type natriuretic peptide assays: cross-reactivity with different BNP, NT-proBNP, and proBNP peptides. Clin. Chem. 63, 351–358 (2017).
Semenov, A. G. et al. Searching for a BNP standard: glycosylated proBNP as a common calibrator enables improved comparability of commercial BNP immunoassays. Clin. Biochem. 50, 181–185 (2017).
Vasile, V. C. & Jaffe, A. S. Natriuretic peptides and analytical barriers. Clin. Chem. 63, 50–58 (2017).
Mueller, C. et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur. J. Heart Fail. 21, 715–731 (2019).
Yancy, C. W. et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Failure Society of America. J. Am. Coll. Cardiol. 70, 776–803 (2017).
Clerico, A., Passino, C. & Emdin, M. When gonads talk to the heart sex hormones and cardiac endocrine function. J. Am. Coll. Cardiol. 58, 627–628 (2011).
Taki, M. et al. Sex differences in the prognostic power of brain natriuretic peptide and N-terminal pro-brain natriuretic peptide for cardiovascular events — the Japan morning surge-home blood pressure study. Circ. J. 82, 2096–2102 (2018).
Krim, S. R. et al. Racial/ethnic differences in B-type natriuretic peptide levels and their association with care and outcomes among patients hospitalized with heart failure: findings from Get With The Guidelines-Heart Failure. JACC Heart Fail. 1, 345–352 (2013).
Redfield, M. M. et al. Plasma brain natriuretic peptide concentration: impact of age and gender. J. Am. Coll. Cardiol. 40, 976–982 (2002).
Ibrahim, I. et al. Superior performance of N-terminal pro brain natriuretic peptide for diagnosis of acute decompensated heart failure in an Asian compared with a Western setting. Eur. J. Heart Fail. 19, 209–217 (2017).
Madamanchi, C., Alhosaini, H., Sumida, A. & Runge, M. S. Obesity and natriuretic peptides, BNP and NT-proBNP: mechanisms and diagnostic implications for heart failure. Int. J. Cardiol. 176, 611–617 (2014).
Srisawasdi, P., Vanavanan, S., Charoenpanichkit, C. & Kroll, M. H. The effect of renal dysfunction on BNP, NT-proBNP, and their ratio. Am. J. Clin. Pathol. 133, 14–23 (2010).
Davidovski, F. S. & Goetze, J. P. ProANP and proBNP in plasma as biomarkers of heart failure. Biomark. Med. 13, 1129–1135 (2019).
Maisel, A. et al. Mid-region pro-hormone markers for diagnosis and prognosis in acute dyspnea: results from the BACH (Biomarkers in Acute Heart Failure) trial. J. Am. Coll. Cardiol. 55, 2062–2076 (2010).
Odermatt, J. et al. The natriuretic peptide MR-proANP predicts all-cause mortality and adverse outcome in community patients: a 10-year follow-up study. Clin. Chem. Lab. Med. 55, 1407–1416 (2017).
Lindberg, S. et al. MR-proANP improves prediction of mortality and cardiovascular events in patients with STEMI. Eur. J. Prev. Cardiol. 22, 693–700 (2015).
Salo, P. P. et al. Genome-wide association study implicates atrial natriuretic peptide rather than B-type natriuretic peptide in the regulation of blood pressure in the general population. Circ. Cardiovasc. Genet. 10, e001713 (2017).
Matsuo, A., Nagai-Okatani, C., Nishigori, M., Kangawa, K. & Minamino, N. Natriuretic peptides in human heart: novel insight into their molecular forms, functions, and diagnostic use. Peptides 111, 3–17 (2019).
Costello-Boerrigter, L. C. et al. Secretion of prohormone of B-type natriuretic peptide, proBNP1-108, is increased in heart failure. JACC Heart Fail. 1, 207–212 (2013).
Huntley, B. K. et al. Pro-B-type natriuretic peptide-1-108 processing and degradation in human heart failure. Circ. Heart Fail. 8, 89–97 (2015).
Rubattu, S. & Volpe, M. Natriuretic peptides in the cardiovascular system: multifaceted roles in physiology, pathology and therapeutics. Int. J. Mol. Sci. 20, E3991 (2019).
de Oliveira, I. M., Oliveira, B. D., Scanavacca, M. I. & Gutierrez, P. S. Fibrosis, myocardial crossings, disconnections, abrupt turns, and epicardial reflections: do they play an actual role in human permanent atrial fibrillation? A controlled necropsy study. Cardiovasc. Pathol. 22, 65–69 (2013).
Goldman, M. E. et al. Pathophysiologic correlates of thromboembolism in nonvalvular atrial fibrillation: I. Reduced flow velocity in the left atrial appendage (the Stroke Prevention in Atrial Fibrillation [SPAF-III] study). J. Am. Soc. Echocardiogr. 12, 1080–1087 (1999).
Breitenstein, A. et al. Increased prothrombotic profile in the left atrial appendage of atrial fibrillation patients. Int. J. Cardiol. 185, 250–255 (2015).
Schnabel, R. B. et al. Multiple biomarkers and atrial fibrillation in the general population. PLoS One 9, e112486 (2014).
Zuo, K. et al. Correlation of left atrial wall thickness and atrial remodeling in atrial fibrillation: study based on low-dose-ibutilide-facilitated catheter ablation. Medicine 98, e15170 (2019).
Hijazi, Z. et al. Cardiac biomarkers are associated with an increased risk of stroke and death in patients with atrial fibrillation: a randomized evaluation of long-term anticoagulation therapy (RE-LY) substudy. Circulation 125, 1605–1616 (2012).
Hijazi, Z. et al. N-terminal pro-B-type natriuretic peptide for risk assessment in patients with atrial fibrillation: insights from the ARISTOTLE trial (apixaban for the prevention of stroke in subjects with atrial fibrillation). J. Am. Coll. Cardiol. 61, 2274–2284 (2013).
Hijazi, Z. et al. Repeated measurements of cardiac biomarkers in atrial fibrillation and validation of the ABC stroke score over time. J. Am. Heart Assoc. 6, e004851 (2017).
Ruff, C. T. et al. Cardiovascular biomarker score and clinical outcomes in patients with atrial fibrillation: a subanalysis of the ENGAGE AF-TIMI 48 randomized clinical trial. JAMA Cardiol. 1, 999–1006 (2016).
Dudink, E. A. et al. The biomarkers NT-proBNP and CA-125 are elevated in patients with idiopathic atrial fibrillation. J. Atr. Fibrillation 11, 2058 (2018).
Galie, N. et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 37, 67–119 (2016).
Kovacs, G. et al. Definition, clinical classification and initial diagnosis of pulmonary hypertension: updated recommendations from the Cologne Consensus Conference 2018. Int. J. Cardiol. 272S, 11–19 (2018).
Hoeper, M. M. et al. A global view of pulmonary hypertension. Lancet Respir. Med. 4, 306–322 (2016).
Lewis, G. D. et al. Pulmonary vascular hemodynamic response to exercise in cardiopulmonary diseases. Circulation 128, 1470–1479 (2013).
Helgeson, S. A., Imam, J. S., Moss, J. E., Hodge, D. O. & Burger, C. D. Comparison of brain natriuretic peptide levels to simultaneously obtained right heart hemodynamics in stable outpatients with pulmonary arterial hypertension. Diseases 6, E33 (2018).
Benza, R. L. et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 122, 164–172 (2010).
Galie, N. et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Rev. Esp. Cardiol. 69, 177 (2016).
Bender, A. T. & Beavo, J. A. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol. Rev. 58, 488–520 (2006).
Hobbs, A. J. et al. Neprilysin inhibition for pulmonary arterial hypertension: a randomized, double-blind, placebo-controlled, proof-of-concept trial. Br. J. Pharmacol. 176, 1251–1267 (2019).
Baliga, R. S. et al. Synergy between natriuretic peptides and phosphodiesterase 5 inhibitors ameliorates pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 178, 861–869 (2008).
Baliga, R. S. et al. Intrinsic defence capacity and therapeutic potential of natriuretic peptides in pulmonary hypertension associated with lung fibrosis. Br. J. Pharmacol. 171, 3463–3475 (2014).
Wilkins, M. R. et al. Recent advances in pulmonary arterial hypertension. F1000Res. 7, 1128 (2018).
Ponikowski, P. et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 37, 2129–2200 (2016).
Hildebrandt, P. et al. Age-dependent values of N-terminal pro-B-type natriuretic peptide are superior to a single cut-point for ruling out suspected systolic dysfunction in primary care. Eur. Heart J. 31, 1881–1889 (2010).
Januzzi, J. L. et al. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the International Collaborative of NT-proBNP study. Eur. Heart J. 27, 330–337 (2006).
Maisel, A. S. et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N. Engl. J. Med. 347, 161–167 (2002).
Fernandez-Ruiz, I. Mechanisms of sacubitril-valsartan benefit in HFrEF. Nat. Rev. Cardiol. 16, 648 (2019).
McMurray, J. J. et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 371, 993–1004 (2014).
Balmforth, C. et al. Outcomes and effect of treatment according to etiology in HFrEF: an analysis of PARADIGM-HF. JACC Heart Fail. 7, 457–465 (2019).
Packer, M. et al. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation 131, 54–61 (2015).
Ibrahim, N. E. et al. Effect of neprilysin inhibition on various natriuretic peptide assays. J. Am. Coll. Cardiol. 73, 1273–1284 (2019).
Lainchbury, J. G. et al. Regional plasma levels of cardiac peptides and their response to acute neutral endopeptidase inhibition in man. Clin. Sci. 95, 547–555 (1998).
Zile, M. R. et al. Prognostic implications of changes in N-terminal pro-B-type natriuretic peptide in patients with heart failure. J. Am. Coll. Cardiol. 68, 2425–2436 (2016).
Testa, M. et al. Circulating levels of cytokines and their endogenous modulators in patients with mild to severe congestive heart failure due to coronary artery disease or hypertension. J. Am. Coll. Cardiol. 28, 964–971 (1996).
Yucel, T., Memis, D., Karamanlioglu, B., Sut, N. & Yuksel, M. The prognostic value of atrial and brain natriuretic peptides, troponin I and C-reactive protein in patients with sepsis. Exp. Clin. Cardiol. 13, 183–188 (2008).
D’Elia, E. et al. Neprilysin inhibition in heart failure: mechanisms and substrates beyond modulating natriuretic peptides. Eur. J. Heart Fail. 19, 710–717 (2017).
Jhund, P. S. & McMurray, J. J. The neprilysin pathway in heart failure: a review and guide on the use of sacubitril/valsartan. Heart 102, 1342–1347 (2016).
Miners, J. S., Barua, N., Kehoe, P. G., Gill, S. & Love, S. Aβ-degrading enzymes: potential for treatment of Alzheimer disease. J. Neuropathol. Exp. Neurol. 70, 944–959 (2011).
Cannon, J. A. et al. Dementia-related adverse events in PARADIGM-HF and other trials in heart failure with reduced ejection fraction. Eur. J. Heart Fail. 19, 129–137 (2017).
Cannon, J. A., McMurray, J. J. & Quinn, T. J. ‘Hearts and minds’: association, causation and implication of cognitive impairment in heart failure. Alzheimers Res. Ther. 7, 22 (2015).
Pandharipande, P. P. et al. Long-term cognitive impairment after critical illness. N. Engl. J. Med. 369, 1306–1316 (2013).
Solomon, S. D. et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N. Engl. J. Med. 381, 1609–1620 (2019).
Januzzi, J. L. Jr et al. Association of change in N-terminal pro-B-type natriuretic peptide following initiation of sacubitril-valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. JAMA 322, 1085–1095 (2019).
Desai, A. S. et al. Effect of sacubitril-valsartan vs enalapril on aortic stiffness in patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA 322, 1077–1084 (2019).
Schelbert, E. B. et al. Myocardial fibrosis quantified by extracellular volume is associated with subsequent hospitalization for heart failure, death, or both across the spectrum of ejection fraction and heart failure stage. J. Am. Heart Assoc. 4, e002613 (2015).
Gonzalez, A., Schelbert, E. B., Diez, J. & Butler, J. Myocardial interstitial fibrosis in heart failure: biological and translational perspectives. J. Am. Coll. Cardiol. 71, 1696–1706 (2018).
Bishop, J. E. & Laurent, G. J. Collagen turnover and its regulation in the normal and hypertrophying heart. Eur. Heart J. 16 (Suppl. C), 38–44 (1995).
Ellmers, L. J. et al. Ventricular expression of natriuretic peptides in Npr1−/− mice with cardiac hypertrophy and fibrosis. Am. J. Physiol. Heart Circ. Physiol. 283, H707–H714 (2002).
Zile, M. R. et al. Effects of sacubitril/valsartan on biomarkers of extracellular matrix regulation in patients with HFrEF. J. Am. Coll. Cardiol. 73, 795–806 (2019).
Pitt, B. et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study investigators. N. Engl. J. Med. 341, 709–717 (1999).
Zannad, F., Alla, F., Dousset, B., Perez, A. & Pitt, B. Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: insights from the Randomized Aldactone Evaluation Study (RALES). Circulation 102, 2700–2706 (2000).
Pitt, B. et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N. Engl. J. Med. 348, 1309–1321 (2003).
Iraqi, W. et al. Extracellular cardiac matrix biomarkers in patients with acute myocardial infarction complicated by left ventricular dysfunction and heart failure: insights from the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) study. Circulation 119, 2471–2479 (2009).
Zannad, F. et al. Eplerenone in patients with systolic heart failure and mild symptoms. N. Engl. J. Med. 364, 11–21 (2011).
Pankow, K. et al. Structural substrate conditions required for neutral endopeptidase-mediated natriuretic peptide degradation. J. Mol. Biol. 393, 496–503 (2009).
Shen, L., Jhund, P. S. & McMurray, J. J. V. Declining risk of sudden death in heart failure. N. Engl. J. Med. 377, 1794–1795 (2017).
Nguyen, M. N., Kiriazis, H., Gao, X. M. & Du, X. J. Cardiac fibrosis and arrhythmogenesis. Compr. Physiol. 7, 1009–1049 (2017).
Liu, C. Y. et al. Association of elevated NT-proBNP with myocardial fibrosis in the Multi-Ethnic Study of Atherosclerosis (MESA). J. Am. Coll. Cardiol. 70, 3102–3109 (2017).
Ramos, H. R., Birkenfeld, A. L. & de Bold, A. J. Interacting disciplines: cardiac natriuretic peptides and obesity: perspectives from an endocrinologist and a cardiologist. Endocr. Connect. 4, R25–R36 (2015).
Pivovarova, O. et al. Insulin up-regulates natriuretic peptide clearance receptor expression in the subcutaneous fat depot in obese subjects: a missing link between CVD risk and obesity? J. Clin. Endocrinol. Metab. 97, E731–E739 (2012).
Nakatsuji, H. et al. Reciprocal regulation of natriuretic peptide receptors by insulin in adipose cells. Biochem. Biophys. Res. Commun. 392, 100–105 (2010).
Sarzani, R., Dessì-Fulgheri, P., Paci, V. M., Espinosa, E. & Rappelli, A. Expression of natriuretic peptide receptors in human adipose and other tissues. J. Endocrinol. Invest. 19, 581–585 (1996).
Standeven, K. F. et al. Neprilysin, obesity and the metabolic syndrome. Int. J. Obes. 35, 1031–1040 (2011).
Bachmann, K. N. et al. Acute effects of insulin on circulating natriuretic peptide levels in humans. PLoS One 13, e0196869 (2018).
Baldassarre, S. et al. NTproBNP in insulin-resistance mediated conditions: overweight/obesity, metabolic syndrome and diabetes. The population-based Casale Monferrato study. Cardiovasc. Diabetol. 16, 119 (2017).
Khan, A. M. et al. Cardiac natriuretic peptides, obesity, and insulin resistance: evidence from two community-based studies. J. Clin. Endocrinol. Metab. 96, 3242–3249 (2011).
Kim, F. et al. Brain natriuretic peptide and insulin resistance in older adults. Diabet. Med. 34, 235–238 (2017).
Walford, G. A. et al. Circulating natriuretic peptide concentrations reflect changes in insulin sensitivity over time in the Diabetes Prevention Program. Diabetologia 57, 935–939 (2014).
Halfinger, B. et al. Unraveling the molecular complexity of O-glycosylated endogenous (N-terminal) pro-B-type natriuretic peptide forms in blood plasma of patients with severe heart failure. Clin. Chem. 63, 359–368 (2017).
Lewis, L. K. et al. ProBNP that is not glycosylated at threonine 71 is decreased with obesity in patients with heart failure. Clin. Chem. 65, 1115–1124 (2019).
Alcala, M., Calderon-Dominguez, M., Serra, D., Herrero, L. & Viana, M. Mechanisms of impaired brown adipose tissue recruitment in obesity. Front. Physiol. 10, 94 (2019).
Cypess, A. M. et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 360, 1509–1517 (2009).
Saito, M. et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58, 1526–1531 (2009).
van Marken Lichtenbelt, W. D. et al. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 360, 1500–1508 (2009).
Zingaretti, M. C. et al. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 23, 3113–3120 (2009).
Wang, T. J. et al. Impact of obesity on plasma natriuretic peptide levels. Circulation 109, 594–600 (2004).
Seferovic, J. P. et al. Effect of sacubitril/valsartan versus enalapril on glycaemic control in patients with heart failure and diabetes: a post-hoc analysis from the PARADIGM-HF trial. Lancet Diabetes Endocrinol. 5, 333–340 (2017).
Collins, S. A heart–adipose tissue connection in the regulation of energy metabolism. Nat. Rev. Endocrinol. 10, 157–163 (2014).
Glode, A. et al. Divergent effects of a designer natriuretic peptide CD-NP in the regulation of adipose tissue and metabolism. Mol. Metab. 6, 276–287 (2017).
Rorth, R. et al. The prognostic value of troponin T and N-terminal pro B-type natriuretic peptide, alone and in combination, in heart failure patients with and without diabetes. Eur. J. Heart Fail. 21, 40–49 (2019).
Abrahamsson, N., Engstrom, B. E., Sundbom, M. & Karlsson, F. A. Gastric bypass surgery elevates NT-ProBNP levels. Obes. Surg. 23, 1421–1426 (2013).
Gabrielsen, A. M. et al. The effect of surgical and non-surgical weight loss on N-terminal pro-B-type natriuretic peptide and its relation to obstructive sleep apnea and pulmonary function. BMC Res. Notes 9, 440 (2016).
Docherty, N. G., Fandriks, L., le Roux, C. W., Hallersund, P. & Werling, M. Urinary sodium excretion after gastric bypass surgery. Surg. Obes. Relat. Dis. 13, 1506–1514 (2017).
Belluardo, P. et al. Lack of activation of molecular forms of the BNP system in human grade 1 hypertension and relationship to cardiac hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 291, H1529–H1535 (2006).
Macheret, F. et al. Human hypertension is characterized by a lack of activation of the antihypertensive cardiac hormones ANP and BNP. J. Am. Coll. Cardiol. 60, 1558–1565 (2012).
Seven, E. et al. Higher serum concentrations of N-terminal pro-B-type natriuretic peptide associate with prevalent hypertension whereas lower associate with incident hypertension. PLoS One 10, e0117864 (2015).
Newton-Cheh, C. et al. Association of common variants in NPPA and NPPB with circulating natriuretic peptides and blood pressure. Nat. Genet. 41, 348–353 (2009).
Soares-da-Silva, P. & Fernandes, M. H. Synthesis and metabolism of dopamine in the kidney. Effects of sodium chloride, monoamine oxidase inhibitors and α-human atrial natriuretic peptide. Am. J. Hypertens. 3, 7S–10S (1990).
Pandey, K. N. Molecular and genetic aspects of guanylyl cyclase natriuretic peptide receptor-A in regulation of blood pressure and renal function. Physiol. Genomics 50, 913–928 (2018).
Kouyoumdzian, N. M. et al. Atrial natriuretic peptide stimulates dopamine tubular transport by organic cation transporters: a novel mechanism to enhance renal sodium excretion. PLoS One 11, e0157487 (2016).
Gupta, D. K., de Lemos, J. A., Ayers, C. R., Berry, J. D. & Wang, T. J. Racial differences in natriuretic peptide levels: the Dallas Heart Study. JACC Heart Fail. 3, 513–519 (2015).
Seidelmann, S. B. et al. An NPPB promoter polymorphism associated with elevated N-terminal pro-B-type natriuretic peptide and lower blood pressure, hypertension, and mortality. J. Am. Heart Assoc. 6, e005257 (2017).
Mogelvang, R. et al. Discriminating between cardiac and pulmonary dysfunction in the general population with dyspnea by plasma pro-B-type natriuretic peptide. J. Am. Coll. Cardiol. 50, 1694–1701 (2007).
Modin, D. et al. Prognostic value of left atrial functional measures in heart failure with reduced ejection fraction. J. Card. Fail. 25, 87–96 (2019).
Saito, Y. Roles of atrial natriuretic peptide and its therapeutic use. J. Cardiol. 56, 262–270 (2010).
O’Connor, C. M. et al. Effect of nesiritide in patients with acute decompensated heart failure. N. Engl. J. Med. 365, 32–43 (2011).
Buglioni, A. & Burnett, J. C. Jr. New pharmacological strategies to increase cGMP. Annu. Rev. Med. 67, 229–243 (2016).
Cataliotti, A., Costello-Boerrigter, L. C., Chen, H. H., Textor, S. C. & Burnett, J. C. Jr. Sustained blood pressure-lowering actions of subcutaneous B-type natriuretic peptide (nesiritide) in a patient with uncontrolled hypertension. Mayo Clin. Proc. 87, 413–415 (2012).
Cataliotti, A. et al. Oral human brain natriuretic peptide activates cyclic guanosine 3′,5′-monophosphate and decreases mean arterial pressure. Circulation 112, 836–840 (2005).
Strohl, W. R. Fusion proteins for half-life extension of biologics as a strategy to make biobetters. BioDrugs 29, 215–239 (2015).
de Bold, M. K. et al. Characterization of a long-acting recombinant human serum albumin-atrial natriuretic factor (ANF) expressed in Pichia pastoris. Regul. Pept. 175, 7–10 (2012).
Ding, Y. et al. The effects of fusion structure on the expression and bioactivity of human brain natriuretic peptide (BNP) albumin fusion proteins. Curr. Pharm. Biotechnol. 15, 856–863 (2014).
Sezai, A. et al. Continuous low-dose infusion of human atrial natriuretic peptide in patients with left ventricular dysfunction undergoing coronary artery bypass grafting: the NU-HIT (Nihon University working group study of low-dose Human ANP Infusion Therapy during cardiac surgery) for left ventricular dysfunction. J. Am. Coll. Cardiol. 55, 1844–1851 (2010).
McKie, P. M. et al. A human atrial natriuretic peptide gene mutation reveals a novel peptide with enhanced blood pressure-lowering, renal-enhancing, and aldosterone-suppressing actions. J. Am. Coll. Cardiol. 54, 1024–1032 (2009).
Mezo, A. R. et al. Atrial natriuretic peptide-Fc, ANP-Fc, fusion proteins: semisynthesis, in vitro activity and pharmacokinetics in rats. Bioconjug. Chem. 23, 518–526 (2012).
Zhang, S. M. et al. A new chimeric natriuretic peptide, CNAAC, for the treatment of left ventricular dysfunction after myocardial infarction. Sci. Rep. 7, 10099 (2017).
McGregor, A., Richards, M., Espiner, E. A., Yandle, T. G. & Ikram, H. Brain natriuretic peptide administered to man: actions and metabolism. J. Clin. Endocrinol. Metab. 70, 1103–1107 (1990).
Chen, H. H. et al. Novel protein therapeutics for systolic heart failure: chronic subcutaneous B-type natriuretic peptide. J. Am. Coll. Cardiol. 60, 2305–2312 (2012).
Wang, W., Ou, Y. & Shi, Y. AlbuBNP, a recombinant B-type natriuretic peptide and human serum albumin fusion hormone, as a long-term therapy of congestive heart failure. Pharm. Res. 21, 2105–2111 (2004).
Dickey, D. M. & Potter, L. R. Dendroaspis natriuretic peptide and the designer natriuretic peptide, CD-NP, are resistant to proteolytic inactivation. J. Mol. Cell. Cardiol. 51, 67–71 (2011).
Martin, F. L. et al. CD-NP: a novel engineered dual guanylyl cyclase activator with anti-fibrotic actions in the heart. PLoS One 7, e52422 (2012).
Martin, F. L. et al. Experimental mild renal insufficiency mediates early cardiac apoptosis, fibrosis, and diastolic dysfunction: a kidney-heart connection. Am. J. Physiol. Regul. Integr. Comp. Physiol. 302, R292–R299 (2012).
Kawakami, R. et al. A human study to evaluate safety, tolerability, and cyclic GMP activating properties of cenderitide in subjects with stable chronic heart failure. Clin. Pharmacol. Ther. 104, 546–552 (2018).
Bruneau, B. G. Atrial natriuretic factor in the developing heart: a signpost for cardiac morphogenesis. Can. J. Physiol. Pharmacol. 89, 533–537 (2011).
Ichiki, T., Dzhoyashvili, N. & Burnett, J. C. Jr. Natriuretic peptide based therapeutics for heart failure: cenderitide: a novel first-in-class designer natriuretic peptide. Int. J. Cardiol. 281, 166–171 (2019).
Author information
Authors and Affiliations
Contributions
J.P.G., B.G.B., H.R.R., T.O. and M.K.d.B. researched data for the article. All the authors discussed the content of the manuscript. J.P.G., B.G.B., H.R.R., M.K.d.B. and A.J.d.B. wrote the manuscript. All the authors reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Cardiology thanks A. Clerico and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Goetze, J.P., Bruneau, B.G., Ramos, H.R. et al. Cardiac natriuretic peptides. Nat Rev Cardiol 17, 698–717 (2020). https://doi.org/10.1038/s41569-020-0381-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-020-0381-0